INTRODUCTION
The phylogenetic relationships among families and subfamilies within the Dactylogyrinea Bychowsky, 1937 remain unresolved (e.g. Kritsky and Boeger, 1989; Lim, 1998; Lim, Timofeeva and Gibson, 2001; Simková et al. 2003). In the classification of Bychowsky (1957), the Calceostomatidae Parona & Perugia, 1890, Dactylogyridae Bychowsky, 1933, and Diplectanidae Bychowsky, 1957 were included in the Dactylogyrinea. Three subfamilies were included in the Dactylogyridae, i.e. the Linguadactylinae Bychowsky, 1957, Dactylogyrinae and Ancyrocephalinae Bychowsky, 1937. Since then, a series of changes happened in the Dactylogyridae.
Firstly, Gussev (1961) and Price (1967) removed genera originally within the Ancyrocephalinae, and proposed 3 new subfamilies, Ancylodiscoidinae Gussev, 1961, Heteronchocleidinae Price, 1967 and Anacanthorinae Price, 1967. Subsequently, Bychowsky and Nagibina (1978) raised the Ancyrocephalinae to family status including 3 other subfamilies originally within the Dactylogyridae, i.e. the Linguadactylinae, Ancylodiscoidinae and Hareocephalinae Young, 1968. Ogawa (1986) removed Pseudodactylogyrus Gussev, 1965 from the Ancyrocephalinae, and proposed a new subfamily, the Pseudodactylogyrinae, within the Ancyrocephalidae sensuBychowsky and Nagibina, 1978. At the same time, Le Brun, Lambert and Justine (1986) raised the Pseudodactylogyrinae to family status. Phylogenetic analysis of the Dactylogyrinea based on morphological characters was performed by Kritsky and Boeger (1989), and the Ancyrocephalidae sensuBychowsky and Nagibina, 1978 appeared polyphyletic as a result. According to the analysis, the Ancyrocephalidae was proposed to be rejected as a junior synonym of the Dactylogyridae, and Pseudodactylogyridae were to be treated as a subfamily. However, Lim (1998) disagreed with this move and proposed that the Ancyrocephalidae be left intact within the Dactylogyrinea until further studies had been carried out. On the other hand, Lim et al. (2001) proposed that the Ancylodiscoidinae Gussev, 1961, be raised to family status to accommodate ancyrocephalid genera almost all of which are parasites of siluriform fishes. Given the controversies over the systematics of the Dactylogyrinea, we have followed Kritsky and Boeger (1989) who considered 3 families, namely the Diplectanidae, Pseudomurraytrematidae Kritsky, Mizelle and Bilqees, 1978 and Dactylogyridae are to be recognized within the Dactylogyrinea, with 9 subfamilies included in the Dactylogyridae.
The Ancyrocephalinae has been a catch-all group ever since its formation (Lim et al. 2001), and one of its genera, Haliotrema was also considered as a taxonomic waste-basket (Klassen, 1994a; Kritsky and Stephens, 2001). Some previous studies have considered Haliotrema as a polyphyletic taxon based on morphological analyses (Klassen, 1994a,b; Kritsky and Stephens, 2001; Kritsky and Boeger, 2002). In fact, some species of Haliotrema from hosts of specific families appeared to form monophyletic groups. For example, Young (1968) proposed 6 species groups for 32 Haliotrema species based on morphological features and host occurrences. Euzet and Suriano (1977) created Ligophorus for species parasitizing on the Mugilidae, and they transferred H. vanbenedenii and H. mugilinus to Ligophorus as new combinations. Kritsky and Boeger (2002) erected Euryhaliotrema to which they transferred 8 Haliotrema species from the Lutjanidae and 1 Haliotrema species from the Haemulidae. Plaisance and Kritsky (2004) created Euryhaliotrematoides and Aliatrema for parasites of the Chaetodontidae, and they transferred 3 Haliotrema species to Euryhaliotrematoides.
Though a number of authors have viewed Haliotrema as a polyphyletic taxon, the validity of all species groups and/or genera, and their inter-relationships, still require further investigation. The morphological phylogenetic analysis of the Haliotrema by Klassen (1994a) showed a clear lack of resolution due to the limited number of morphological characters (see Plaisance, Bouamer and Morand, 2004). Molecular analyses have been suggested by Plaisance et al. (2004) to help resolve taxonomic problems such as the limits of the genus, and also to test the monophyly of the groups already delineated in Haliotrema by Young (1968).
The sequences of partial large subunit (LSU) ribosomal DNA (rDNA) have been successfully used to study phylogenetic relationships of the monogeneans at the high level (Mollaret et al. 1997; Littlewood, Rohde and Clough, 1998; Mollaret, Jamieson and Justine, 2000a; Jovelin and Justine, 2001), as well as at familial, subfamilial and generic levels (Mollaret, Lim and Justine, 2000b; Chisholm et al. 2001; Justine et al. 2002; Olson and Littlewood, 2002; Whittington et al. 2004; Wu et al. 2005a). Given the controversies over the systematics of the Ancyrocephalinae and Haliotrema, the objectives of the present study were to analyse the phylogenetic relationships within the Ancyrocephalinae, and to test the radiation of Haliotrema, using the D1-D2 domains of LSU rDNA because this rDNA region has been effectively used to estimate phylogenetic relationships within the Diplectanidae (see Wu et al. 2005a).
MATERIALS AND METHODS
Parasite samples
Monogeneans were removed from the gills of freshly killed fish and preserved immediately in 75% ethanol. Parasites were fixed in Bleasure's glue (Acacia gum 17·25%, glycerin 13·79%, chloral hydrate 34·48%, distilled water 34·48%) and their sclerotized parts examined using a dissecting microscope equipped with phase-contrast and digital image analysis (Olympus BX51, CoolSNAP-Pro). Individuals were identified to species and classified based on morphological characters, mainly on the sclerotized parts of the haptors and reproductive organs, according to the existing keys and species descriptions (see Yamaguti, 1963; Zhang, Yang and Liu, 2001). In total, 32 species representing 15 recognized genera from 3 families were examined in the present study; their family names, host species, geographical origins and GenBank Accession numbers are listed in Table 1.
Table 1. List of monogenean species used in this study with host species, locality (in China), and GenBank Accession numbers (Asterisks indicate species sequenced in this study.)

DNA extraction, PCR and DNA sequencing
Prior to DNA extraction, individual parasites were removed from Bleasure's glue, placed in 0·5 ml Eppendorf tubes and dipped in 200 μl of TE9 (500 mM Tris-HCl, 200 mM EDTA, and 10 mM NaCl, pH 9·0) for 2–3 h. They were then transferred into 0·2 ml tubes containing 20 μl of lysis buffer (0·45% NP-40, 0·45% Tween-20, 1 mM EDTA, 10 mM Tris-HCl and 20 μg/ml proteinase K) and incubated at 65 °C for 1 h, followed by incubation at 95 °C for 15 min to inactivate the proteinase K. This lysate (8 μl) was used as template in PCR reactions to amplify the D1-D2 domains of the LSU rDNA, using primers C1 (forward; 5′-ACCCGCTGAATTTAAGCAT-3′) and D2 (reverse; 5′-TGGTCCGTGTTTCAAGAC-3′) (Wu et al. 2005b). PCR reactions (50 μl) were performed in 1·5 mM MgCl2; PCR buffer (100 mM Tris-HCl, 500 mM KCl, 0·8% NP-40, pH 8·8) (TakaRa); 200 μM of each dNTP; 0·8 μM of each PCR primer and 2·5 units of Ex Taq polymerase (TakaRa) in a thermocycler (MJ Research) using the following conditions: an initial denaturation at 94 °C for 5 min, followed by 30 cycles of 94 °C for 1 min (denaturation); 56 °C for 1 min (annealing) and 72 °C for 1 min (extension), followed by a final extension at 72 °C for 5 min. Control samples with host (fish) DNA or without genomic DNA (no-DNA controls) were included in each PCR run, but in each case amplicons were detected. Aliquots (5 μl) of amplicons were detected in 1% agarose gels, stained with ethidium bromide, and photographed upon trans-illumination. The remaining 45 μl of each amplicon was purified over a spin column (TakaRa Agarose Gel DNA Purification Kit ver. 2.0) and subjected to automated DNA sequencing (ABI 3730 DNA Sequencer, Shanghai United Gene Inc.) using the same primers (individually) as used for PCR. All sequences are available from DDBJ, EMBL, and GenBank under the Accession numbers shown in Table 1.
Phylogenetic analyses
DNA sequences were edited and aligned with SeqMan II (DNASTAR, Madison, WI) and Clustal X (Thompson et al. 1997) using default parameters and verified visually. Saturation level was assessed by plotting the proportion of differences for transitions (in ordinate) versus transversions (in abscissa) between pairs of species. The relationship was almost linear, revealing the absence of saturation in the data and allowing the use of all substitutions of the whole alignment for phylogenetic reconstruction. Level of sequence variation (not shown) based on uncorrected pairwise distance was calculated using MEGA ver. 3.0 (Kumar, Tamura and Nei, 2004).
For phylogenetic analysis, 6 diplectanids were used as multiple outgroups based on the previous phylogenetic hypotheses (e.g. Kritsky and Boeger, 1989; Boeger and Kritsky, 2001; Simková et al. 2003). Phylogenetic trees were inferred using the maximum-likelihood (ML) and maximum parsimony (MP) with PAUP* ver. 4.0b10 (Swofford, 2001), and the neighbour-joining (NJ) and minimum evolution (ME) with MEGA ver. 3.0 (Kumar et al. 2004). For the ML analysis, the GTR+I+G substitution model (rmat=0·7670 3·9700 1·9653 0·4476 5·0513) with invariable sites (pinvar=0·2395), base frequencies (A 0·1893 C 0·1954 G 0·3010) and the shape parameter of the gamma distribution (α=1·4319) was selected under the Akaike Information Criterion (AIC) implemented in Modeltest ver. 3.5 (Posada and Crandall, 1998). The analyses were performed using the heuristic option, 10 random-addition replicates and the tree-bisection-reconnection (TBR) algorithm. The MP analyses were performed with the heuristic algorithm using equal weighting for all substitutions. Heuristic search was conducted with TBR branch swapping and 10 random-addition replicates. Kimura 2-parameter model was used to estimate distances for the NJ and ME analyses. The robustness of the inferred phylogeny was assessed using a bootstrap procedure with 1000 replications for the MP, NJ and ME analyses. Replicates were restricted to 100 for estimating the ML nodal support because of limited computing time. The purpose of using these different methodologies was to compare their resolution abilities for inferring the phylogenetic relationships in the present study.
RESULTS
The lengths of the D1-D2 domains of the LSU rDNA for all parasite specimens were aligned over a consensus length of 642 bp after removing gaps and ambiguously aligned positions. No sequence variation was detected among individuals of species collected from different host species (2 species, namely H. subancistroides and H. Sinodiplectanotrema argyromus) (Table 1). Usually, molecular divergences among species representing different genera were higher than that among species representing the same genera, particularly within the same family. For example, Pseudorhabdosynochus lantauensis and P. epinepheli differed by 2·7%, and Diplectanum blairense and D. sillagonum by 6% in the D1-D2 domains, whereas sequence divergences ranged from 21·5 to 22·3% among the species representing these 2 genera. However, there were also exceptions. For example, 9·4% molecular divergence was observed between Metahaliotrema mizellei and H. subancistroides, slightly lower than that (10·6%) between M. geminatohamula and M. mizellei. In the present study, inter-specific sequence divergence among species of Haliotrema was the most variable, ranging from 0·3% (H. platycephali vs H. johnstoni) to 30·6% (Haliotrema sp. ZHDDa vs H. fleti). Murraytrema pricei and S. argyromus was the least diverged inter-genus species pair (3·1%), and the second least diverged inter-genus species pair was Cichlidogyrus sclerosus and Scutogyrus longicornis (5·3%).
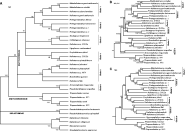
The phylogenetic tree obtained using maximum parsimony (MP) method is presented in Fig. 1A. Six monophyletic clades (Clade 1-Clade 6) were defined on the bootstrap 50% majority-rule consensus MP tree, based on their comparatively robust bootstrap support. Although the 50% majority-rule consensus MP tree could not effectively depict the relationships within the Dactylogyridae, relationships among the 3 families examined in the present study could be well depicted as (Diplectanidae, (Ancylodiscoididae, Dactylogyridae)). However, we can get more details about the relationships within the Dactylogyridae from the maximum-likelihood (ML) analysis under selected model, as well as the neighbour-joining (NJ) analyses using Kimura 2-parameter (K2P) distance (Fig. 1B). As there were no obvious topological differences between the NJ and the bootstrap 50% majority-rule consensus ML trees, only the NJ tree (only ingroups are shown) is presented in Fig. 1B, including the bootstrap values with 100 replications for the ML analysis. In this figure, the 4 most terminal clades (Clade 1-Clade 4) in the Dactylogyridae cluster to show monophyly and the Ligophorus vanbenedenii appears to be the sister group to these clades (Clade 1-Clade 4). The Clade 6 (Pseudodactylogyrus anguillae and Ancyrocephalus mogurndae) appears to be the most basal branch in the Dactylogyridae, and the Clade 5 (Bravohollisia gussevi, Haliotrema sp. MTY, H. fleti, H. platycephali and H. johnstoni) to be the second most.

Fig. 1. Molecular phylogenetic trees obtained utilizing partial LSU rDNA (D1-D2 domains) sequences using diplectanids as multiple outgroups. (A) Maximum parsimony (MP) bootstrap 50% majority-rule consensus tree. Bootstrap values (1000 replicates) are shown along the branches. (B) Partial neighbour-joining (NJ) tree using Kimura 2-parameter (K2P) distance. Bootstrap values shown along the branches are based on 100 replicates for the maximum likelihood (ML) analysis and 1000 replicates for the NJ analysis. (C) Partial minimum evolution (ME) tree obtained based on the K2P distances. Bootstrap values for 1000 replicates indicated at each node.
However, the phylogenetic position of L. vanbenedenii was changed when we used the minimum evolution (ME) method under the K2P model (Fig. 1C; only ingroups are shown). It appeared as the sister group of Clade 4 (Euryhaliotrema johnii, Haliotrema sp. ZHDDb and H. spirotubiforum), although with relatively low bootstrap support (BP=48). Hence, the L. vanbenedenii was merged into the monophyletic group comprising Clade 1 to Clade 4. Though surprising, it seems reasonable when we consider the comparatively low molecular divergences among L. vanbenedenii and species from Clade 4 (18·5–19·4%). The relationship between Clade 5 and Clade 6 was the same as that of the NJ tree, and notably, all methods used in the present study generated identical topologies at the family level as detailed above. Pseudodactylogyrus anguillae appeared as the sister species to A. mogurndae and Clade 6 as the most basal branch in all 4 phylogenetic analyses, indicating the polyphyly of the Ancyrocephalinae.
DISCUSSION
Overview
Olson and Littlewood (2002) suggested that both D1 and D2 domains of LSU rDNA might best be used for resolving relationships within families, and also they pointed out the prime candidate families, including the Dactylogyridae, for studying with these rDNA fragments. Previous studies have also supported the robust phylogenetic resolution ability within the Diplectanidae (e.g. Wu et al. 2005a). In the present study, the phylogenetic reconstructions resulted in the same general pattern among the families as that of previous molecular investigations (e.g. Mollaret et al. 2000b; Olson and Littlewood, 2002), thus confirming the validity of using the D1-D2 LSU rDNA for inferring the phylogenetic relationships within the Dactylogyridae.
Lim et al. (2001) gave a thorough review of the Ancylodiscoidinae Gussev, 1961, and proposed to raise it to full family status and to include within it all the 4-anchored monogeneans from the Old World siluriforms with sac-like and/or dactylogyrid-type of seminal vesicles. This was consistent with Gussev's (1961) hypothesis that, despite the observed differences in the morphologies of the seminal vesicles, monogeneans of siluriforms are related. However, both Lim et al. (2001) and Gussev's (1961) suggestions were not based on any phylogenetic analyses, and they totally ignored any possibility for host switching, which has been shown to be prevalent during the evolutionary history of monogeneans (e.g. Boeger and Kritsky, 1997; Boeger, Kritsky and Pie, 2003). Therefore, the phylogenetic position of the Ancylodiscoididae sensuLim et al. (2001) still remained controversial (e.g. Wu et al. 2000; Zhang, Qiu and Ding, 2001; Simková et al. 2003). In the present study, Thaparocleidus species were used as the representatives of the Ancylodiscoididae, and all 4 analyses inferred the same interrelationship pattern: (Diplectanidae, (Ancylodiscoididae, Dactylogyridae)). This result did not contradict that of Olson and Littlewoood (2002) using D1 LSU rDNA sequences, and Mollaret et al. (2000b), though, in the latter study, small sample numbers (only 4 ancyrocephalids were included) were used with low bootstrap support. However, it appears completely incongruent with that of some other morphological and molecular analyses. Kritsky and Boeger (1989) presented, among others, a sister relationship between the Pseudodactylogyrinae and the Ancylodiscoidinae. Simková et al. (2003) reported their results inferred from the partial 18S rDNA sequences, which indicated the sister-group relationships between the Thaparocleidus species and 1 clade comprising 3 ancyrocephalids (namely, Cleidodiscus pricei, Ancyrocephalus percae and Urocleidus similis). The present phylogenetic analyses based on D1-D2 LSU rDNA provide a robust tool for further test and determination of the phylogenetic position of the Ancylodiscoididae. Lim et al. (2001) also predicted that in the future the Ancylodiscoididae may require subdivision based on the form of the seminal vesicles. Therefore, molecular analyses are required in further studies of the Ancylodiscoididae and to test the interrelationships among subfamilies listed by Lim et al. (2001). As for the Pseudodactylogyrinae, incongruence still exists between the results of the previous studies (Kritsky and Boeger, 1989; Simková et al. 2003) and that of the present study. More taxa of this subfamily should be included in future studies in order to better understand their relationships with the Ancyrocephalinae and Ancylodiscoididae.
The radiation of Haliotrema
Haliotrema was erected by Johnston and Tiegs in 1922, and a major revision of the genus diagnosis was made by Young (1968). Haliotrema now has become a taxonomic group including more than 100 species which exhibit very different morphologies and parasitize a large number of hosts (representing 33 families and 6 orders) with a wide range of ecology and morphology (Plaisance et al. 2004). Klassen (1994a) characterized Haliotrema as a taxonomic waste-bucket containing numerous species from teleost fishes throughout the warm seas. Such characterization seems reasonable when considering that the wide host range is unusual for monogeneans (Kritsky and Stephens, 2001). However, some species of Haliotrema from hosts of specific families were observed to form monophyletic groups. For example, Young (1968) proposed 6 species groups for 32 Haliotrema species based on morphological features and host occurrences. Several genera, such as LigophorusEuzet and Suriano, 1977, EuryhaliotremaKritsky and Boeger, 2002, Euryhaliotrematoides and AliatremaPlaisance and Kritsky, 2004, were then erected to restrict the size of the Haliotrema by re-examining the morphological characters. Although a number of authors have viewed Haliotrema as a polyphyletic taxon, the validity of each species groups or genus and their interrelationship still remain unresolved. The morphological phylogenetic analysis of Haliotrema by Klassen (1994a) showed a clear lack of resolution due to the limited number of morphological characters (see Plaisance et al. 2004). The present study examined 9 Haliotrema species and 13 other dactylogyrids belonging to 9 different genera. Nine Haliotrema species were dispersed to form 4 clades together with species from other genera.
In Clade 1, two Haliotrema species, one (H. subancistroides) from the Gerridae and the other (H. geminatohamula) from the Leiognathidae, and 2 Metahaliotrema species both from the Scatophagidae form a monophyletic cluster. A well-supported group comprising Clade 1 and Clade 2 indicated a close relationship between Protogyrodactylus species and the Haliotrema and Metahaliotrema species group (Clade 1). However, evidence from molecular analyses only was insufficient to combine the latter 2 genera into 1 genus, though our results imply the non-monophyly of the Metahaliotrema in the ML, NJ and ME analyses. Two Haliotrema species both from Lutjanus argentimaculatus (Lutjanidae) were separated to form 2 clades, Haliotrema sp. ZHDDa was sister to 2 species from tilapia to form Clade 3, and Haliotrema sp. ZHDDb was clustered with E. johnii and H. spirotubiforum to form Clade 4. In the ME tree, Clade 3, Ligophorus vanbenedenii and Clade 4 grouped together to show monophyly, and a monophyletic group comprising only Clade 3 and Clade 4 was presented in the NJ tree. In conclusion, Clade 3 and Clade 4 displayed a more intimate relationship than other clades. Previous molecular investigations also indicated similar relationships although small sample numbers were examined. For example, (Cichlidogyrus, (Ligophorus, Euryhaliotrema)) was presented in the study of Olson and Littlewoods (2002) using D1 LSU rDNA sequences, and Mollaret et al. (2000b) reported their similar results as (Cichlidogyrus, (Tetrancistrum, (Ligophorus, Euryhaliotrema))). Taken together, it seems understandable and reasonable to recognize the sister relationships between the L. vanbenedenii and Clade 4. Hence, we prefer to consider the ME tree as the most reliable reconstruction of relationships among these taxa.
Four Haliotrema species and 1 Bravohollisia species formed a monophyletic group (i.e. Clade 5). This clade appeared as the second most basal branch within the Dactylogyridae, indicating a comparatively distant relationship with other Haliotrema species groups. In Clade 5, B. gussevi appeared as the sister-group to H. fleti in both distance methods (ME and NJ) and the ML analyses with moderate bootstrap supports, but remained ambiguous in the MP analysis. Therefore, in future studies more taxa of the Bravohollisia should be added to better investigate their relationships with Haliotrema species in Clade 5.
Four Haliotrema species in Clade 5 were observed to share similar morphological characters, especially in the male copulatory organ (MCO), which was shown as horn-like shaped with a wide-increasing base. We suggest that 4 other species, namely H. spirale, H. macassarensis, H. chenhsintaoi, and H. bihamulatum that also have horn-like shaped MCO and are from similar host ranges, be included in further studies. In the present study, the homology in morphology was also detected in the Clade 4, where 3 species were all found parasitizing the Lutjanus, and Euryhaliotrema-type MCO was observed in Haliotrema sp. ZHDDb and H. spirotubiforum, except that H. spirotubiforum lacks an accessory piece in its copulatory complex, while all other species of Euryhaliotrema have one. Taking into consideration the host occurrence, morphological and molecular evidences, we propose to transfer the H. spirotubiforum and the undetermined Haliotrema sp. ZHDDb to Euryhaliotrema as new combinations. However, we could not do such combinations for Haliotrema sp. ZHDDa in Clade 3 and 2 Haliotrema species in Clade 1 according mainly to the morphology of MCO and/or host occurrence. Taken together, one evolutionary hypothesis that speciation follows host switching and subsequent radiation may be used to explain the radiation of Haliotrema. Recent studies of the speciation of gyrodactylid monogenean flatworms indicated that switching hosts can indeed lead to rapid adaptation (e.g. Ziętara and Lumme, 2002; Boeger et al. 2003; Meinilä et al. 2004). However, in future evolutionary studies on this interesting genus, more information about their biogeography and ecology is needed.
The way forward: phenetics versus phylogeny
Phylogenetic analyses of the dactylogyrids using morphological and molecular characters will be beneficial to assess relationships within and between subfamilies. However, conclusions on phylogenetic and biogeographical patterns have to wait for taxonomic problems to be at least partially resolved (Klassen, 1994a). Conversely, phylogenetic information is also helpful to deal with taxonomic questions. More weight on haptoral sclerite morphology for identification purposes was placed in the early literature and later studies where genera were based on highly diverse haptoral morphologies of ancyrocephalids (e.g. Gussev, 1978, 1985). As indicated by Beverley-Burton and Klassen (1990), much of the taxonomic confusion seen in the Nearctic Ancyrocephalidae is due to considering the haptoral sclerites to be essential in specific identification, as well as to a reliance on vaginal characters that are difficult, if not impossible, to be seen in preserved material. Beverley-Burton and Suriano (1981) and Suriano and Beverley-Burton (1981) hypothesized that several distinct ‘MCO types exist among Nearctic ancyrocephalids and that species with a particular MCO type often parasitize a particular major host taxon. Our present molecular phylogenetic analyses have some taxonomic implications for the diagnosis of genera. Here we present our opinions on some genera included in this study, namely the Metahaliotrema, Haliotrema, Protogyrodactylus, Scutogyrus, Cichlidogyrus and Sinodiplectanotrema.
The Metahaliotrema Yamaguti, 1953 was erected based mostly on the absence of vagina and 2 bars whose centre parts link together to form a natural arthrosis, by which it can be differentiated with other genera. Beverley-Burton and Klassen (1990) considered that the expression vagina absence is often meaningless, as it may reflect lack of sclerotization and/or the quality of the observers microscope. However, Kritsky and Boeger considered the absence of a vagina to be a valid derived character state (by personal communication with Beverley-Burton and Klassen, 1990). We re-examined 2 Haliotrema species (in Clade 1) and 4 Protogyrodactylus species (in Clade 2), and found that all these species share vagina-absence. Our present results indicated an intimate relationship among Metahaliotrema, Haliotrema and Protogyrodactylus. Therefore, we propose that further studies be performed to reconsider the validity of the Metahaliotrema and Protogyrodactylus, with morphological and molecular evidences.
Scutogyrus was defined for Cichlidogyrus longicornis minus Dossou, 1982, and differentiated from the Cichlidogyrus mainly by different morphology of the haptor, in particular the transverse bar (Pariselle and Euzet, 1995). If the hypothesis of Beverley-Burton and Klassen (1990) is followed, then we should focus more on reproductive characters. In fact, these 2 genera share similar morphological MCO characters and host occurrences. Given the close molecular phylogenetic relationships between the Cichlidogyrus and Scutogyrus species pair (only 5·3% divergence was detected), 2 hypotheses could be used to explain the possible relationships of these 2 genera. (1) These 2 genera have diverged recently and under radiation separately, considering the low divergence (5·3%) between their D1-D2 LSU rDNA sequences and their identical/similar parasite-host associations; (2) Scutogyrus might be treated as the synonym of Cichlidogyrus because of the notably low divergence (5·3%), as the inter-genus species pair divergences in the D1-D2 LSU rDNA are usually above 10%. Hence, more taxa (at least 1 more species) of each genus should be used in further molecular investigations, if we want to draw a final conclusion.
With the development of the molecular genetic analyses, more and more hidden and cryptic taxonomic problems have been resolved (e.g. Wu et al. 2005a,b). Based on results of the present study, we propose to erect a new genus to accommodate the Haliotrema species with horn-like shaped MCO. Another 4 species of Haliotrema, namely H. spirale, H. macassarensis, H. chenhsintaoi, and H. bihamulatum which also have horn-like shaped MCO and are from similar host ranges, were not available in the present analyses. We consider that these species should be included in future studies when we describe the proposed new genus from a morphological perspective. However, the classification of other Haliotrema species groups remains a question. We may have to combine morphological and molecular evidences when addressing such questions. Though we have attempted to collect as many Haliotrema species as possible from different hosts in the South China Sea, some Haliotrema species groups and close genera such as Euryhaliotrematoides and Aliatrema were not available in the present study. Therefore, a wider range of taxa and more DNA markers displaying various evolutionary rates should be used in estimating phylogenetic relationships among species within the Ancyrocephalinae in further studies.
Project support was provided in part by the National Natural Science Foundation of China (grant No. 30170124) to A.X.L., and by the China National Science Fund for Distinguished Young Scientists (grant No.30225033) to X.Q.Z. The authors are grateful to Professor Zhang Jian-Ying for advice and suggestions, to Professor Antoine Pariselle and Professor L. H. Susan Lim for their assistance in the identification of some specimens, and to Professor Delane C. Kritsky and Professor Laetitia Plaisance for providing some references.