INTRODUCTION
Patellid limpets are the dominant grazers of NE Atlantic rocky shores (Hawkins & Hartnoll, Reference Hawkins and Hartnoll1983). Their role in preventing the colonization of the shore by canopy-forming fucoids has received a considerable amount of attention by scientists over the last 50 years (e.g. Southward & Southward, Reference Southward and Southward1978; Hawkins & Hartnoll, Reference Hawkins and Hartnoll1983; Jenkins et al., Reference Jenkins, Hawkins and Norton1999; Thompson et al., Reference Thompson, Norton and Hawkins2004). Experimental studies have shown that removal of limpets invariably leads to proliferation of macroalgae (ephemeral green algae first, then fucoids) (see Coleman et al., Reference Coleman, Underwood, Benedetti-Cecchi, Aberg, Arenas, Arrontes, Castro, Hartnoll, Jenkins, Paula, Della Santina and Hawkins2006), suggesting that limpets' grazing is an important factor controlling macroalgal cover on rocky shores. It is known that limpets consume macroalgal propagules whilst grazing the substrate, thereby preventing the establishment of new algal populations in areas with high limpet densities. Hence, their foraging activity promotes spatial variability in intertidal assemblages, i.e. the co-occurrence of algal canopies and unvegetated areas, with their respective associated biodiversities (Coleman et al., 2006). On NE Atlantic rocky shores, Patella vulgata is the main intertidal grazer, and can reach densities over 100 individuals m−2 (Hawkins & Hartnoll, Reference Hawkins and Hartnoll1983). Patella vulgata is generally regarded as a generalist grazer, that consumes different components of epilithic biofilm (i.e. benthic microalgae, germinating algal sporelings, bacteria and their associated matrix of organic matter) (Hawkins et al., Reference Hawkins, Watson, Hill, Harding, Kyriakides, Hutchinson and Norton1989). Patella vulgata usually colonizes bare rock areas adjacent to macroalgae beds formed by the canopy-forming fucoid Ascophyllum nodosum, because understorey red algal turfs prevent limpets from foraging (Jenkins et al., Reference Jenkins, Hawkins and Norton1999). Ascophyllum nodosum dominates sheltered to semi-exposed rocky shores along the coasts of the northern Atlantic. Even though this algae is consumed by some herbivorous gastropods, such as the periwinkle Littorina obtusata (Sarà et al., Reference Sarà, De Pirro, Romano, Rumolo, Sprovieri and Mazzola2007), it is usually considered of poor nutritive value for herbivores, due to the concentration of herbivore-deterrent secondary metabolites within its tissues (Pavia & Toth, Reference Pavia and Toth2000), and direct grazing of A. nodosum by limpets is thought to be quite rare. Some events of dramatic herbivory by limpets, leading to local eradication of A. nodosum, have nevertheless been reported on western European coasts (Le Roux, Reference Le Roux2005), but seem to remain local phenomena (Davies et al., Reference Davies, Johnson and Maggs2007). Trophic supply from A. nodosum has been suggested to play a role in sustaining growth rates and lower mortality in limpet populations during winter (Davies et al., Reference Davies, Johnson and Maggs2008). Limpets often aggregate at the vicinity of A. nodosum fronts or solitary individuals (Davies et al., Reference Davies, Johnson and Maggs2007), where they have been observed to play a role in increasing the vulnerability of plants to wave-induced breakage (Davies et al., Reference Davies, Johnson and Maggs2007), but are also to be found further away. It is not clear, to our knowledge, how the variability of limpets' direct environment affects their trophic resource, that is, their consumption of A. nodosum and/or other food sources.
Stable isotopes have been used to investigate trophic relationships on rocky shores (Bode et al., Reference Bode, Alvarez-Ossorio and Varela2006; Sarà et al., 2007; Riera et al., Reference Riera, Escaravage and Leroux2009; Golléty et al., Reference Golléty, Riera and Davoult2010; Richoux & Ndhlovu, in press). Although a trophic link between Patella vulgata and Ascophyllum nodosum has been suggested in one of these studies (Golléty et al., Reference Golléty, Riera and Davoult2010), most of them did not find any relationships between limpets and macroalgae. In fact, the diet of gastropods is tightly linked to the morphology of their radula (Steneck & Watling, Reference Steneck and Watling1982). Limpets in the genus Patella are characterized by a docoglossan radula, which is adapted to the consumption of epilithic biofilm, leathery macroalgae (e.g. Ascophyllum nodosum) and encrusting macroalgae (Steneck & Watling, Reference Steneck and Watling1982). The diet of this gastropod is therefore likely to vary in time and space according to the availability of its potential food sources.
Because rocky shores are naturally highly heterogeneous ecosystems (McGuiness & Underwood, Reference McGuiness and Underwood1986), primary producers (i.e. canopy-forming Fucales, understorey macroalgae, benthic biofilm) are likely to vary at the small spatial scale. Here, we formulate the hypothesis that the diet of limpets cannot be totally understood without a small-scale perspective, particularly in relation to the spatial distribution of Ascophyllum nodosum. Since Patella vulgata has been considered to be responsible for the loss of A. nodosum canopies in different areas of Europe (Le Roux, Reference Le Roux2005; Davies et al., Reference Davies, Johnson and Maggs2007), we expected that limpets found in the immediate vicinity of macroalgal fronts would strongly rely on A. nodosum and its associated epiphytic flora for food, while those found far from these fronts would rely mainly on alternative sources, such as benthic biofilm or ephemeral macroalgae.
MATERIALS AND METHODS
Sampling procedure
Field sampling was carried out in July 2010 on a semi-sheltered rocky shore of Porspoder (Western Brittany, France, 04°46.505W 48°29.230N). This rocky shore is characterized by the co-occurrence of Ascophyllum nodosum beds and bare rock zones, forming unvegetated patches within A. nodosum beds, including patches of sponges (Hymeniacidon perlevis and Halichondria panicea) localized in crevices and underneath the algal canopy. Limpets were abundant everywhere on bare rock areas, but did aggregate at the the edges of A. nodosum beds (later called fronts). Eight fronts were selected for sampling at mid-shore level. The distance between the different fronts sampled ranged from a few metres to about 10 metres. A tape measure was deployed perpendicular to each front, and all limpets within the first 10 cm from the tape were removed from the substrate, their distance to the algal front noted, and sealed individually in labelled plastic bags. Limpets were sampled up to 6 m from A. nodosum fronts, which was the maximal distance found between limpets and algal fronts. Preliminary studies (Grall, unpublished data) indicate that most small individuals keep moving until they find a suitable place to settle. Hence, in order to sample established adults, only individuals larger than 30 mm were kept for analysis. For the eight fronts, 103 individuals were collected.
At each front, samples of A. nodosum and other abundant macroalgae (i.e. Fucus serratus, Ulva spp., Ceramium sp., Vertebrata lanosa) were also collected. Epilithic biofilm was sampled by brushing the substrate (0.05 m2) using a thin brush, and then resuspended in filtered (0.7 μm) seawater (Riera et al., Reference Vander Zanden and Rasmussen2009; Schaal et al., Reference Schaal, Riera and Leroux2009). The brush was carefully rinsed with fresh water between each sampling to prevent any contamination between two samples. Three samples of water were also collected at incoming tide to characterize the isotopic signature of the suspended particulate organic matter (POM). All samples were stored in a coolbox until being brought back to the laboratory.
Sample processing
Once in the laboratory, limpets were starved overnight in filtered seawater and killed by freezing (−20°C). They were then dissected, and a piece of muscle tissue was cut off from the foot, as well as the entire digestive gland. Muscle and digestive gland samples were freeze-dried, and ground into a fine and homogeneous powder using an automatic grinder.
Sponges were carefully checked for the presence of any carbonate particles, briefly acidified, rinsed with distilled water and freeze-dried, before being ground into powder. Macroalgae were cleaned of any visible epiphytes, and also freeze-dried and ground into powder. Samples of POM and biofilm were filtered once back in the laboratory through pre-combusted (450°C, 4 h) GF/F filters. Biofilm samples were briefly acidified to remove any carbonate contamination. The filters were then freeze-dried and the material retained on the filter scraped using a clean scalpel. All samples were stored frozen (−20°C) until further processing. Powdered samples were then placed in tin capsules before isotope ratio mass spectrometry analysis.
Isotopic analyses were performed by Mylnefield Research Services Ltd. (Dundee, Scotland) using a SerCon 20/20 Geo Isotope Ratio Mass Spectrometer with ANCA-GSL Combustion/Reduction interface. The data are expressed in ‰ in the standard δ unit:
with R = 13C/12C for carbon and 15N/14N for nitrogen. These abundances were calculated in relation to the certified reference materials Vienna Pee Dee Belemnite-limestone (carbon) and atmospheric dinitrogen (nitrogen).
Data analysis
Limpets were classified in six classes of distance to front for data analysis (0–20 cm, 20–50 cm, 50–100 cm, 100–200 cm, 200–300 cm, >300 cm) (Table 1). Analysis of variance was used to compare the stable isotope ratios of limpets among those different classes, after checking for normality (Shapiro–Wilk's tests) and homogeneity of variances (Levene's tests). Post-hoc Tukey's tests were then performed to identify differences. Difference were considered as significant at P < 0.05.
Table 1. δ13C and δ15N of limpets (Patella vulgata) foot and digestive glands measured at various distances from the closest Ascophyllum nodosum front.

The contribution of each potential food source to the diet of Patella vulgata was estimated using the Bayesian stable isotope mixing model SIAR (Parnell et al., Reference Parnell, Inger, Bearhop and Jackson2010; available at http://cran.r-project.org/web/packages/siar/index.html). This model estimates the contribution of different potential food sources to the diet of a consumer, taking into account the variability of their respective stable isotope signature, as well as the variability of trophic enrichment. Different potential food sources were considered: Fucales (regarded as the average isotopic ratios of Ascophyllum nodosum and Fucus serratus), the Chlorophyta Ulva spp. (two species were present, foliose and filamentous) and the Rhodophyta Vertebrata lanosa, epiphyte of A. nodosum. Because limpets have sometimes been reported to ingest encrusting invertebrates (Camus et al., Reference Camus, Daroch and Opazo2008), the two main sponges (Halichonria panicea and Hymeniacidon perlevis), as well as benthic biofilm, were also considered as potential food sources for SIAR modelling. Because limpets are exclusive grazers, POM was not considered as a potential food source. The isotopic fractionation occurring between a consumer and its source is known to be variable according to the taxa or the environment considered (Vander Zanden & Rasmussen, Reference Vander Zanden and Rasmussen2001). Consequently, we considered for this study a δ15N fractionation of 1.2 ± 0.8‰, according to Vanderklift & Ponsard (Reference Vanderklift and Ponsard2003). For δ13C, the fractionation considered was 0.5 ± 0.13‰, according to McCutchan et al. (Reference McCutchan, Lewis, Kendall and McGrath2003).
RESULTS
Primary producers and potential food sources
Primary producers displayed similar δ13C and δ15N over the different Ascophyllum nodosum fronts considered. Consequently, the data (both primary producers and limpets) from all the fronts were pooled together for subsequent analyses.
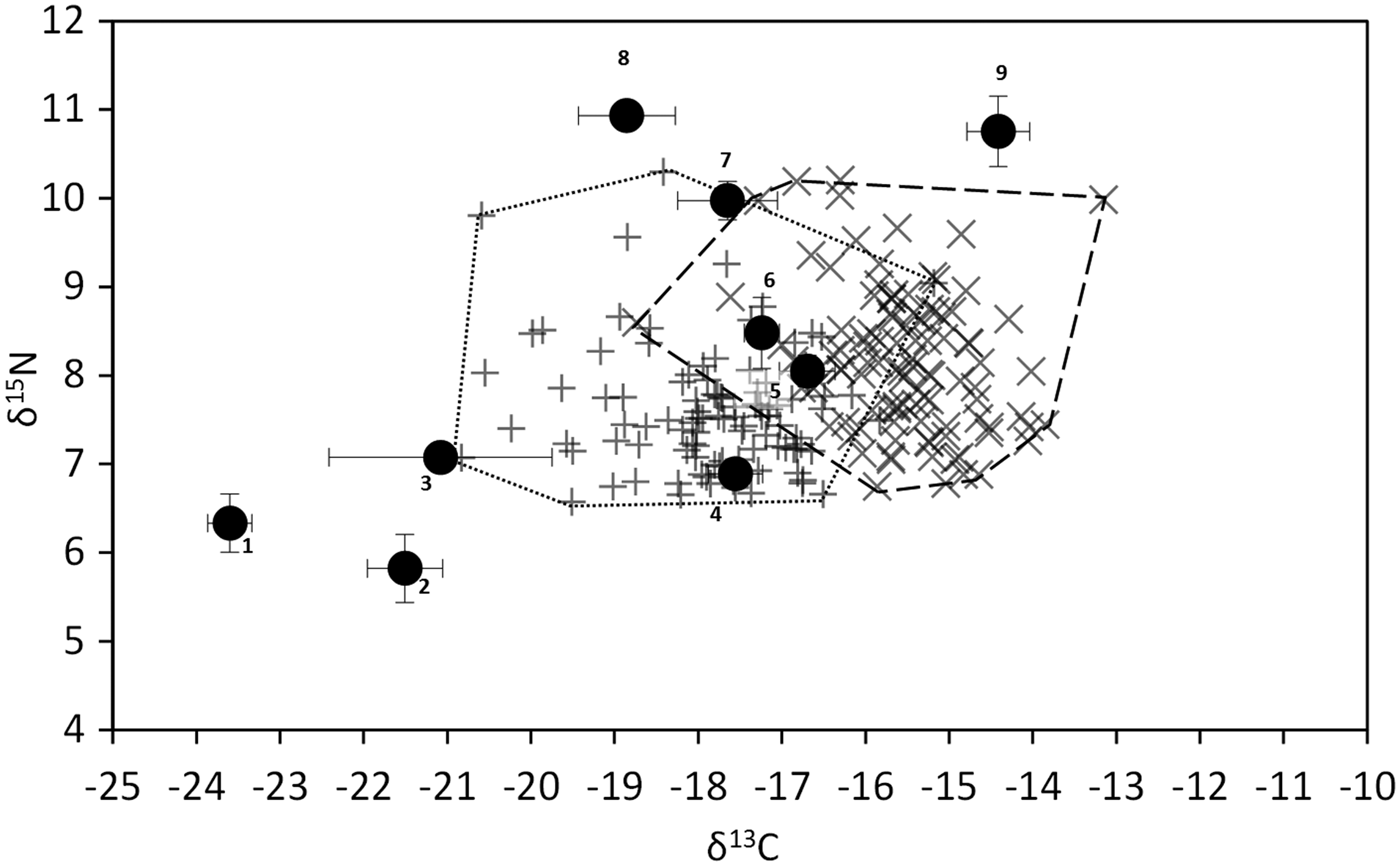
The δ13C of potential food items ranged from −23.6‰ (suspended particulate organic matter, POM) to −14.4‰ (Ulva sp.) (Figure 1). Among co-occurring potential food sources, A. nodosum was the second most 13C-enriched (−16.7‰), but its δ13C was close to those displayed by the Phaeophya Fucus serratus and the Rhodophyta Ceramium sp. (−17.6 and −17.2‰, respectively). The δ13C of biofilm was much lower than most macroalgae (−21.5‰). δ15N values ranged from 5.8‰ (Biofilm) to 10.8‰ (Ulva sp.). Most primary producers’ δ15N comprised between 5.8 and 8.5‰. The two sponges (Halichondria panicea and Hymeniacidon perlevis) and the Chlorophyta Ulva sp. displayed higher δ15N than other potential food sources.
Fig. 1. δ13C vs δ15N of the most abundant primary producers and sponges (black dots), and of the limpet Patella vulgata. (+, fine dashes) = muscle, (×, long dashes) = digestive gland. 1, Suspended particulate organic matter; 2, benthic biofilm; 3, Vertebrata lanosa; 4, Fucus serratus; 5, Ascophyllum nodosum; 6, Ceramium sp.; 7, Hymeniacidon perleve; 8, Halichondria panicea; 9, Ulva sp.
Limpets’ isotopic ratios and diet
The isotopic ratios of muscle tissue were consistently 13C-enriched (2.2 ± 0.6‰) compared with digestive glands (Figure 2).Overall, the δ13C of muscle comprised between −18.7 and −13.1‰, while the δ13C of digestive glands ranged from −20.8 to −15.2‰. While no significant difference was found among the different categories for the δ13C of muscle (one-way ANOVA, F 5,97 = 1.76, P = 0.13), some significant differences occurred for the digestive glands (one-way ANOVA, F 5,97 = 5.35, P < 0.001), mostly due to the differences between samples next to the front (0–20 cm) and those further away (>20 cm) (post-hoc Tukey's pairwise comparisons, P < 0.05). The opposite pattern was observed for δ15N, with no difference being observed among distances for digestive gland tissues (one-way ANOVA, F 5,97 = 1.85, P = 0.11), while some significant differences were observed for muscle (one-way ANOVA, F 5,97 = 14.97, P < 0.001), revealing a pattern of 15N-enrichment in the most distant groups from the front (post-hoc Tukey's pairwise comparisons, P < 0.05).

Fig. 2. Differences of δ13C and δ15N observed between muscle and digestive gland in Patella vulgata along the Ascophyllum nodosum front–bare rock gradient. Letters indicate the results of pairwise comparisons realized among each tissue/isotope category.
The results of SIAR modelling of Patella vulgata's diet showed that, regardless of the tissues analysed or the distance from an Ascophyllum nodosum front, Fucales (i.e. A. nodosum and F. serratus) and the biofilm formed the major part of the diet (Figure 3). SIAR modelling, either based on muscle or digestive gland tissues, indicated a higher contribution of both Fucales and biofilm at the vicinity of A. nodosum fronts, while the diet of limpets collected more than 200 cm from the fronts appeared more diversified, including small but significant amounts of the Chlorophyta Ulva sp. and Enteromorpha sp. (maximal estimated contribution around 35% of the diet) (Figure 4).

Fig. 3. Contribution of the different potential food sources to the diet of Patella vulgata at the immediate vicinity of Ascophyllum nodosum fronts and more than 300 cm away, estimated through the use of SIAR based on the stable isotope ratios of muscle and digestive gland tissues. Boxplots indicate the 5, 25, 75, 95 percentiles, and the credibility intervals of contributions’ distribution.

Fig. 4. Variations in the contribution of Ascophyllum nodosum and epilithic biofilm to the diet of Patella vulgata along the Ascophyllum nodosum front–bare rock gradient estimated through the use of SIAR based on the stable isotope ratios of muscle and digestive gland tissues. Boxplots indicate the 5, 25, 75, 95 percentiles, and the credibility intervals of contributions’ distribution.
DISCUSSION
This study is, to our knowledge, the first one to investigate the diet of Patella vulgata with a microscale spatial perspective. Recent studies have highlighted the need to consider small spatial scales in order to fully understand the diets of benthic invertebrates, and the possible factors influencing their diets (Guest & Connolly, Reference Guest and Connolly2004; Kon et al., Reference Kon, Kurokura and Hayashizaki2007; Schaal et al., Reference Schaal, Riera and Leroux2011). These studies have emphasized the limits of trophic pattern generalization without a thorough consideration of local-scale structural features of the environment that are likely to affect food source availability. Here, we showed that the diet of P. vulgata varies at the centimetre scale, with respect to its distance to macroalgal beds.
In this study, we analysed both muscle and digestive glands of limpets, each reflecting the food assimilated over different time spans (Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983). Although turnover times have mostly been investigated in the different tissues of fish, and few studies have addressed this issue in molluscs, isotopic ratios from the digestive glands are expected to correspond to the diet assimilated recently (i.e. from days to weeks; Raikow & Hamilton, Reference Raikow and Hamilton2001), while muscle is known to integrate the food assimilated over a longer period (from weeks to months; McIntyre & Flecker, Reference McIntyre and Flecker2006). This difference is of particular importance, because Ascophyllum nodosum can experience strong seasonal variation in both δ13C and δ15N (Golléty et al., Reference Golléty, Riera and Davoult2010). Consequently, the stable isotope ratios measured for muscle tissue are likely to be representative of the assimilation of food sources consumed over a longer time period and whose δ13C and δ15N differ from food assimilated leading up to and during the course of the sampling period. In the case of a single time study, such as the present one, the analysis of fast-turnover tissues, such as digestive glands, may therefore be more relevant and representative of the potential food sources analysed during the sampling.
In spite of the higher relevance of considering the digestive glands of limpets to infer the composition of their diet in single time studies, the storage of lipids in this tissue is likely to affect the conclusions of stable isotope analyses. Molluscs typically store lipids in their digestive gland and their gonads (Wenne & Polak, Reference Wenne and Polak1989). Among biochemical compounds in animals, lipids are known to be 13C-depleted compared with proteins (De Niro & Epstein, Reference De Niro and Epstein1977), which sometimes requires the application of correction factors to the stable isotope ratios of these tissues (e.g. Bodin et al., Reference Bodin, Le Loc'h and Hily2007). We did not measure the lipid content in limpet tissues during the present study. We are, however, confident that any potential variability in the lipid content of digestive glands would only marginally affect our conclusions. For instance, lipid content in lake fish varying from 3% (northern pike) to 26% (lake trout) only resulted in a 2‰ δ13C difference (Post et al., Reference Post, Layman, Arrington, Takimoto, Quattrochi and Montaña2007), which is much lower than the isotopic variability observed in this study. Moreover, using correction factors accounting for the effects of lipids on stable isotope ratios would yield higher δ13C for digestive glands, potentially higher than the most 13C-enriched potential food source. Finally, it is likely that lipids contained in digestive glands affect the δ13C in the same way along the A. nodosum front–bare substrate gradient. Hence, the variability in the diet of limpets along this gradient is also unlikely to be affected by this potential bias.
Although the SIAR diet modelling based on the two different tissues leads to contrasting conclusions, there is some consistency in the contribution of both Ascophyllum nodosum and biofilm to the diet of Patella vulgata along the A. nodosum front–bare substrate gradient. According to our initial hypothesis, the contribution of A. nodosum was higher at the front vicinity (less than 50 cm) than far away from it (more than 200 cm). However, even the diet of limpets sampled more than 3 m away from the front was still significantly based on this alga. Limpets predominantly graze during high tide and at night, making a complete loop centred around their home scar, where they come back before emersion (Evans & Williams, Reference Evans and Williams1991; Noël et al., Reference Noël, Hawkins, Jenkins and Thompson2009). Although a consistent lag between P. vulgata's resting area and foraging area has already been reported around rockpools (Noël et al., Reference Noël, Hawkins, Jenkins and Thompson2009), this could hardly explain the reliance of limpets on A. nodosum more than 3 m away from their resting area (L.M.-L.J. Noël, personal communication). Even though limpets have sometimes been observed foraging more than 2 m away from their resting area (J. Grall, personal observation), such a consistent directional foraging would involve energetic expenses that would seriously reduce the advantage of feeding on a potentially higher nutritive value source. The trapping of drifting macroalgae fragments is known to be an important feature of limpets’ feeding behaviour (Bustamante & Branch, Reference Bustamante and Branch1996).The significant contribution of A. nodosum to the diet of limpets colonizing bare substrate far from the fronts studied here might thus indicate that P. vulgata is able to trap detached fragments of this algae and to readily feed on them, as previously suggested by Lorenzen (Reference Lorenzen2007). This behaviour can play a significant role in sustaining important populations of limpets in unvegetated areas (Bustamante & Branch, Reference Bustamante and Branch1996).
Surprisingly the contribution of the biofilm to the diet of Patella vulgata did not match our initial expectations, since it was highest at the immediate vicinity of A. nodosum fronts, and only contributed about 20% of the diet for the furthest individuals (based on digestive gland stable isotope ratios). The high contribution of biofilm at the vicinity of A. nodosum challenges the conclusions of Davies et al. (Reference Davies, Johnson and Maggs2007), who found that this alga, when present, was the prime food source of P. vulgata. A possible alternative explanation for this unexpected high contribution of biofilm is the secretion by A. nodosum of high amounts of extracellular polymeric substances (EPS) (Khailov & Burlakova, Reference Khailov and Burlakova1969), which can stimulate the growth of the microbial epilithic biofilm, through providing dissolved organic matter that is assimilated by bacteria (Golléty et al., Reference Golléty, Migné and Davoult2008), making this source locally more abundant and more nutritive. The 15N-enrichment observed in limpets’ muscle along the front-bare rock gradient might also be explained to some extent by a slight omnivory in limpets colonizing bare rock. Apart from the sponges Halichondria panicea and Hymeniacidon perlevis, no potential animal dietary items (especially barnacle settlers and recruits) were sampled. This was contrary to some studies where limpets have been reported to derive a small portion of their diet from animal items (Hawkins et al., Reference Hawkins, Watson, Hill, Harding, Kyriakides, Hutchinson and Norton1989; Hill & Hawkins, Reference Hill and Hawkins1991; Camus et al., Reference Camus, Daroch and Opazo2008). However, we did not observe any significant barnacle recruitment during sampling or in the weeks before on the sampling site, which limits the possibility that other animal sources contribute to the diet of P. vulgata. Moreover, one could reasonably argue that other sessile animals would be isotopically more or less similar to sponges, because they all are suspension-feeders. Results from SIAR modelling clearly show that these items do not contribute to the diet of limpets. Consequently, the diet of limpets at the vicinity of A. nodosum fronts is almost only composed of this alga and biofilm, while limpets colonizing bare substrate are characterized by a more diversified diet, including a lower contribution of A. nodosum and biofilm, and a significant contribution of green algae (Ulva sp., Enteromorpha sp.).
Overall, our results highlight the microscale variability in the diet of the limpet Patella vulgata. Although a recent study pointed out the microscale variability occurring in the diet of suspension-feeding invertebrates colonizing rocky shores (Schaal et al., Reference Schaal, Riera and Leroux2011), this is, to our knowledge, the first time that this variability is observed in a field study for rocky shore-associated grazers. The differences in the diet of limpets along the Ascophyllum nodosum front–bare substrate gradient illustrate the need to consider small-scale features of ecosystems, that are particularly likely to affect community structure and functioning in heterogeneous habitats, such as rocky shores (Le Hir & Hily, Reference Le Hir and Hily2005).This study shows that limpets are able to assimilate a wide array of food sources, thanks to their feeding apparatus (Steneck & Watling, Reference Steneck and Watling1982), and their ability to cope with the presence of herbivore-deterrent secondary metabolites in their diet. These characteristics make limpets generalist and opportunistic consumers. Together with their ability to exclude their space competitors from the substrate through removal and/or consumption, these constitute key factors likely to explain their dominance on eastern Atlantic rocky shores.
CONCLUSIONS
North Atlantic sheltered rocky shores are typically dominated by canopies of Ascophyllum nodosum. This species is considered as a foundation species (sensu Bruno & Bertness, Reference Bruno, Bertness, Bertness, Gaines and Hay2001), enhancing both local algal and animal diversity (Jenkins et al., Reference Jenkins, Hawkins and Norton1999). Rocky shore-associated communities are thought to be characterized by a predominance of detritus-based food chains (Bustamante & Branch, Reference Bustamante and Branch1996; Riera et al., 2009; Schaal et al., Reference Schaal, Riera and Leroux2009). The main reasons for this functional predominance are the low regulative effect of littorinid grazers (Jenkins et al., Reference Jenkins, Moore, Burrows, Garbary, Hawkins, Ingólfsson, Sebens, Snelgrove, Wethey and Woodin2008), which have long been thought to be the main grazers of A. nodosum, and the high content of herbivore-deterrent secondary metabolites found in this alga's tissues (Pavia & Toth, Reference Pavia and Toth2000). However, this study, along with recent works on the relationships between A. nodosum and Patella vulgata (Le Roux, Reference Le Roux2005; Davies et al., Reference Davies, Johnson and Maggs2007, 2008; Lorenzen, Reference Lorenzen2007), seriously challenges this paradigm, not only pointing out a strong trophic link between P. vulgata and A. nodosum, but also suggesting an important top-down regulation of A. nodosum by limpet grazing. The dominance of A. nodosum on northern Atlantic rocky shores has long been a mystery for ecologists, considering its extremely low growth rate and recruitment intensity (Vadas et al., Reference Vadas, Wright and Miller1990). The main reason commonly advanced was related to the relative absence of efficient grazers, which allowed the persistence of A. nodosum stands for long periods. Considering the consistent evidence of A. nodosum overgrazing by limpets, this explanation cannot stand any longer. Further effort should therefore be devoted in future to the understanding of ecological factors allowing the maintenance of extensive stands of A. nodosum in the northern Atlantic.
ACKNOWLEDGEMENTS
We would like to thank LM-LJ Noël, AJ Davies and an anonymous reviewer for their constructive comments on the different versions of this manuscript.