One pill makes you larger, and one pill makes you small
And the ones that mother gives you, don’t do anything at all
Go ask Alice, when she’s ten feet tall
(“White Rabbit,” sung by Jefferson Airplane, written by Grace Wing Slick)
Diseases without Good Treatments
As evidence continues to mount of an epidemic of opioid-related deaths in the United States, the entire world is plagued by addictions to multiple other drugs including ethanol, cocaine, amphetamines, and nicotine; the ever-present and increasing scourges of Alzheimer disease and other dementias; debilitating neurological disorders including Parkinson disease; and a multitude of mental health disorders.
Depression and anxiety, for example, are the world’s most common mental disorders. The World Health Organization estimates that, globally, the total number of people with depression exceeds 300 million. Depression is not only the single largest contributor to global disability (7.5% of all years lived with disability in 2015), but it is also the major contributor to the nearly 800,000 suicides worldwide annually. It is most common in older adulthood (55 to 74 years), and women (7.5%) are more commonly affected than men (5.5%).Footnote 1 In the United States, almost 7% of adults suffer from at least one major depressive episode each year, and more than 19% suffer from anxiety disorders. Almost 13% of 12- to 17-year-old adolescents have had at least one major depressive episode, and almost one-third have had anxiety disorders in their lifetime. Severe impairment accompanied depressive episodes in about two-thirds of the individuals in both groups.Footnote 2
Posttraumatic stress disorder (PTSD) affects about 3.6% of U.S. adults and 5% of 13- to 18-year-old adolescents annually.Footnote 3 In the United States, 15.1 million adults ages 18 and older (6.2%) and more than 600,000 adolescents 12 to 17 years of age (2.5%) have alcohol abuse disorder. About 88,000 people per year die from alcohol-related causes, and alcohol-impaired driving fatalities account for about 10,000 annual deaths (31% of all driving fatalities).Footnote 4 Parkinson disease affects about 1 million Americans, and approximately 60,000 new patients receive the diagnosis annually. More than 10 million people worldwide have the disease.Footnote 5
Opioid addiction and overdoses have become epidemic in the United States. From July 2016 through September 2017, opioid overdoses increased 30% across 45 states, with a 70% increase in the Midwest and a 54% increase in large cities.Footnote 6 Cigarette smoking is the single largest preventable cause of death and disease in the United States, killing more than 480,000 Americans each year. Nearly 29 million American adults smoke daily.Footnote 7
Current Treatments
Currently, available treatments for these widespread disorders are often costly, complex, and only marginally effective, and many have numerous side effects—some of which are lethal. Studies, including those highlighted below (Table), have shown that a variety of psychedelic and similar U.S. Drug Enforcement Administration Schedule I drugs may offer better treatment options than those that currently exist. The large population affected by these disorders deserve to have regulatory barriers eased to permit a variety of robust studies that may point the way to new, more effective, and safer treatments—or even cures.
Relaxing regulations governing research on Schedule 1 drugs may also provide the opportunity to discover applications to medical conditions that investigators have overlooked. A well-known example is thalidomide, which was banned for causing severe birth defects (phocomelia) but was reintroduced when research showed that it was an immunomodulator and angiogenesis inhibitor effective for treating leprosy, multiple myeloma, and AIDS. Likewise, acetylsalicylic acid (aspirin) was used as an analgesic and anti-inflammatory drug for a century before its use as an antiplatelet agent and major treatment for acute myocardial infarctions became accepted practice.
If some of these drugs are approved for clinical practice, an entire new range of possibilities exist. Clinicians often use medications “off-label,” meaning that they use the drug for a purpose for which it has not been approved. According to the Federal Drug Administration, once a drug is approved, “healthcare providers generally may prescribe the drug for an unapproved use when they judge that it is medically appropriate for their patient. . . [because] there might not be an approved drug to treat your disease or medical condition. . . [or] you may have tried all approved treatments without seeing any benefits.”Footnote 8 Some medications that are widely and successfully used “off-label” include sildenafil (Viagra®) for pulmonary arterial hypertension, amitriptyline for neurological pain and other disorders, methotrexate for multiple inflammatory disorders, and multiple cancer drugs that are used for cancers for which they have not been specifically approved.
Schedule 1 Drug Uses, Research, and Restrictions
In 1970, the United States enacted the Comprehensive Drug Abuse Prevention and Control Act.Footnote 9 As of May 2018, the list contains 204 Schedule 1 drugs.Footnote 10 In 1971, the United Nations also published a list of controlled drugs that was adopted by numerous countries. It now contains 26 Schedule I drugs.Footnote 11
Between 1950 and the mid-1960s, before U.S. and international government restrictions were enacted, psychiatric researchers trying to alleviate their patients’ disorders tested a variety of psychedelic drugs, most commonly psilocybin, lysergic acid diethylamide (LSD), and mescaline. Researchers worldwide published more than a thousand articles and several dozen books discussing their experience with about 40,000 patients. They also held 6 international conferences on psychedelic drug therapy.Footnote 12
While early trials had suboptimal designs and small sample sizes, they tended to show that the tested medications helped those with substance abuse and neuroses, but not those with established psychoses.Footnote 13,Footnote 14 Early researchers who used LSD to treat alcohol abuse, for example, expected patients to experience a physiologically safe delirium, reasoning that most alcoholics stopped drinking after a bout of delirium tremens. While their therapy suggests at least short-term benefit, patients described a “mystical” or “mind manifesting” experience, leading to the term “psychedelic” from the Greek words psychē (ψ υ χ ή, “soul”) and dēloun (δ η λ ο ῦ ν, “to make visible, to reveal”), meaning “mind-revealing.”Footnote 15,Footnote 16 About this time, a German psychiatrist introduced the term “psycholytic” therapy, meaning the use of low (not psychedelic state–inducing) drug doses together with psychotherapy.Footnote 17 For a short time, the therapy was legalized in Switzerland.Footnote 18 Currently, but without psychiatric support, using microdoses of Schedule 1 drugs has become fashionable in some parts of the United States.Footnote 19
The public fascination with psychedelic drugs used for recreational purposes has continued since the 1950s, with many millions of Americans experiencing the effects of LSD, psilocybin (mushrooms), mescaline, other hallucinogens, and MDMA (Ecstasy; 3,4-methylenedioxymethamphetamine), which shares features with psychedelic drugs.Footnote 20 Interestingly, they have been shown to be relatively safe—especially compared to alcohol and opiates.Footnote 21
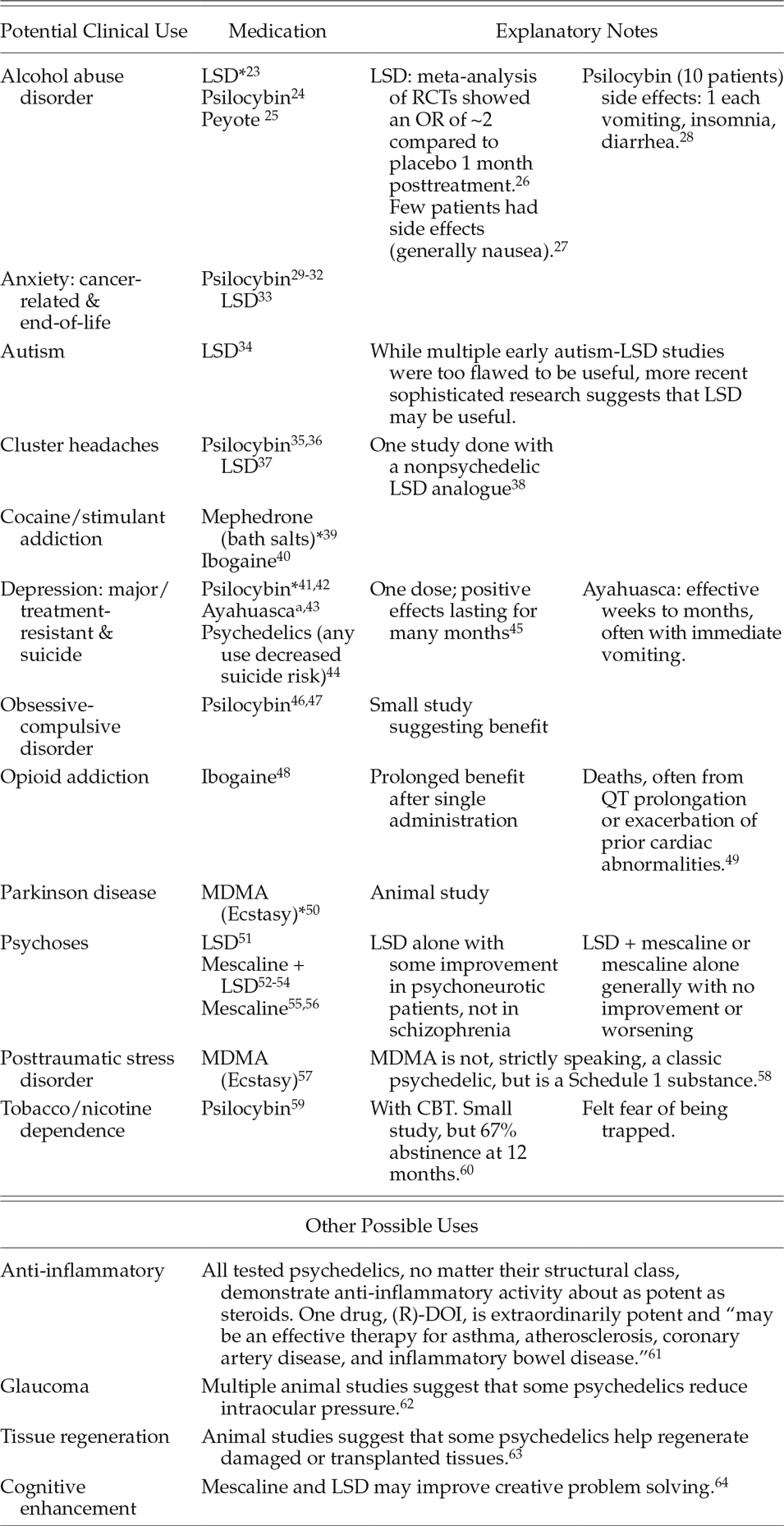
Following decades without substantive clinical research in psychedelics, a small but increasing number of human neuroimaging, psychology, and psychopharmacology studies have been published and shown promising results (Table 1). In addition, scientists and drug companies have overcome substantial barriers to obtain approval for clinicians to prescribe certain Schedule I drugs as medications for serious or unmet medical needs. One example is dronabinol (Marinol®; synthetic delta-9-THC in an oily capsule formulation), which was approved in 1985 for AIDS-related anorexia and later approved for chemotherapy-related nausea and vomiting. Others include gamma hydroxybutyrate (Xyrem®) in 2004 for narcolepsy and the cannabis-derived nabiximol (Sativex®; delta-9-tetrahydrocannabinol and cannabidiol) for spasticity, which is approved in Canada and Europe and under review for approval in the United States.Footnote 22
Table 1. Potential Clinical Uses for Selected Schedule 1 Drugs

Abbreviations: CBT, cognitive behavioral therapy; LSD, lysergic acid diethylamide; MDMA, 3,4-methylenedioxymethamphetamine (Ecstasy); RCT, randomized clinical trials; (R)-DOI, 2,5-dimethoxy-4-iodoamphetamine.
* Studies suggest a positive effect.
a Plant-based decoction containing N,N-dimethyltryptamine (DMT).
Getting U.S. Food and Drug Administration permission to develop and conduct clinical trials on psychedelic and related drugs requires not only overcoming significant political hurdles, but also targeting serious or unmet medical needs. That is the direction in which current researchers are heading. Yet designing scientifically acceptable studies may prove difficult.
Issues Complicating Research
While the pharmaceutical industry and academia routinely investigate new drug classes to address diseases with inadequate or unmet treatments, they have avoided researching most Schedule 1 drugs, especially the psychedelics. This is due to several factors, including the difficulty of obtaining samples, potentially negative publicity, financial issues (funding for academia and marketing difficulties and a potential lack of patent protection for companies), and potential negative repercussions from a large-scale diversion of marketed drugs for recreational purposes, as now occurs with opioids, stimulants, benzodiazepines and Z-drugs. Another hurdle they face is obtaining institutional review board (IRB) approval for any studies.
IRBs review any clinical trials on humans, and their criteria is based on well-known ethical codes and regulations (Nuremberg Code [1947], Declaration of Helsinki [2000], Belmont Report [1979], Council for International Organizations of Medical Sciences Guidelines [2002], U.S. Common Rule [1991]). Their principle requirements are that the research (1) must have social and clinical value and be sufficiently important to justify any risk or inconvenience to study subjects, (2) be scientifically valid, and (3) encompass subjects that are informed of and, whenever possible, protected from foreseeable risks.
Social and Clinical Value
The value of studying a potentially useful group of drugs seems obvious. The medical conditions listed in the Table currently have inadequate treatments or unmet needs. Far from “orphan” or rare diseases, these conditions plague large portions of the U.S. and world populations. The extensive prior human research provides suggestive evidence for the efficacy of selected Schedule 1 drugs, particularly psychedelics, for these conditions.
Scientifically Valid
To ethically study a new drug, researchers must define a target disease or symptom, understand its natural history and pathophysiology, and preferably know the drug’s mechanism of action. Then they must design scientifically valid and feasible studies that reliably show whether the drug is both safe and effective at different doses.Footnote 65 Although researchers understand the diseases and some of the pathophysiology they are targeting, fulfilling the other research elements may be problematic.
Although chemical drug activity is known, the mechanism for any psychedelic’s therapeutic effect has yet to be discovered. That is not unusual for some new medications, but researchers still need to elucidate a dose-response relationship; duration of action; and toxicity, efficacy and safety at the levels used.Footnote 66 This may be complicated by the process of legally obtaining (or needing to manufacture) the drugs with techniques and chemical compositions that meet regulatory standards.
Finally, and perhaps most difficult, is the need for double-blind studies, which provide reliable endpoints and meaningful outcome assessments. Blinding of psychedelic drugs is extremely difficult, since the drugs’ subjective effects are so evident. Moreover, for psychiatric conditions, such as depression or obsessive-compulsive disorder, outcome measures can be subjective. Historical trials with these drugs generally had too few patients, often used subjective reported outcomes, and provided too few details to be replicated. More recent trials have attempted to overcome these limitations, and validated improvement measures now exist for most of the entities that need to be initially tested.
Protection from Foreseeable Risks
IRBs and researchers are obligated to minimize, as much as possible, any physical, psychological, economic, or social risks and to maximize the benefits associated with the study.Footnote 67 Unlike most tested drugs, many of the Schedule 1 compounds have been used extensively by large populations for extensive recreational and religious experiences over past decades, if not centuries. Their relatively good safety record suggests that any risks associated with many of the conditions being considered for treatment may far outweigh any harms associated with these drugs.Footnote 68 Those using most of these drugs (ibogaine is the exception) seemed to experience as few or fewer adverse consequences than those using the most commonly used legal psychoactive (alcohol, caffeine, nicotine) and nonpsychoactive (aspirin, acetaminophen, ibuprofen) substances. In fact, one might wonder, given the extensive experience with some of these agents, if it would be reasonable to begin most trials at Phase I or even Phase 2. Of course, the study participant populations will need to be carefully selected and the studies carefully monitored so that if the medication proves ineffective or has unreasonable side effects, the study can be terminated early.Footnote 69
Conclusions/Recommendations
A variety of diseases lack effective prevention, treatment, or cure. We must ask ourselves as a society if we are actively working to ameliorate or cure these disorders or are obstructing valuable research avenues. Limited studies suggest that many may benefit from the use of currently restricted Schedule I (illegal) drugs.
In most cases, experience shows that these drugs pose little or even much less risk than do legal psychoactive (alcohol, caffeine, nicotine) and non-psychoactive (aspirin, acetaminophen, ibuprofen) substances. We need adequate scientific studies that can demonstrate their efficacy for specific conditions. At present, legal, financial, and scientific barriers (i.e., limited knowledge of diseases and objective outcomes) prevent adequate exploration of these potentially useful modalities.
In cases where Schedule I drugs are better than the available alternatives, or for those instances where no alternatives exist, fairness to the population demands that the Schedule I drugs be adequately studied and, if they or their congeners are shown to be effective, made available, with the proper caveats, instructions, and protections that other potentially abused prescribed medications (e.g., narcotics) receive.
The current situation harms both individual patients and society. Without permission and funding to study important groups of drugs, patients not only lack possible interventions for serious ailments but also must grapple with less effective treatments with serious side effects and increased mortality from nontreatment of some conditions; society at large, meanwhile, wastes resources on drug enforcement and forgoes potential tax revenue from legalization. It’s time that we have the courage to “ask Alice” what medical benefits lie on the other side of the psychedelic door.