Introduction
Chagas disease (CD) is a neglected parasitic infection that affects more than six million people worldwide, most of them living in the Americas in extreme poverty without access to diagnosis or proper treatment (WHO, 2015; Chatelain, Reference Chatelain2016). Caused by the protozoan Trypanosoma cruzi, CD is a silent and progressive disease with two phases: acute and chronic (Chatelain, Reference Chatelain2016). During the acute phase, the patient may present mild flu-like symptoms or not, and after 6–9 weeks, enters the long-lasting chronic phase that is predominantly indeterminate (70% of the diagnosed cases), opposing to 30–40% of the patients, that can, years or decades later, develop clinic signs characterized by cardiac and/or digestive pathology (Prata, Reference Prata2001; Rassi et al., Reference Rassi, Rassi and Marin-Neto2010). The therapy for CD is based in two drugs, benznidazole (BZ) and nifurtimox, both in use for more than five decades. However, both present severe side-effects, limited efficacy on the later chronic phase besides the occurrence of naturally resistant parasite strains to these nitroderivatives (Bermudez et al., Reference Bermudez, Davies, Simonazzi, Real and Palma2016).
Nowadays, drug repurposing strategies have been used in attempt to find alternative therapies for diseases that lack aetiological or adequate drug treatment (Nwaka and Hudson, Reference Nwaka and Hudson2006). The approach is especially relevant for the neglected diseases reducing time and budget in drug discovery process (Ashburn and Thor, Reference Ashburn and Thor2004). In addition to that, combined therapy is as well an interesting strategy for enabling the simultaneous action towards more than one target, to overcome natural or acquired resistance (Ashburn and Thor, Reference Ashburn and Thor2004; Sun et al., Reference Sun, Sanderson and Zheng2016; Cha et al., Reference Cha, Erez, Reynolds, Kumar, Ross, Koytiger, Kusko, Zeskind, Risso, Kagan, Papapetropoulos, Grossman and Laifenfeld2018).
Imatinib (IMB) is a rationally designed BCR-ABL tyrosine kinase (TK) inhibitor broadly used for neoplasias, such as chronic myeloid leukaemia and gastrointestinal stromal tumour (Rix et al., Reference Rix, Hantschel, Dürnberger, Remsing Rix, Planyavsky, Fernbach, Kaupe, Bennett, Valent, Colinge, Köcher and Superti-Furga2007; Cruz-Rico et al., Reference Cruz-Rico, Garrido-Acosta, Anguiano-Robledo, Rodríguez-Wong, Pérez-Cruz, Sánchez Navarrete, Ruiz-Pérez and Montes-Vera2013; Musumeci et al., Reference Musumeci, Schenone, Grossi, Brullo and Sanna2015). New analogues and derivatives of IMB have been synthesized and screened not only for neoplasias, but also for parasitic pathologies like malaria (Pathak et al., Reference Pathak, Colah and Ghosh2015), schistosomiasis (Beckmann et al., Reference Beckmann, Long, Scheld, Geyer, Caffrey and Grevelding2014; Buro et al., Reference Buro, Beckmann, Oliveira, Dissous, Cailliau, Marhöfer, Selzer, Verjovski-Almeida and Grevelding2014), filariasis (O'Connell et al., Reference O'Connell, Bennuru, Steel, Dolan and Nutman2015), leishmaniasis (Wetzel et al., Reference Wetzel, McMahon-Pratt and Koleske2012), human African trypanosomiasis (Behera et al., Reference Behera, Thomas and Mensa-Wilmot2014) and CD (Engel et al., Reference Engel, Ang, Chen, Arkin, McKerrow and Doyle2010).
The scope of our study was to explore drug repositioning by evaluating the potential trypanocidal activity of IMB, also assessing its use in combination with BZ. The phenotypic activity of 14 novel IMB derivatives against the different forms and strains of T. cruzi was also investigated.
Materials and methods
Drugs
BZ (Fig. 1) was purchased from Laboratório Farmacêutico do Estado de Pernambuco (Recife, Pernambuco, Brazil). IMB mesylate (Fig. 1) and the derivatives (1a–c, 1e, 2a–e and 3a–e – Fig. 2) were provided by Dr Núbia Boechat from the Laboratório de Síntese Orgânica – Instituto de Tecnologia em Fármacos/Fundação Oswaldo Cruz (Farmanguinhos/Fiocruz) (Rio de Janeiro, Rio de Janeiro, Brazil) and synthesized as reported (Azevedo et al., Reference Azevedo, Bastos, Vasconcelos, Hoelz, Silva Junior, Dantas, de Almeida, de Oliveira, Gomes, Maia and Boechat2017). Stock solutions in dimethyl sulfoxide were prepared w/v with the final concentration never exceeding 0.6%, which does not exert mammalian host cell toxicity as reported (Timm et al., Reference Timm, Da Silva, Batista, Farahat, Kumar, Boykin and Soeiro2014). The highest concentration was 50 µm as some of the derivative molecules slightly precipitated (light microscopy observation) using higher concentrations.

Fig. 1. Chemical structures of benznidazole (A) and imatinib mesylate (B).

Fig. 2. Chemical structures of imatinib derivatives.
Mammalian cell cultures
Mouse fibroblasts from L929 cell line (4 × 103 cell well−1 into 96-well plates) were cultivated in RPMI-1640 medium (pH 7.2–7.4) without phenol red (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) and 2 mm glutamine at 37 °C (Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). Primary cardiac cell cultures (cc) were prepared from hearts isolated from 18–20 days old mice embryos and seeded in 96- and 24-well plates previously coated with 0.01% gelatin (Meirelles et al., Reference Meirelles, de Araujo-Jorge, Miranda, de Souza and Barbosa1986).
Parasites
Trypomastigotes of Tulahuen strain (DTU VI) expressing the β-galactosidase gene from Escherichia coli were obtained from the supernatant of infected L929 cell line cultures, as described (Buckner et al., Reference Buckner, Verlinde, La Flamme and Van Voorhis1996; Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). Bloodstream trypomastigotes (BT) of Y strain (DTU II) were obtained at the parasitaemia peak from infected Swiss Webster mice and then resuspended in Dulbecco modified Eagle medium (DMEM), supplemented with 10% FBS (Meirelles et al., Reference Meirelles, de Araujo-Jorge, Miranda, de Souza and Barbosa1986; Batista et al., Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales, Stephens, Boykin and Soeiro2010). Culture-derived trypomastigotes (CT) of the Y strain were obtained from the supernatant of L929 cc (10:1 parasite:cell ratio) as described (Batista et al., Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales, Stephens, Boykin and Soeiro2010).
Cell toxicity assessment
Non-infected cc and L929 cultures were incubated at 37 °C for 24–48 and 96 h, respectively, with increasing concentrations of each compound (up to 50 µm) diluted in RPMI. The morphology, and the spontaneous contractibility of cc was evaluated by light microscopy and cellular viability was determined by the PrestoBlue (cc) and AlamarBlue (L929) tests, as standardized (Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010; Simões-Silva et al., Reference Simões-Silva, Nefertiti, De Araújo, Batista, Da Silva, Bahia, Menna-Barreto, Pavão, Green, Farahat, Kumar, Boykin and Soeiro2016). The results were expressed as the difference in reduction between treated and non-treated cells according to the manufacturer's instructions, and the value of LC50 (minimum concentration that reduces the cellular viability by 50%) was determined. Also, toxicity on mammalian cells was assessed using combination schemes of IMB with BZ as described below (item 2.5.2). Selectivity index was expressed as the ratio between the mean values of the CC50 for host cells, and the EC50 for the parasites or infected cc (SI: CC50/EC50). All SIs were done comparing always the same time of drug exposure for each parasites and mammalian cells: 24 h for BT and CT, 48 h for intracellular and extracellular amastigote forms of Y strain, and 96 h for intracellular forms of Tulahuen strain, as reported (Batista et al., Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales, Stephens, Boykin and Soeiro2010; Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
Trypanocidal activity
Monotherapy
BT and CT of Y strain (5 × 106 mL−1) were incubated for 2 and 24 h at 37 °C in RPMI with 1:3 serial dilutions of the compounds (0–50 µm) for determination of parasite death rates, through the direct quantification of live parasites by light microscopy. The EC50 (compound concentration that reduces the number of parasites by 50%) was then calculated (Timm et al., Reference Timm, Da Silva, Batista, Farahat, Kumar, Boykin and Soeiro2014; Simões-Silva et al., Reference Simões-Silva, Nefertiti, De Araújo, Batista, Da Silva, Bahia, Menna-Barreto, Pavão, Green, Farahat, Kumar, Boykin and Soeiro2016). For the analyses of compound effect on intracellular forms, T. cruzi-infected L929 cultures (Tulahuen strain) were incubated for 96 h at 37 °C with each compound using 1:3 serial dilutions (0–50 µm) in RPMI. After the incubation, 500 µ m chlorophenol red-β-D-galactopyranoside in 0.5% Nonidet P40 was added to each well (final concentration 50 µm) and the plate incubated for 18 h at 37 °C. The absorbance was measured at 570 and 600 nm using a spectrophotometer. Controls with uninfected cells and infected cells, both treated only with vehicle and/or with BZ, were run in parallel for each plate in order to determine the EC50 values (Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). To test the potency against other parasite strain, the selected compounds were evaluated on the infection of cc using the Y strain. After 24 h of interaction (10:1 parasite and host cell ratio), the cc were incubated for 48 h using 1:3 serial dilutions of the compounds (0–50 µm), rinsed with saline, fixed with Bouin, stained with Giemsa and examined by light microscopy (Simões-Silva et al., Reference Simões-Silva, Nefertiti, De Araújo, Batista, Da Silva, Bahia, Menna-Barreto, Pavão, Green, Farahat, Kumar, Boykin and Soeiro2016). The percentage of infected host cells was determined, as well as the number of parasites per cell and the infection index, which represents the product of the multiplication between the percentage of infection and the number of parasites per cell. Then, the EC50 values were determined based on the infection indexes.
Regarding the effect on extracellular amastigotes (Y strain), the parasites were purified from the supernatant of highly infected cardiac cc (parasite: host cell ratio: 50:1) (De Souza et al., Reference De Souza, Nefertiti, Bailly, Lansiaux and Soeiro2010). Then, 5 × 106 parasites mL−1 were incubated for 48 h at 37 °C with increasing concentrations of the studied compounds (⩽50 µm) and parasite death rates quantified by light microscopy for EC50 determination (De Souza et al., Reference De Souza, Nefertiti, Bailly, Lansiaux and Soeiro2010). The maximum time of drug exposure was set according to the characteristics of parasite viability [for trypomastigotes the maximum was 24 h, since longer in vitro cultivation decreases viability (BT) or induces cell differentiation (CT)], and due to the previous standardized protocols for each parasite strain (Batista et al., Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales, Stephens, Boykin and Soeiro2010; Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010).
Combined therapy
Drug interactions were investigated using a fixed-ratio method (Fivelman et al., Reference Fivelman, Adagu and Warhust2004) by combining IMB with BZ, in L929 cc infected with Tulahuen strain expressing β-galactosidase, according to the same protocol above described. Predetermined EC50 values were used to determine the top concentrations of the individual ratios ensuring that it fell in the midpoint of a seven-point 2-fold dilution series. The fixed ratios of 5:0, 4:1, 3:2, 2:3, 1:4 and 0:5 were used, as reported (Simões-Silva et al., Reference Simões-Silva, Nefertiti, De Araújo, Batista, Da Silva, Bahia, Menna-Barreto, Pavão, Green, Farahat, Kumar, Boykin and Soeiro2016).
Determination of FIC index, isobologram preparation and nature of drug interaction
Fractional inhibitory concentrations (FICs) and the sum of FICs (∑FICs) were calculated as follows: FIC of IMB = EC50 of IMB in combination/EC50 of IMB in monotherapy. The same equation was applied to the partner drug BZ. The ∑FICs = FICIMB + FICBZ. An overall ∑FICs was determined and used to classify the nature of each interaction. Isobolograms were built by plotting the EC50 of IMB against the EC50 of BZ (Diniz et al., Reference Diniz, Urbina, de Andrade, Mazzeti, Martins, Caldas, Talvani, Ribeiro and Bahia2013; Simões-Silva et al., Reference Simões-Silva, Nefertiti, De Araújo, Batista, Da Silva, Bahia, Menna-Barreto, Pavão, Green, Farahat, Kumar, Boykin and Soeiro2016). ∑FICs ⩽ 0.5 = synergism; 0.5 < ∑FICs ⩽ 4.0 = additive (no interaction); ∑FICs > 4.0 = antagonism.
Statistical analysis
The assays were done in at least duplicate and the results were on average from three independent experiments. Analysis of variance tests were performed and the significance level set for P ⩽ 0.05 only for those compounds that presented higher potency than the reference drugs.
Results
Following our well-established flowchart, the activity against intracellular forms of T. cruzi (Tulahuen strain – DTU VI) was done using L929-infected cc treated or not with IMB and BZ in serial dilutions (0–50 µ m) and readout using colorimetric analysis. As presented in Table 1, IMB displayed a moderate trypanocidal activity (EC50 = 24.8 ± 7.4 µm) in comparison to BZ (EC50 = 4.1 ± 1.3 µm). When mammalian cells viability was evaluated, IMB exhibited higher toxicity than BZ after 96 h of incubation (CC50 = 38.3 ± 0.2 and >50 µm, respectively) (Table 1). To expand the analysis to other parasite strains and other mammalian host cells, IMB was screened against intracellular forms of the Y strain (DTU II) inside cc, besides evaluating its potential cardiotoxicity profile. After 48 h of drug exposure, IMB and BZ were not toxic up to 50 µm (Table 1). The readout of Giemsa-stained T. cruzi-infected cc by light microscopy showed the trypanocidality of IMB, being able to reduce both the percentage of infected host cells as well as the number of parasites per infected cell (data not shown). Regarding the infection index, the EC50 values for IMB and BZ were 20.0 ± 4.4 and 2.8 ± 1.9 µ m, respectively (Table 1). Aiming to validate the trypanocidal activity of IMB directly against amastigotes without the possible drug influence on the mammalian host cell machinery, free parasites released from a highly infected cardiac culture were incubated also for 48 h. The results confirmed the in vitro moderate effect of IMB, reaching EC50 values of 30.0 ± 4.4 µ m (Table 1).
Table 1. In vitro trypanocidal activity (EC50 – μ m) of benznidazole and imatinib against amastigote forms (Tulahuen and Y strains of Trypanosoma cruzi, intra and extracellular sources), as well as cytotoxicity (CC50 – μm) on different mammalian cells and their corresponding selectivity indexes (SI)

nd, not determined.
a SI: CC50/EC50 obtained after 96 h of drug exposure.
b Soeiro et al. (Reference Soeiro, de Souza, da Silva, da Batista, Batista, Pavão, Araújo, Aiub, da Silva, Lionel, Britto, Kim, Sulikowski, Hargrove, Waterman and Lepesheva2013).
c SI: CC50/EC50 obtained after 48 h of drug exposure.
d Extracellular amastigotes obtained from the supernatant of infected cardiac cells.
IMB was assayed against CT and BT of the Y strain and the data showed EC50 values of 43.3 ± 19.0 and 33.6 ± 4.9 µm for CT and BT, respectively, after 24 h of incubation (Table 2).
Table 2. In vitro trypanocidal activity (EC50 and EC90 – μ m) of benznidazole and imatinib against bloodstream (BT), culture-derived (CT) trypomastigotes from Trypanosoma cruzi Y strain, cytotoxicity on cardiac cells (CC50 – μ m) and the corresponding selectivity indexes (SIa)

nd, not determined.
a SI: CC50/EC50 obtained after 24 h of drug exposure.
Since BZ and IMB present distinct modes of action, which means acting on different targets, IMB was combined with BZ for drug interaction assays with fixed-ratio and serially diluted concentrations (Fig. 3, Table 3). The isobologram shows that the interaction between both drugs was additive (mean ∑FICs = 0.91) (Fig. 3), and all the ∑FIC for each combination ratio demonstrated that none of them was synergistic (Table 3). The best non-toxic ratio was two parts of IMB to three parts of BZ, with the ∑FIC = 0.68 (Table 3). Toxicity upon L929 cell lines was only observed for the highest concentrations, reaching 91% of loss of cellular viability for 200 µm IMB + 4.8 µm BZ (ratio 4:1, data not shown).

Fig. 3. Isobologram against intracellular forms of Trypanosoma cruzi Tulahuen strain in L929 cell line. The EC50 of each compound is plotted on the abscissa and the ordinate, respectively. ∑FICs ⩽ 0.5 = synergism; 0.5 < ∑FICs ⩽ 4.0 = additive (no interaction); ∑FICs < 4.0 = antagonism.
Table 3. Mean of ∑FICs of interaction between imatinib (IMB) and benznidazole (BZ) on intracellular forms of Trypanosoma cruzi Tulahuen strain in L929 cell line

Top drug concentrations: 4 + 1 = 200 µm of IMB plus 4.8 µm of BZ; 3 + 2 = 150 µm of IMB plus 9.6 µm of BZ; 2 + 3 = 100 µm of IMB plus 14.4 µm of BZ; 1 + 4 = 50 µm of IMB plus 19.2 µm of BZ
The moderate activity of IMB against different forms and strains of T. cruzi motivated the next step performing the phenotypic screening of 14 new analogues. The compounds (Fig. 2) were assayed under the same in vitro established models above reported. The toxicity assessment against L929 cell line cultures demonstrated that 1a, 1b, 2c, 3a and 3d were not toxic up to 50 µm after 96 h of incubation (Table 4). However, the other compounds displayed distinct levels of mammalian cells toxicity ranging from 8 to 48 µm (Table 4). When the derivatives were screened using L929-infected cc (Tulahuen-β galactosidase strain), ten derivatives, listing 1a, 2a–e, 3a and 3c–e, presented considerable activity, with EC50 values below 10 µm (Table 4). The derivatives 1a, 2d and 3c were most potent (EC50 ⩽ 3.8 µm) than BZ, being at least 6-fold more active than IMB (Tables 1 and 4, P ⩽ 0.05).
Table 4. In vitro trypanocidal activity (EC50) of imatinib derivatives against intracellular forms (Tulahuen strain, 96 h of drug exposure) and bloodstream trypomastigotes (Y strain, 24 h of drug exposure), as well as the cytotoxic concentration for mammalian cells (CC50, 24 and 96 h) and the corresponding selectivity index (SI)
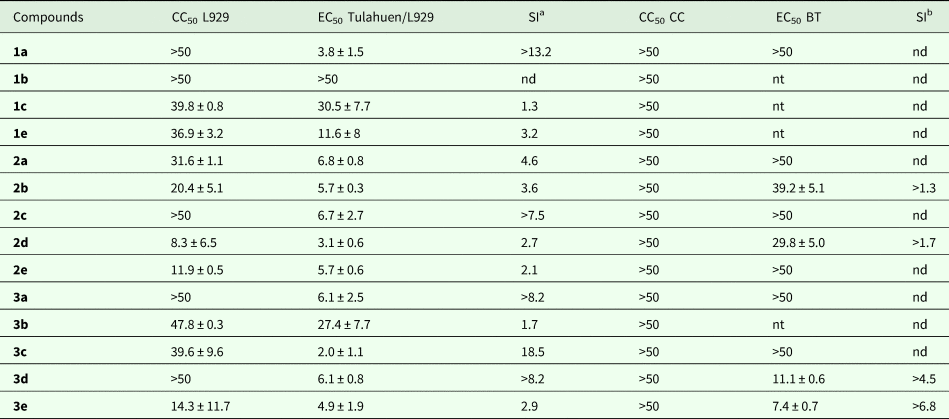
nt, not tested; nd, not determined.
a SI: CC50/EC50 obtained after 96 h of drug exposure.
b SI: CC50/EC50 obtained after 24 h of drug exposure.
The toxicity assays performed on cc for 24 h revealed that none of the derivatives was cardiotoxic up to 50 µm (Table 4). Then, the ten compounds active against intracellular forms (Tulahuen strain) were tested against BT (Y strain). Compounds 1a, 2a, 2c, 2e, 3a and 3c showed no trypanocidal activity up to 50 µ m (Table 4). The best trypanocidal molecule was 3e (EC50 = 7.4 µm), being 5-fold more active than IMB (P ⩽ 0.05) (Tables 2 and 4). This derivative investigated on T. cruzi-infected cc (Y strain) displayed EC50 values of 1.3 ± 0.4 µm with SI >38 (Table 5), in the same activity range of BZ (Table 1).
Table 5. In vitro trypanocidal activity (EC50 and EC90 − μM) of 3e, an imatinib derivative, against intracellular amastigote forms of the Y strain of Trypanosoma cruzi in mice primary cardiac cell culture and selectivity index (SI)

a SI: CC50/EC50 obtained after 48 h of drug exposure.
Discussion
The focus of the study was to access the phenotypic profile of IMB and the 14 novel derivatives, by using different strategies recommended for the drug discovery of CD: drug repurposing and combination, thus aiming to contribute for the identification of novel therapies more specific towards the parasite and less dangerous to mammalian cells.
IMB is a TK inhibitor with high selectivity for ABL kinases, mostly present in tumourigenic cells (Cruz-Rico et al., Reference Cruz-Rico, Garrido-Acosta, Anguiano-Robledo, Rodríguez-Wong, Pérez-Cruz, Sánchez Navarrete, Ruiz-Pérez and Montes-Vera2013; Musumeci et al., Reference Musumeci, Schenone, Grossi, Brullo and Sanna2015). The activity of IMB has also been demonstrated against several pathogens, including helminths (Beckmann et al., Reference Beckmann, Long, Scheld, Geyer, Caffrey and Grevelding2014; Buro et al., Reference Buro, Beckmann, Oliveira, Dissous, Cailliau, Marhöfer, Selzer, Verjovski-Almeida and Grevelding2014; O'Connell et al., Reference O'Connell, Bennuru, Steel, Dolan and Nutman2015) and protozoa (Wetzel et al., Reference Wetzel, McMahon-Pratt and Koleske2012; Behera et al., Reference Behera, Thomas and Mensa-Wilmot2014; Pathak et al., Reference Pathak, Colah and Ghosh2015), suggesting the repurposing potential of this drug.
As suggested for CD flowchart screenings (Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010), the toxicity profile of IMB and derivatives was explored using different host cells (primary cardiac cells and fibroblasts of L929 cell lines). The trypanocidal effect was investigated upon the relevant forms of the parasite for mammalian infection (amastigotes and trypomastigotes) (Brener and Chiari, Reference Brener and Chiari1963) obtained from different sources (culture-derived and BT, intracellular and extracellular amastigotes) and from distinct strains, which are susceptible (Tulahuen strain) and the partially naturally resistant to nitroderivatives (Y strain) belonging to different discrete typing units – DTUS VI and II, respectively (Zingales et al., Reference Zingales, Miles, Campbell, Tibayrenc, Macedo, Teixeira, Schijman, Llewellyn, Lages-Silva, Machado, Andrade and Sturm2012).
Our present results demonstrate the moderate activity of IMB against T. cruzi in vitro using different parasite strains and forms, corroborating previous studies reported against strains obtained from chronic chagasic patients (CA-I/72 clone and PSD-1), and clones derived from insect (Sylvio-X10/7 clone and its parental strain) (Engel et al., Reference Engel, Ang, Chen, Arkin, McKerrow and Doyle2010; Dichiara et al., Reference Dichiara, Marrazzo, Prezzavento, Collina, Rescifina and Amata2017). Analogues of TK are present in protozoa such as trypanosomatids, including T. cruzi, and have been related to several parasite metabolic processes besides playing a role during host cell invasion (Melo et al., Reference Melo, Tucci, Nogueira, de Meirelles and Pereira2014). IMB was moderately active against Plasmodium falciparum (Pathak et al., Reference Pathak, Colah and Ghosh2015) and Leishmania amazonensis (Wetzel et al., Reference Wetzel, McMahon-Pratt and Koleske2012), being the enzyme inhibition demonstrated. However, IMB was inactive against Trypanosoma brucei gambiense, although some of its analogues showed mild potency, especially lapatinib (Behera et al., Reference Behera, Thomas and Mensa-Wilmot2014), which was used as scaffold for new molecules further tested against L. major, T. brucei and T. cruzi with promising results (De Rycker et al., Reference De Rycker, Thomas, Riley, Brough, Miles and Gray2016; Devine et al., Reference Devine, Thomas, Erath, Bachovchin, Lee, Leed, Rodriguez, Sciotti, Mensa-Wilmot and Pollastri2017).
In our studies, besides assessing the trypanocidal activity of IMB, 14 novel derivatives were studied using well-established in vitro methodologies for drug screening in T. cruzi (Romanha et al., Reference Romanha, De Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). These new derivatives have the phenylamino-pyrimidine group as their main pharmacophore fragment and presented antitumour activity in vitro (Moreno et al., Reference Moreno, Cabanas and Hitt2010). The series 1a–e has hybrid molecules developed from IMB and sunitinib, another TK inhibitor. The molecules of series 2a–e have 2-oxo-2-phenylacetamides, the ones of series 3a–e have 3,2-difluoro-2-phenylacetamides, and isatins were used as starting molecules in all series (Azevedo et al., Reference Azevedo, Bastos, Vasconcelos, Hoelz, Silva Junior, Dantas, de Almeida, de Oliveira, Gomes, Maia and Boechat2017).
The TDR (Special Programme for Research and Training in Tropical Diseases, World Health Organization) establishes criteria for the determination of antiparasitic activity and selectivity of compounds in test: EC50 lower than 4.0 µ m are ‘active’, between 4.0 and 60 µm are ‘moderately active’ and higher than 60 µ m are ‘inactive’ against T. cruzi amastigotes (Papadopoulou et al., Reference Papadopoulou, Bloomer, Rosenzweig, O'Shea, Wilkinson, Kaiser, Chatelain and Ioset2015). Accordingly, although IMB presented a moderate anti-parasitic activity (against different parasite forms and strains of T. cruzi), with EC50 values 4- to 6-fold higher than the reference drug (BZ). Some of the studied derivatives displayed an enhanced anti-T. cruzi effect, being active in micromolar range, which represents a dramatic improvement in the trypanocidal activity in relation to the scaffold molecule already reported in the literature (Engel et al., Reference Engel, Ang, Chen, Arkin, McKerrow and Doyle2010). The compounds 1a, 2a–e, 3a and 3c–e presented trypanocidal effects under 10 µ m against intracellular forms of the Tulahuen strain, being more potent than IMB (EC50 = 38.3 µm) and 3d and 3e exhibited superior/similar effect as BZ when assayed against BT. However, poor selectivity was achieved for most of them.
Moving forward, as combined therapy has been largely used for different diseases with promising results (Sun et al., Reference Sun, Sanderson and Zheng2016), we investigated the trypanocidal effect of the IMB plus BZ. Although according to the sum of FICs (∑FICs), the combination was additive, the FIC values differed among the fixed-ratio proportions suggesting that a combination of two parts of IMB to three parts of BZ might have a better predictive in vivo outcome than the monotherapies. Another interesting feature was the low toxicity profile of the combination IMB + BZ. L929 cellular viability dropped by 91% only when higher concentrations of IMB were used, such as 200 µm IMB + 4.4 µm BZ. The strategy of combining compounds acting on distinct targets is promising as reported for other drug combinations (Simões-Silva et al., Reference Simões-Silva, Nefertiti, De Araújo, Batista, Da Silva, Bahia, Menna-Barreto, Pavão, Green, Farahat, Kumar, Boykin and Soeiro2016; Santos et al., Reference Santos, Lionel, Peres, Batista, da Silva, de Oliveira, da Silva, Batista, Souza, Andrade, Neves, Braga, Patrick, Bakunova, Tidwell and Soeiro2018), improving parasite killing. In this sense, approaches for the synthesis and development of novel IMB derivatives using possibly a hybrid molecule composed by IMB derivatives (such as compound 3e) and BZ could be performed aiming to improve the activity and selectivity of these compounds as anti-T. cruzi agents.
Author ORCIDs
Maria Soeiro, 0000-0003-0078-6106.
Acknowledgements
The authors would like to thank MSc Ludmila Fiuza and Dr Gabriel Melo de Oliveira (LBC/IOC/Fiocruz) for their technical support.
Financial support
The fundings were provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Rio de Janeiro (FAPERJ), PAEF and Instituto Oswaldo Cruz (IOC/Fiocruz). MNCS and MTB are CNPq fellows. MNCS and NB are Cientista do Nosso Estado CNE FAPERJ.
Conflict of interest
None.
Ethical standards
Swiss mice (21–23 g) were provided by the Instituto de Ciência e Tecnologia em Biomodelos (ICTB/Fiocruz) (Rio de Janeiro, Brazil). They were kept in a conventional room at 20–24 °C under a 12/12 h light/dark cycle. The animals were supplied with sterilized water and chow ad libitum. The procedures complied to the guidelines of the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA LW16/14).











