Introduction
The taxonomic position and ranking of the taxa named Eruca vesicaria (L.) Cav., E. sativa Miller and E. pinnatifida (Desf.) Pomel are controversial. Treated as three separate species by Greuter et al. (Reference Greuter, Burdet and Long1986), more recently E. vesicaria and E. sativa have been united as subspecies under the older, accepted name E. vesicaria (Gómez-Campo, Reference Gómez-Campo, Castroviejo, Aedo, Gómez-Campo, Laínz, Montserrat, Morales, Muñoz Garmendia, Nieto Feliner, Rico, Talavera and Villar1993, Reference Gómez-Campo and Gómez-Campo1999; Tutin, Reference Tutin, Tutin, Burges, Chater, Edmondson, Heywood, Moore, Valentine, Walters and Webb1993; Jalas et al., Reference Jalas, Suominen and Lampinen1996). Gómez-Campo (Reference Gómez-Campo2003) concluded that although it was difficult to ascribe specimens to a particular subspecies based on morphological traits, the presence/absence of persistent sepals and the length of lower fruit pedicels were appropriate as discriminators. Eruca pinnatifida has long been considered a subspecies of E. vesicaria (Maire, Reference Maire1965). For the remainder of this paper, these three taxa will be referred to as E. vesicaria subsp. sativa, subsp. vesicaria and subsp. pinnatifida.
Subspecies sativa is cultivated in southern Europe, north and north-east Africa, the Middle East, central Asia and north and central India. It occurs as a wild plant in the Mediterranean area, north and north-east Africa, the Balkans and the Near and Middle East to Afghanistan, but its true native range is difficult to ascertain since it has been introduced or escaped and naturalized in several European and Asian countries and even on other continents, including North and South America and Australia (Al-Shehbaz, Reference Al-Shehbaz1985; Specht and Diederichsen, Reference Specht, Diederichsen and Hanelt2001). It is also a cosmopolitan weed and a host for several fungi and viruses that attack other cruciferous crops (Al-Shehbaz, Reference Al-Shehbaz1985). Its earliest cultivation dates back to the ancient Romans and Greeks. The pods of cultivated forms are more robust and their seeds are larger than those of the wild forms (Gómez-Campo and Prakash, Reference Gómez-Campo, Prakash and Gómez-Campo1999). It is currently grown in Europe and infrequently in North America as a pungent or non-pungent salad (arugula, rocket, rúcula), spice (in sauces and prepared mustard) or medicinal plant; it is also cultivated extensively in central and western Asia for seed oil, called ‘jamba-oil’ in India (Al-Shehbaz, Reference Al-Shehbaz1985; Yaniv et al., Reference Yaniv, Schafferman and Amar1998; Specht and Diederichsen, Reference Specht, Diederichsen and Hanelt2001). The oil is used for human nutrition, as lamp oil, as a lubricant, and for medicinal and cosmetic purposes (Al-Shehbaz, Reference Al-Shehbaz1985; Yaniv et al., Reference Yaniv, Schafferman and Amar1998). In Asia, the seed cake and the entire plant are also used as fodder for domestic animals. The seed oil content of subsp. sativa ranges from 22 to 41% and is rich in erucic acid (Al-Shehbaz, Reference Al-Shehbaz1985; Yaniv et al., Reference Yaniv, Elber, Zur and Schafferman1991, Reference Yaniv, Schafferman and Amar1998; Yadava et al., Reference Yadava, Friedt and Gupta1998; Mandal et al., Reference Mandal, Yadav, Singh, Begum, Suneja and Singh2002), which makes this species a potential future source of industrial oil (Yaniv et al., Reference Yaniv, Schafferman and Amar1998). The seed also contains significant amounts of 4-methylthiobutyl glucosinolate (glucoerucin), which has both direct and indirect antioxidant activity and may be of value for human nutrition (Barillari et al., Reference Barillari, Canistro, Paolini, Ferroni, Pedulli, Iori and Valgimigli2005) in addition to several potential non-food uses (Tiyagi and Alam, Reference Tiyagi and Alam1995; Angelini et al., Reference Angelini, Lazzeri, Galletti, Cozzani, Macchia and Palmieri1998). Subsp. vesicaria from Spain is not cultivated (Gómez-Campo and Prakash, Reference Gómez-Campo, Prakash and Gómez-Campo1999), while the North African subsp. pinnatifida, found in eastern Morocco and central regions of Algeria and Tunisia, is sometimes cultivated as a leaf vegetable or grown as a fresh forage in oases in the Sahara (Specht and Diederichsen, Reference Specht, Diederichsen and Hanelt2001).
Molecular markers, including random amplified polymorphic DNA (RAPD), amplified fragment length polymorphisms (AFLP) and simple sequence repeat polymorphisms (SSR), are increasingly being utilized as tools for assessing genetic diversity in crop species (Powell et al., Reference Powell, Morgante, Andre, Hanafey, Vogel, Tingey and Rafalski1996). The AFLP technique produces highly reproducible, dominant markers well suited for estimating levels of genetic diversity (Vos et al., Reference Vos, Hogers, Bleeker, Reijans, van de Lee, Hornes, Frijters, Pot, Peleman, Kuiper and Zabeau1995). The technique has been applied to assess genetic variation in various crucifer oilseeds including Brassica carinata A. Braun (Warwick et al., Reference Warwick, Gugel, McDonald and Falk2006), B. juncea (Srivastava et al., Reference Srivastava, Gupta, Pental and Pradhan2001; Burton et al., Reference Burton, Ripley, Potts and Salisbury2004), B. napus (Lombard et al., Reference Lombard, Baril, Dubreuil, Blouet and Zhang2000; Sobotka et al., Reference Sobotka, Dolanská, Čurn and Ovesná2004), B. nigra L. Koch (Negi et al., Reference Negi, Sabharwal, Bhat and Lakshmikumaran2004), B. rapa (Zhao et al., Reference Zhao, Wang, Deng, Lou, Wu, Sun, Xu, Vromans, Koornneef and Bonnema2005) and Raphanus sativus (Huh and Ohnishi, Reference Huh and Ohnishi2002; Muminović et al., Reference Muminović, Merz, Melchinger and Lübberstedt2005). The purpose of this study was to evaluate patterns and levels of genetic diversity in the vesicaria–sativa–pinnatifida complex based on AFLP polymorphisms, and to evaluate agronomic and seed quality data from accessions of E. vesicaria subsp. sativa grown in the field in western Canada.
Materials and methods
Plant material
Accession numbers and sources of seed for 184 Eruca accessions are listed in Supplementary Table 1 (available online only at http://journals.cambridge.org). Most accessions of subsp. sativa were received from the United States Department of Agriculture (USDA) GRIN system (as E. sativa Mill.) or the Agriculture and Agri-Food Canada (AAFC) Saskatoon Research Centre (SRC). A further 14 accessions of subsp. sativa, nine of subsp. vesicaria and one of subsp. pinnatifida, were collected from natural habitats from various regions of Spain and Morocco; one accession of subsp. sativa from Mexico was obtained from the AAFC Eastern Cereal and Oilseed Research Centre (ECORC) collection (Ottawa). A sub-sample of 46 accessions was subjected to AFLP analyses (Supplementary Table 1, Fig. 1); including representatives from all three subspecies, with accessions of subsp. sativa chosen to represent a broad range of geographic diversity for origin, including Afghanistan, Cyprus, Egypt, India, Iran, Morocco, Pakistan, Spain and Turkey. For the field trials, 159 accessions of subsp. sativa from the USDA and the AAFC-SRC were tested.

Fig. 1 Genetic similarity among 46 accessions of Eruca vesicaria based on AFLP data. The dendrogram was produced using UPGMA clustering of pairwise similarity distances between accessions. Accession numbers are given in Supplementary Table 1 (available online only at http://journals.cambridge.org).
AFLP protocols
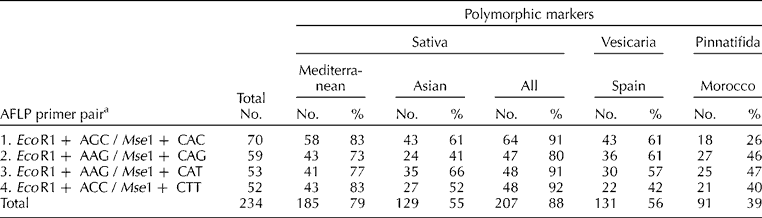
Plants were grown in a controlled environment. Two to three young leaves were collected from each of 10 individuals per accession and stored at − 80°C; two bulked samples per accession (b1 and b2) were prepared with equal amounts of leaf tissue from five individuals. Genomic DNA was extracted from lyophilized and ground material using the Nucleon PhytoPure DNA Isolation Kit (GE Healthcare Bio-Sciences Corp., Piscataway, New Jersey, USA) according to the manufacturer's directions. All bulked DNA samples were diluted to 50 ng/μl. AFLPs were generated based on the protocol of Vos et al. (Reference Vos, Hogers, Bleeker, Reijans, van de Lee, Hornes, Frijters, Pot, Peleman, Kuiper and Zabeau1995) as detailed in Warwick et al. (Reference Warwick, Gugel, McDonald and Falk2006). Four selective primer pairs were used (Table 1). The amplified products were separated by polyacrylamide gel electrophoresis (PAGE) in an automated sequencer (LI-COR, Lincoln, Nebraska, USA) and infrared gel images analysed using a GeneIR (Scanalytics) program.
Table 1 Number of markers detected for each of four AFLP primer pairs and numbers and frequencies of polymorphic markers detected within the clusters observed in Fig. 1: Sativa (Mediterranean: 18 accessions of subsp. sativa; Asian: 15 accessions of subsp. sativa; All Sativa: 33 accessions of subsp. sativa, excluding three from Morocco); Vesicaria (Spain: nine accessions of subsp. vesicaria); and Pinnatifida (Morocco: four accessions, including one of subsp. pinnatifida and three of subsp. sativa from Morocco)
a The core sequences of primers for the selective amplification were as follows: 5′GACTGCGTACCAATTC3′ for the EcoR1 primer and 5′GATGAGTCCTGAGTAA3′ for the Mse1 primer. Each primer contained three selective nucleotides at the 3′ end (e.g. EcoR1+ AGC contained the core sequence plus AGC at the end).
Table 2 Means, standard deviation (SD) and ranges of values for agronomic and seed quality traits of 159 accessions of Eruca vesicaria subsp. sativa grown in field trials at Saskatoon, Saskatchewan, averaged for 1997 and 1998

a Includes 2-propenyl, 3-butenyl, 4-pentenyl, 2-hydroxy-3-butenyl, 2-hydroxy-4-pentenyl, 2-phenylethyl, 3-methylthiopropyl, 5-methylthiopentyl, 4-hydroxybenzyl, 3-indolylmethyl, 4-hydroxy-3-indolylmethyl and other unidentified glucosinolates.
b Includes C16:1 (palmitoleic), C20:2 (eicosadienoic), C22:2 (docosadienoic), C24:1 (nervonic) and other unidentified fatty acids.
c Includes C14:0 (myristic), C16:0 (palmitic), C18:0 (stearic), C20:0 (arachidic), C22:0 (behenic) and C24:0 (lignoceric).
Table 3 Means±standard deviation by cluster for 14 traits recorded for 159 accessions of Eruca vesicaria subsp. sativa in field trials at Saskatoon, Saskatchewan, in 1997 and 1998

a Clusters based on UPGMA clustering of pairwise similarity distances between accessions (Fig. 2).
b DTF, days to flower; DTM, days to mature; CH, crop height (cm); LS, lodging severity (1–5); BS, blackleg severity (0–5); SW, thousand seed weight (g); OIL, % seed oil; PRO, % seed protein; MTB, 4-methylthiobutyl glucosinolate (μmol/g whole seed); Fatty acids (% of total): C18:1, oleic; C18:2, linoleic; C18:3, linolenic; C20:1, eicosenoic; C22:1, erucic.
Field trials
The 159 subsp. sativa accessions (Supplementary Table 1, Fig. 2) were sown in mid-May at the AAFC-SRC Research Farm, Saskatoon, Saskatchewan, Canada (52°07′N 106°38′W) in 1997 and 1998. Each plot consisted of two rows, 18 cm apart and 3 m in length with 100 seeds per row. Plots were 1 m apart and flanked by two rows of barley. Limited seed availability in 1997 allowed only single plots of each accession to be sown that year; in 1998, two replicate plots of each accession were sown using seed harvested from isolation plots grown in 1997. Agronomic and seed quality traits of interest to plant breeders were evaluated on a plot basis as follows:
● Days to flower: the number of days from seeding to when about 20% of the plants had open flowers.
● Days to maturity: the number of days from seeding to when about 90% of the pods had changed colour and seeds were firm, equivalent to a moisture content of about 25%.
● Crop height: the mean of two measurements taken near the centre of each plot after flowering and prior to swathing maturity.
● Lodging severity was determined after flowering and prior to swathing maturity using a visual rating scale of 1 (plants erect) to 5 (plants flat on ground).
● Blackleg severity was evaluated on plants at swathing maturity in a field where disease inoculum was present. Westar, a B. napus cultivar highly susceptible to blackleg, was sown in single rows to provide and monitor disease pressure. A random sample of 25 plants per accession per replicate (one replicate in 1997; two replicates in 1998) was uprooted; each plant was cut through the hypocotyl and/or tap root and scored for blackleg severity using a rating scale of 0 (no diseased tissue visible in the cross-section) to 5 (diseased tissue occupies 100% of cross-section with significant constriction of affected tissues; plant dead). Mean blackleg severity over all replicates was calculated for each accession.
● Seed weight: a random sample of 500 seeds was dried to < 5% moisture content and weighed.
● Oil content was determined using continuous wave, low-resolution nuclear magnetic resonance (AOCS, 2000).
Protein content was analysed by the Dumas Total Combustion Method (AOCS, 1999).
● Fatty acid composition of the seed oil was determined by gas chromatography (AOCS, 1997) following preparation of fatty acid methyl esters by base-catalysed methanolysis (Thies, Reference Thies1971).
● Glucosinolate composition and content of the seed meal were determined by gas chromatography (Sosulski and Dabrowski, Reference Sosulski and Dabrowski1984).

Fig. 2 Similarity among 159 accessions of Eruca vesicaria subsp. sativa based on 1997 and 1998 field evaluation data collected at Saskatoon, Saskatchewan, Canada. The dendrogram was produced using UPGMA clustering of pairwise similarity distances between accessions. Accession numbers are given in Supplementary Table 1 (available online only at http://journals.cambridge.org).
Data analysis
AFLP bands were scored as either present (1) or absent (0) for all samples. Fragments showing the same electrophoretic mobility were treated as identical. Pairwise genetic similarity measures between each accession were obtained from the binary matrix with the Numerical Taxonomy and Multivariate Analysis package in NTSYSpc version 2.02 (Rohlf, Reference Rohlf1997) using the SIMQUAL option. The Sokal and Michener's simple matching (SM) coefficient and the Jaccard (J) coefficient were computed. Dendrograms were generated from each similarity matrix using the unweighted pair-group arithmetic average (UPGMA) clustering procedure in a sequential agglomerative hierarchical combinatorial (SAHN) strategy. The degree of relationship between the two similarity matrices (SM and J) was measured using the product–moment correlation r [ = normalized Mantel statistic Z (Mantel, Reference Mantel1967)] between the two similarity matrices calculated using the MXCOMP function in NTSYS. A cophenetic correlation procedure was also used to compare the matrix of ultrametric distances (generated from the tree matrix) with the pairwise similarity matrix. An analysis of molecular variance (AMOVA) was performed using Arlequin version 3.01 (Excoffier et al., Reference Excoffier, Laval and Schneider2006) to partition the total genetic variation among and within the clusters observed in the UPGMA analysis. The ordination method principal components analysis (PCA), available in NTSYS, was also used to confirm the results of cluster analysis (data not shown).
Means, standard deviations and ranges were calculated in Microsoft Excel for each trait of the 159 subsp. sativa accessions (see Table 2). The data, with the exception of ‘Other glucosinolates’, ‘Other fatty acids’, ‘Total saturated fatty acids’ (excluded as minor components) and ‘Total glucosinolates’ (excluded as a derived value), were analysed using NTSYS. The data were subjected to linear transformation using the STAND option to reduce the effects of different scales of measurement for the different characters. Pairwise distances between accessions were then calculated using the SIMINT option in NTSYS. Two distance coefficients were compared including DIST, the average taxonomic distance and EUCLID, the Euclidean distance. Both coefficients yielded identical matrices. Dendrograms were generated from the DIST matrix using the UPGMA clustering procedure in the SAHN strategy in NTSYS. A cophenetic correlation procedure was used to compare the matrix of ultrametric distances (generated from the tree matrix) with the pairwise similarity matrix. The results of cluster analysis and the relative importance of the 14 variables in separating the accessions were confirmed with PCA. The agronomic data were standardized (STAND) and a correlation matrix among the variables computed (SIMINT, CORR). The first three principal component axes (i.e. eigenvectors) were extracted from the correlation matrix (EIGEN) and a visual three-dimensional plot of variables defining these axes produced. Means and standard deviations were calculated for each of the resultant clusters.
Results
AFLP analysis
A total of 234 polymorphic AFLP fragments were generated [Table 1, Supplementary Table 2 (available online only at http://journals.cambridge.org)]. For each accession, the data from the two bulked samples (b1 and b2) were combined to give a single score of fragment presence/absence in a given accession. Fragment size ranged from 54 to 357 bp (Supplementary Table 2). About 20% of the 234 markers occurred at greater than 80% frequency (i.e. in 37 of the 46 accessions), while less than 10% of the markers were found in a single accession. A comparable number of markers (52–70) were generated per primer pair.
The distance matrices based on the SM and J similarity coefficients were highly correlated (r = 0.943). The high correlation between the SM- and J-based matrices showed that the allelic relationship between the absence and presence of a given band can be assumed (Lombard et al., Reference Lombard, Baril, Dubreuil, Blouet and Zhang2000). Since the cophenetic value for the similarity matrices generated for the SM and J coefficients was so high, only the SM data were used to generate the dendrograms presented here. A single dendrogram (Fig. 1) was obtained using the SM coefficient for pairwise distance calculations between accessions. A single dendrogram was also obtained with the J coefficient (data not shown, but available upon request from the senior author). The cophenetic correlation was also high (r = 0.955 and 0.922) for the SM and J coefficient matrix and the cluster matrix, indicating a good fit of the cluster analysis to the SM and J similarity matrix, respectively. Both analyses revealed four main clusters: ‘Sativa’ (Mediterranean + Mexico, 18 accessions of subsp. sativa), ‘Sativa’ (Asia, 15 accessions of subsp. sativa), ‘Vesicaria’ (Spain, nine accessions of subsp. vesicaria) and a Moroccan ‘Pinnatifida’ cluster (four accessions, including one accession of subsp. pinnatifida and three accessions of subsp. sativa from Morocco). Within the Sativa cluster, several accessions clustered by country of origin, the most notable being those from Spain and those from India/Pakistan. In contrast, one accession from Turkey was in the Mediterranean and the other in the Asian cluster. AMOVA (Supplementary Table 3, available online only at http://journals.cambridge.org) and PCA (data not shown) of the molecular data also revealed the same four clusters. For the AMOVA analysis, the accessions were placed in groups corresponding to either three (Sativa, Vesicaria, Pinnatifida) or four (Sativa – Mediterranean, Sativa – Asian, Vesicaria, Pinnatifida) clusters. Both AMOVA results indicated significant differences among the groups (P = 0.03).
Similarity values based on the SM coefficient averaged 0.705 (0.562–0.915) among the 33 accessions of the Sativa cluster, with a slightly lower mean and greater range among the 18 Mediterranean (+Mexico) accessions (0.733; 0.653–0.858) versus the 13 Asian accessions (0.823; 0.735–0.897). Similarity values among accessions within the Moroccan Pinnatifida cluster averaged 0.697 (0.650–0.722), while accessions in the Vesicaria cluster were the most similar (0.850; 0.803–0.931).
The three subspecies varied in diversity levels, with accessions in the Sativa cluster having the greatest percentage of polymorphic loci (88%) compared with the Vesicaria (56%) and Pinnatifida (39%) clusters (Table 1). Differences were also detected between the two Sativa groups, with greater diversity in the Mediterranean (79%) versus Asian (55%) accessions. Levels of diversity can also be assessed by numbers of unique alleles (Supplementary Table 2). The Sativa accessions were the most diverse, with 39 of the 234 AFLP markers unique to the Sativa cluster; 11 of these were confined to the Mediterranean accessions, whereas only one was unique to the Asian accessions. Eight AFLP markers were unique to the Pinnatifida cluster, while the Vesicaria cluster had only one unique AFLP marker. The genetic variation within and among the clusters was further examined by performing AMOVA on the molecular data (Supplementary Table 3). Accessions were placed in groups corresponding to either three (Sativa, Vesicaria, Pinnatifida) or four (Sativa – Mediterranean, Sativa – Asian, Vesicaria, Pinnatifida) clusters. Among-group variation accounted for approximately 32 and 37% of the total variation in the two analyses, respectively, and among accessions within-group variation accounted for 37 and 29% of the total variation, respectively. The discriminating power of each primer pair was high, as each primer pair analysed separately was able to uniquely identify all 46 accessions tested.
Agronomic traits
Means and ranges of values for agronomic and seed quality traits, averaged for years 1997 and 1998, are given in Table 2. In general, considerable variation was observed for most traits. Individual ANOVA performed on each of the variables indicated statistically significant differences among accessions (P < 0.05) for all traits except ‘Days to maturity’ and ‘Other glucosinolates’.
The among-accession pairwise similarity matrices generated for the DIST and EUCLID coefficients were identical (r = 1.00), and only the DIST matrix was used for generating the dendrograms presented here. The cophenetic correlation was high (r = 0.86) for the DIST pairwise similarity matrix and the cluster matrix, indicating a good fit of the cluster analysis to the pairwise DIST matrix. A single dendrogram (Fig. 2) was generated using the UPGMA clustering procedure. Six main clusters were evident; two clusters were represented by one or two accessions and one cluster contained the majority (123) of the accessions. Accessions did not group by geographic origin.
Principal components analysis, often used to determine the relative importance of classification variables, constructs a new set of orthogonal coordinate axes, such that the projection of points onto the axes have maximum variance. In our trials, the first three principal components explained 66.4% of the total variation in the data: 43.4% in the first, 12.5% in the second and 10.5% in the third (Supplementary Table 4, available online only at http://journals.cambridge.org). Five principal components explained 81.5% of the total variation. In the first principal component, high loadings were estimated for most traits; few traits had high loadings in the second and third principal components (Supplementary Table 4). Traits with larger loadings (+ or − ) contribute proportionally more towards explaining the total variation accounted for in that particular eigenvector. Loadings associated with agronomic traits were similar in magnitude to those associated with seed quality traits. No single trait clearly dominated in separating the accessions into clusters. Cluster means for each of the 14 traits are given in Table 3. Cluster 6, consisting of a single accession (S10), was the most distinct as it was early to flower and mature, short in stature, large seeded with a high oil content, and had high proportions of oleic and eicosenoic acid.
Differences were also detected between the Mediterranean and Asian groups. Thirteen Mediterranean accessions (103 d to mature, 96 cm tall, 28.0% oil, 2.3 g/1000 seeds) were compared with 141 Asian accessions (100 d to mature, 87 cm tall, 28.5% oil, 2.8 g/1000 seeds); differences between the groups were statistically significant for crop maturity, height and thousand seed weight, but not for oil content (least significant difference, P = 0.05).
Discussion
AFLP analysis
The results of this study confirmed the separation of subspecies sativa, vesicaria (native to Spain) and pinnatifida (native to North Africa) and clearly showed that the three accessions of sativa from Morocco are closer to pinnatifida, and that the Spanish native vesicaria was more similar to pinnatifida than to sativa. The results also indicated a separation of Mediterranean and Asian subsp. sativa accessions, which likely reflects differences in their wild or cultivated condition or perhaps in their primary usage, vegetable versus seed oil, in these two regions. The introduced weedy subsp. sativa collection from Mexico was confirmed as Mediterranean (most likely from Spain) in origin, consistent with the suggestion by Rollins (Reference Rollins1993) that subsp. sativa is the adventive form in North America and not vesicaria. Indeed, subsp. sativa is an especially abundant and widespread weed in central and southern Mexico, where it commonly forms solid stands covering many hectares (Rollins, Reference Rollins1993). The three subspecies are weedy in the west Mediterranean region, but only subsp. sativa has spread to other regions, either as a crop or as a weed.
The level of AFLP polymorphism observed in the subsp. sativa accessions was as high or higher than those observed in some Brassica oilseed crops: 79% polymorphism in 426 AFLP markers for 18 accessions of B. nigra (Negi et al., Reference Negi, Sabharwal, Bhat and Lakshmikumaran2004), 91% polymorphism in 524 markers for 161 accessions of B. rapa (Zhao et al., Reference Zhao, Wang, Deng, Lou, Wu, Sun, Xu, Vromans, Koornneef and Bonnema2005), 62% polymorphism in 1251 markers for 30 lines of B. juncea (Srivastava et al., Reference Srivastava, Gupta, Pental and Pradhan2001), 35% polymorphism in 751 markers for 92 B. juncea breeding lines (Burton et al., Reference Burton, Ripley, Potts and Salisbury2004) and 23% polymorphism in 296 markers for 66 accessions of B. carinata (Warwick et al., Reference Warwick, Gugel, McDonald and Falk2006). The four EcoR1 or Mse1 primer pairs showed similar amounts of polymorphisms for the Sativa accessions, ranging from 80 to 92%. Similar AFLP polymorphism frequencies among primer pairs have been reported in other Brassica studies, including a B. napus study with 17 primer pairs (Lombard et al., Reference Lombard, Baril, Dubreuil, Blouet and Zhang2000), a B. juncea study with 10 primer pairs (Burton et al., Reference Burton, Ripley, Potts and Salisbury2004) and a B. carinata study with four primer pairs (Warwick et al., Reference Warwick, Gugel, McDonald and Falk2006).
Information on genetic diversity and/or genetic relatedness among genotypes of the E. vesicaria subspecies is currently limited. The present study demonstrated the utility of AFLP markers in assessing genetic relationships between cultivars relative to their genetic origin, and was certainly effective at the regional level. AFLPs also are important markers for fingerprinting cultivars and may be useful in distinguishing Eruca accessions. Each primer pair in the present study showed high discriminating power, and was able to uniquely identify all 46 accessions tested. Lombard et al. (Reference Lombard, Baril, Dubreuil, Blouet and Zhang2000) showed that AFLP markers had high discriminating power to easily identify cultivars of B. napus for plant registration and protection purposes, as did Warwick et al. (Reference Warwick, Gugel, McDonald and Falk2006) for B. carinata. AFLP markers could also serve as a valuable breeding tool, with genetic distance information used to facilitate the identification of diverse parents to cross in hybrid combinations in order to maximize heterosis (Charcosset and Moreau, Reference Charcosset and Moreau2004). Information on genetic relatedness can also be used for germplasm conservation and management of genetic resources, including identification of core collections.
Agronomic traits
In general, considerable variation was observed for most agronomic and seed quality traits in this study. Unlike the results of the AFLP analysis, accessions did not group by geographic origin and no single trait clearly dominated in separating the accessions into clusters. Divergent genotypes, as identified by their assignment to different clusters, may have good breeding value and maximum variability may be achieved by utilizing such genotypes for crosses. Selection from the Asian group is likely the most appropriate for initial development of Canadian oilseed lines, as Asian accessions tended to have higher oil contents, were earlier maturing, shorter and had larger seed.
Variability in agronomic and seed quality traits has been reported previously for E. vesicaria subsp. sativa. Many of these studies were conducted with relatively few accessions grown in one (Yaniv et al., Reference Yaniv, Schafferman and Amar1998; Mandal et al., Reference Mandal, Yadav, Singh, Begum, Suneja and Singh2002) or several (Singh and Rajput, Reference Singh and Rajput1993a, Reference Singh and Rajputb; Gurgar et al., Reference Gurgar, Sharma and Singh1999) environments, or with seed collected from several sites (Yaniv et al., Reference Yaniv, Elber, Schafferman, Ben-Moshe and Zur1995). Given the significant effects of environment and genotype × environment on the expression of agronomic and seed quality traits in this species (Singh and Rajput, Reference Singh and Rajput1993a, Reference Singh and Rajputb; Gurgar et al., Reference Gurgar, Sharma and Singh1999), it is inappropriate to draw broad conclusions regarding species diversity from trials with few accessions. A larger number of accessions was evaluated by Sodani et al. (Reference Sodani, Sastry and Nehra1990), who grouped 99 accessions into 13 clusters based primarily on yield components; geographic origin was not correlated with assignment of accessions to a cluster. Most (69) accessions were grouped in a single cluster and a comparison of cluster means for different agronomic and seed quality traits indicated differences in maturity, plant morphology, yield components and oil content. Yadava et al. (Reference Yadava, Friedt and Gupta1998) evaluated 100 accessions and reported large variations in oil content and fatty acid profile including erucic, oleic, linoleic, linolenic and eicosenoic acid.
All the accessions evaluated in this study took fewer days to flower (38–48 d) and mature (93–109 d) than reported for other accessions evaluated in India (Sodani et al., Reference Sodani, Sastry and Nehra1990) (51–56 d to 50% flowering, 119–124 d to mature) and Israel (Yaniv et al., Reference Yaniv, Schafferman and Amar1998) (60–88 d to flower, 145–163 d to mature), although the study with the fewest (10) accessions (Yaniv et al., Reference Yaniv, Schafferman and Amar1998) had the most variability for these traits. Most accessions in our study were also taller, and height (71–120 cm) and seed weight (1.5–3.6 g/1000) were more variable, than reported by Sodani et al. (Reference Sodani, Sastry and Nehra1990) (57–84 cm tall, 2.3–3.9 g/1000 seeds) and Yaniv et al. (Reference Yaniv, Schafferman and Amar1998) (1.3–1.9 g/1000 seeds). Seed oil contents in our study (25–31%) were similar to those reported by Yaniv et al. (Reference Yaniv, Schafferman and Amar1998) (25–29%) and Mandal et al. (Reference Mandal, Yadav, Singh, Begum, Suneja and Singh2002) (24–30%), but lower than those reported by Sodani et al. (Reference Sodani, Sastry and Nehra1990) (32–36%), Yadava et al. (Reference Yadava, Friedt and Gupta1998) (32–41%), Gurgar et al. (Reference Gurgar, Sharma and Singh1999) (26–39%) and Sun et al. (Reference Sun, Pan, Liu, Meng, Zhang, Wang and Zeng2004) (23–38%). Seed protein contents in our study ranged from 34–42% and were slightly higher than those reported by Sun et al. (Reference Sun, Pan, Liu, Meng, Zhang, Wang and Zeng2004) (30–38%).
Large variations in fatty acid profiles among accessions were also revealed in our study. Erucic acid was the major fatty acid found in seed oil of all accessions, ranging from 40 to 48% of the total fatty acids. Oleic (12–19%), linoleic (8–10%), linolenic (9–14%) and eicosenoic (7–11%) acids were also present in significant amounts. Values reported in other studies (Yaniv et al., Reference Yaniv, Elber, Zur and Schafferman1991; Das et al., Reference Das, Tyagi and Singhal2001; Mandal et al., Reference Mandal, Yadav, Singh, Begum, Suneja and Singh2002) generally fell within these ranges, but significantly more variation in erucic acid content was reported by Yadava et al. (Reference Yadava, Friedt and Gupta1998) (27–52%) and Yaniv et al. (Reference Yaniv, Schafferman and Amar1998) (33–45%). Erucic acid contributes to the unpalatable flavour of the seed oil and is linked to animal health problems (Yadava et al., Reference Yadava, Friedt and Gupta1998; Das et al., Reference Das, Tyagi and Singhal2001).
The primary glucosinolate in seeds of the accessions evaluated in our study was 4-methylthiobutyl glucosinolate, which comprised about 98% of the total glucosinolates and generally agreed with previous reports (Das et al., Reference Das, Tyagi and Singhal2001, Reference Das, Tyagi and Kaur2003; Barillari et al., Reference Barillari, Canistro, Paolini, Ferroni, Pedulli, Iori and Valgimigli2005). In subsp. sativa, 4-methylthiobutyl glucosinolate is responsible for the pungent flavour of pressed oil-cake (Das et al., Reference Das, Tyagi and Singhal2001); in leaves, however, 4-mercaptobutyl glucosinolate predominates and likely contributes significantly to their unique flavour (Bennett et al., Reference Bennett, Mellon, Botting, Eagles, Rosa and Williamson2002). Many glucosinolates reportedly have beneficial effects in human and animal nutrition, but the presence of glucosinolates in seed meal (oil-cake) generally has a negative effect on palatability and health when consumed in high concentrations (Rosa, Reference Rosa and Gómez-Campo1999). The continuing practice of feeding Eruca oil-cake to animals (Al-Shehbaz, Reference Al-Shehbaz1985; Das et al., Reference Das, Tyagi and Kaur2003), and its potential use in fish diets (Fagbenro, Reference Fagbenro2004), suggests a need for research to reduce glucosinolate levels in this crop.
Accessions of E. vesicaria subsp. sativa that are resistant (Tewari et al., Reference Tewari, Bansal, Tewari, Gómez-Campo, Stringam and Thiagarajah1996) and susceptible (Li et al., Reference Li, Barbetti and Sivasithamparam2005) to blackleg have been reported. Blackleg disease pressure was low in both years of our study and mean blackleg severity values for B. napus cv. Westar (susceptible check; data not shown) ranged from 2.2 to 2.3 on a scale of 0–5, which is too low to identify resistant germplasm conclusively. Almost all (98%) of the accessions tested in our trials were less susceptible to blackleg than Westar and the remainder were similar to Westar. A random selection of plants of both species that had symptoms of blackleg yielded identical colonies of Phoma lingam (Tode ex Fr.) Desmaz., the anamorph of Leptosphaeria maculans, confirming that infection by the blackleg fungus did occur in E. vesicaria subsp. sativa.
The successful cultivation of a large number of E. vesicaria subsp. sativa accessions in our study suggests that this species could be grown as an oilseed crop in western Canada. There is good potential for crop improvement, as the species is reported to have tolerance to cold (as seedlings), heat, drought (Sun et al., Reference Sun, Pan, Liu, Meng, Zhang, Wang and Zeng2004) and salt (Ashraf, Reference Ashraf1994), resistance to various pests (Singh et al., Reference Singh, Ellis, Pink and Phelps1994; Rana et al., Reference Rana, Khokhar and Singh1995; Curto et al., Reference Curto, Dallavalle and Lazzeri2005) and diseases (Tewari and Conn, Reference Tewari and Conn1993; Tewari et al., Reference Tewari, Bansal, Tewari, Gómez-Campo, Stringam and Thiagarajah1996; Bansal et al., Reference Bansal, Tewari, Tewari, Gómez-Campo and Stringam1997; Singh and Kolte, Reference Singh and Kolte1999), and male-sterility (Matsuzawa et al., Reference Matsuzawa, Mekiyanon, Kaneko, Bang, Wakui and Takahata1999) and self-incompatibility alleles for developing hybrid production systems (Verma et al., Reference Verma, Malik and Dhir1977; Sun et al., Reference Sun, Pan, Liu, Meng, Zhang, Wang and Zeng2005). Developing subsp. sativa cultivars with higher yield and seed oil content (25–31%, this study) is also necessary to be competitive with existing crops that have much higher oil contents (rapeseed: 40–45%; flax: 35–45%) (Lühs and Friedt, Reference Lühs, Friedt and Murphy1994) and with other potential new oilseed crops currently under investigation, including B. carinata (25–36%) (Warwick et al., Reference Warwick, Gugel, McDonald and Falk2006) and Crambe abyssinica Hochst. ex R.E. Fries (31–39%) (Warwick and Gugel, Reference Warwick and Gugel2003). Sustainable markets for the oil and meal must be developed and could include both edible and industrial products. Erucic acid and glucosinolate levels would need to be reduced significantly to make the oil and meal acceptable for human and animal nutrition; similar breeding efforts were successful in developing canola from rapeseed (Lühs and Friedt, Reference Lühs, Friedt and Murphy1994). Conversely, breeding efforts may be needed to increase the levels of some antinutritive compounds in order to make subsp. sativa more attractive as an industrial crop. The oil has high repellent activity against some insects that infest stored grain and could replace more toxic pesticides for this purpose (Mohiuddin et al., Reference Mohiuddin, Qureshi, Qureshi, Nasir and Khatri1990). The meal has potential use as a low environmental impact soil fumigant to suppress soilborne plant pathogenic fungi, nematodes (Tiyagi and Alam, Reference Tiyagi and Alam1995) and germination of weed seeds (Angelini et al., Reference Angelini, Lazzeri, Galletti, Cozzani, Macchia and Palmieri1998). Such soil fumigant properties could also be improved by developing genotypes with even higher levels of glucosinolates.
Acknowledgements
Assistance with the field trials was provided by Z. Bainas and D. Hennigan, AAFC-SRC. We gratefully acknowledge Dr J. P. Raney and his technical staff, AAFC-SRC, for conducting the seed quality analyses. We also thank Drs E. Small and J. Cayouette, AAFC-ECORC, and Dr F. Katepa-Mupondwa, AAFC-SRC, for providing helpful comments on an earlier draft of this manuscript.