Short-term peripheral venous catheters (PVCs) are among the most commonly used invasive devices in healthcare settings worldwide. Reference Helm, Klausner, Klemperer, Flint and Huang1-Reference Mermel3 As reported in a recent systematic review, ~200 million PVCs are being inserted each year in the United States. Reference Mermel3 According to point-prevalence studies, PVCs accounted for 80%, 90%, and 95% of all intravascular devices placed in hospitalized patients in France, in Scotland, and Spain, respectively. Reference Mermel3
The high prevalence of PVC insertion results in considerable morbidity, excess length of stay (LOS) and hospital costs, prolonged antibiotics treatments, and bloodstream infections (BSIs). Reference Zhang, Cao and Marsh4
In addition, the overall PVC failure rate ranges from 35% to 50%, Reference Helm, Klausner, Klemperer, Flint and Huang1,Reference Sabri, Szalas, Holmes, Labib and Mussivand2,Reference Wallis, McGrail and Webster5 with such failures being responsible for PVC-related adverse events such as phlebitis, occlusion or mechanical failure, infiltration, dislodgment, and BSIs. Reference Helm, Klausner, Klemperer, Flint and Huang1,Reference Sabri, Szalas, Holmes, Labib and Mussivand2,Reference Wallis, McGrail and Webster5-Reference Abolfotouh, Salam, Bani-Mustafa, White and Balkhy10
Because PVCs have rarely been associated with BSIs, as stated in the 2011 CDC guidelines for the prevention of intravascular catheter-related BSIs, Reference Mermel3,Reference O’Grady, Alexander and Burns11,Reference Maki, Kluger and Crnich12 most studies have been focused on central-line–associated BSIs rather than PVC-related BSIs (PVCR-BSIs), which to date have not been thoroughly analyzed. Reference Zhang, Cao and Marsh4
PVCR-BSIs are confirmed by the presence of positive blood cultures related by clinical data to PVCs in patients who did not have a central line in place. Reference Helm, Klausner, Klemperer, Flint and Huang1 According to the 2016 Infusion Nurses Society standards of practice 13 and the 2017 International Nosocomial Infection Control Consortium (INICC) bundle for the prevention of central- and peripheral-line–related BSIs, there no time limit is recommended for PVC removal. Reference Rosenthal, Kanj and Desse14 In studies from healthcare settings in industrialized countries, the incidence of PVCR-BSI in ICU patients has been reported to be 0.5 per 1,000 PVCs days in ICUs in Australia, Italy, and the United States, Reference Maki, Kluger and Crnich12 and a rate of 0.67 PVCR-BSIs per 1,000 PVCs days has been reported in pediatric and neonatal ICUs in Australia. Reference Worth, Daley, Spelman, Bull, Brett and Richards15 The incidence of PVCR-BSI has not been well documented, and comprehensive data are not available in resource-limited countries nor in resource-rich areas. Although the mentioned percentages reported in high-income countries may seem small, the burden of PVCR-BSI is not a minimal issue in public health. Thus, with this study, we have begun to fill this gap in the literature to contribute to the introduction of strategies targeting the prevention and control of PVCR-BSI.
This prospective surveillance was conducted during 6 years in 141 cities in 42 countries, of Africa, the Americas, Eastern Mediterranean, Europe, South East Asia, and Western Pacific regions between September 1, 2013, and May 31, 2019, in 204 ICUs in 268 hospitals that participate in the INICC. Reference Rosenthal, Al-Abdely and El-Kholy7-Reference Rosenthal, Jarvis and Jamulitrat9,Reference Rosenthal, Maki and Mehta16 It is the first comprehensive study to analyze the incidence rate, bacterial resistance, LOS, and mortality attributable to PVCR-BSI.
Methods
Background of the INICC
The INICC is comprised of a group of hospitals in 210 cities in 54 countries in 6 World Health Organization (WHO) regions: Africa, the Americas, Eastern Mediterranean, Europe, South East Asia, and Western Pacific. The INICC has become the oldest and largest source of aggregate standardized international data on the epidemiology of healthcare-associated infections (HAIs) worldwide. Reference Rosenthal, Al-Abdely and El-Kholy7,Reference Rosenthal17 The INICC focuses on the surveillance and prevention of HAIs in adult, pediatric, and neonatal ICUs, step-down units, and inpatient wards, and on the surveillance and prevention of surgical site infections in surgical procedures hospital-wide.
Study design
This prospective, cohort surveillance study was conducted using an online platform called INICC Surveillance Online System (ISOS). Through ISOS, PVCR-BSI was validated by infection control professionals (ICPs), and the recorded signs and symptoms of infection and the results of cultures, laboratory and radiographic studies, as well as other tests, were scrutinized to assure that the last US Centers for Disease Control and pRevention (CDC)/National Health Safety Network (NHSN) criteria for PVCR-BSIs were met, in accordance with the definition presented below. Reference Rosenthal17,18
INICC methods
The ISOS includes the implementation of the CDC-NSHN methodology, but it adds the collection of other data essential to increase the sensitivity of ICPs to detect PVCR-BSIs and to avoid underreporting. Reference Rosenthal17 According to standard CDC-NSHN methods, numerators are the number of healthcare-acquired infections related to a specific feature and denominators are device days collected from all patients as pooled data, that is, without determining the number of device days related to a particular patient and without collecting features or characteristics of specific patients.
This aspect differs from the ISOS because the design of the cohort study through the ISOS also includes the collection by ICPs of specific data per patient from all patients, both with and without PVCR-BSI. Such data include invasive device utilization, date of admission, date of discharge, LOS, microorganism profile of the HAI, bacterial resistance, and mortality, among several others.
Outcome surveillance data collection and validation
In this study, we investigated the outcome surveillance of PVCR-BSIs in the ICU using the ISOS, which follows the INICC protocol and allows the classification of prospective, active, cohort data into specific module protocols.
The site-specific criteria included reporting instructions and full explanations integral to their adequate application.
ICPs collected daily data on PVCR-BSIs and denominator data such as specific device days in the ICUs, patient days, microorganism profile, and bacterial resistance. All patients with a central line were excluded; only patients with a short-term PVC were included in this study. Midline catheters were not included in the PVC category.
Validation is an essential feature of the ISOS that maximizes the sensitivity and accuracy of surveillance data. Each PVCR-BSI reported by an ICP is validated, that is, scrutinized to be certain that all criteria are satisfied to justify its recording as a PVCR-BSI. The validation process also includes data reported for putatively uninfected patients to permit the detection of unreported but true PVCR-BSIs. To do so, the ISOS shows an online message to the ICPs, asking them to check the criteria for that putative PVCR-BSI. Reference Rosenthal17
Training
The INICC team trained and provided ICPs with manuals, training tools, and tutorial movies that describe in detail how to perform surveillance and upload surveillance data through the ISOS. In addition, investigators attended webinars and had continuous access to a support team at the INICC headquarters in Buenos Aires, Argentina. On a routine basis through the ISOS online platform, the INICC support team ensured that ICPs performed surveillance correctly. The team sent e-mails and online messages to ICPs asking them to check and review surveillance data and specific criteria.
Definitions
We used the US CDC-NHSN definitions for BSI from its 2013 publication and amendments until its latest publication in 2019. 19-22 These definitions do not include the surveillance definition of PVCR-BSI. 19-22 We applied the CDC-NHSN definition for patients who met all the criteria for BSI but who never had central lines or peripherally inserted central catheters, and who only had short-term PVCs before or after the acquisition of a BSI.
Calculation
Data uploaded to ISOS were used to calculate PVCR-BSI rates per 1,000 device days, mortality, and LOS, according to formulas that used device days consisting of the total number of PVC days. Crude excess mortality of PVCR-BSI equaled crude mortality of ICU patients with PVCR-BSI minus crude mortality of patients without PVCR-BSI. Crude excess LOS of PVCR-BSI equaled crude LOS of ICU patients with PVCR-BSI minus crude LOS of patients without PVCR-BSI. The device utilization ratio (DUR) equaled the total number of PVCR days divided by the total number of bed days. To calculate extra LOS and extra mortality, all central-line–associated BSIs were excluded, and only patients with PVCs, with and without BSIs, were included.
Statistical analysis
We used ISOS version 2.0 software (INICC, Buenos Aires, Argentina) to calculate PVCR-BSI rates, DURs, LOS, and mortality. We used EpiInfo version 6.04b software (CDC, Atlanta, GA) and SPSS version 16.0 software (SPSS, IBM, Chicago, IL) for other calculations and analyses. The 95% confidence intervals (CIs) and P values were determined for all outcomes.
Setting
The study was conducted in 727 ICUs from 268 hospitals in 141 cities of the following 42 countries of 6 WHO regions: Argentina, Bahrain, Brazil, Bulgaria, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Egypt, El Salvador, India, Iran, Jordan, Kingdom of Saudi Arabia, Kosovo, Kuwait, Lebanon, Macedonia, Malaysia, Mexico, Mongolia, Morocco, Nepal, New Guinea, Pakistan, Palestine, Panama, People’s Republic of China, Peru, Philippines, Poland, Romania, Russia, Serbia, Slovakia, Sri Lanka, Sudan, Thailand, Tunisia, Turkey, United Arab Emirates, Venezuela, and Vietnam.
Institutional review boards agreed to the study protocol, and patient confidentiality was protected by coding the recorded information, making it identifiable only to the infection control team. All patients admitted to the ICUs during the study period were enrolled in the study with the approval of each hospital’s research ethics committee. In accordance with the INICC charter, the identity of all INICC hospitals and cities remain confidential. Reference Rosenthal17
Results
During the 6-year study period from September 1, 2013, to May 31, 2019, the mean length of participation of the ICUs was 20 months (SD, 27.3 months; range, 1–149 months).
Table 1 shows ICU type and type of ownership for each hospital. Medical-surgical ICUs comprised 38.0% of the total; other ICU types were medical (17.3%), pediatric (9.1%), surgical (8.2%), burn (0.7%), and oncology (0.7%), among others.
Table 1. Type of Intensive Care Unit (ICU) and Hospital Ownership
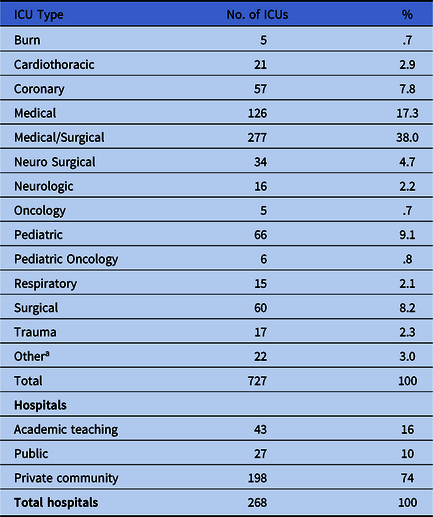
Note. ICU, intensive care unit.
a Includes the following ICU types: cardiac, cardiac surgery, cardiovascular, neurotrauma, post-anesthesia, surgical cardiothoracic, and transplant.
Table 2 presents PVCR-BSI rates and DURs by ICU type. Overall, the PVCR-BSI rate was 2.41 per 1,000 PVC days. The PVCR-BSI rate including only burn and oncology ICUs was 99.45 (ie, 92 PVCR-BSI per 925 PVC days × 1,000). The PVCR-BSI rate without including burn and oncology ICUs was 2.29 (ie, 1,697 PVCR-BSIs per 42,583 PVC days × 1,000).
Table 2. Pooled Means, 95% Confidence Intervals of the Distribution of Short-Term Peripheral Venous Catheter-Related Bloodstream Infections Rates by Type of Location, in Adult and Pediatric Intensive Care Units
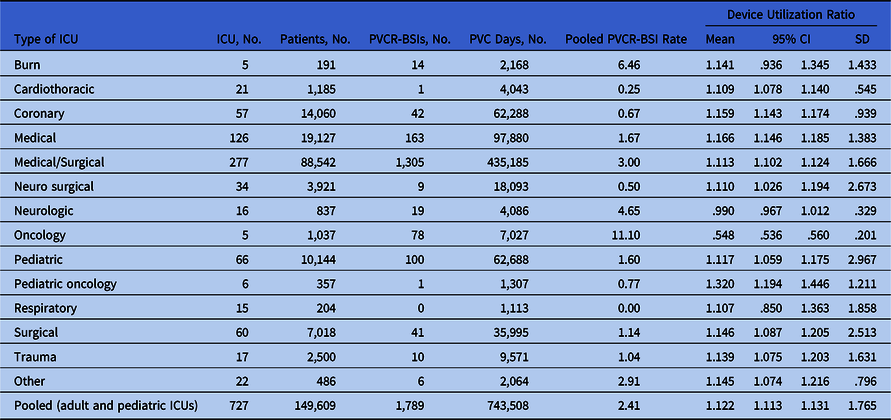
Note. ICU, intensive care unit; PVCR-BSI, short-term peripheral venous catheter-related bloodstream infections; PVC, short-term peripheral venous catheter; DU, device utilization; CI, confidence interval; SD, standard deviation.
Table 3 provides data on crude ICU mortality and crude LOS in patients with and without PVCR-BSI. Mortality without PVCR-BSI was 6.67%, and with PVCR-BSI it was 17.94%. LOS without PVCR-BSI was 4.83 days, and with PVCR-BSI it was 9.85 days.
Table 3. Pooled Means of the Distribution of Crude Mortality and Length of Stay of Intensive Care Unit Patients With Short-Term Peripheral Venous Catheter-Related Bloodstream Infections in Adult and Pediatric Intensive Care Units Combined

Note. PVCR-BSI, short-term peripheral venous catheter-related bloodstream infections; LOS, length of stay; SD, standard deviation; CI, confidence interval.
Figure 1 shows microorganism profile. Overall, 58% were gram-negative bacteria and 42% were gram-positive bacteria.

Fig. 1. Microorganisms profile of short-term peripheral venous catheter-related bloodstream infections.
Table 4 provides data on bacterial resistance of pathogens isolated from patients with PVCR-BSI in adult and pediatric ICUs compared with pathogens from patients with CLAB, as was reported in the last international INICC report of 45 countries. Reference Rosenthal, Bat-Erdene and Gupta23 Pseudomonas aeruginosa related to PVCR-BSI were resistant to fluoroquinolones in 26.93% of these patients versus 20.0% of patients with CLAB. Pseudomonas aeruginosa were resistant to amikacin in 25.00% of patients with PVCR-BSI versus 21.4% of patients with PVCR-BSI and were resistant to imipenem (IPM) or meropenem (MEM) in 25.93% of patients with PVCR-BSI versus 43.48% of patients with CLAB. Resistance of Acinetobacter baumannii to IPM or MEM was 63.15% in patients with PVCR-BSI versus 73.44% in patients with CLAB. The resistance of Klebsiella pneumonia to ceftriaxone or ceftazidime was 75.00% in patients with PVCR-BSI versus 67.54% in patients with CLAB, and resistance to IPM or MEM or ertapenem was 40.35% in patients with PVCR-BSI versus 36.1% in patients with CLAB. The resistance of Escherichia coli to ceftriaxone (CRO) or ceftazidime (CAZ) was 56.99% in patients with PVCR-BSI versus 52.94% in patients with CLAB. Staphylococcus aureus was resistant to oxacillin in 53.66% of patients with PVCR-BSI, which was similar to the resistance in CLAB cases (50.7%).
Table 4. Antimicrobial Resistance Rates in Intensive Care Units Comparing PVCR-BSI with CLAB

Note. PVC, short-term peripheral venous catheter; PVCR-BSI, PVC-related bloodstream infections; infection; CLAB, central line-associated bloodstream infection; FQs, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin, or ofloxacin); OXA, oxacillin; PIP, piperacillin; TZP, piperacillin-tazobactam; AMK, amikacin; VAN, vancomycin; IPM, imipenem; MEM, mer openem; CRO, ceftriaxone; CAZ, ceftazidime; ETP, ertapenem.
a International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012–2017: device-associated module.
Discussion
No comprehensive or representative studies of PVCR-BSI rates at the national level have been conducted in resource-limited countries in any of the 6 WHO regions.
Our study, conducted over 6 years in 727 ICUs of 268 hospitals in 141 cities of 42 countries in the 6 WHO regions with 149,609 patients, is the first comprehensive study in which PVCR-BSI rates per 1,000 device days have been calculated. Reference Alexandrou, Ray-Barruel and Carr6 The overall PVCR-BSI rate was 2.41 per 1,000 PVC days. The incidence of PVCR-BSI has been presented using the number of PVC days in only 2 studies from industrialized countries to our knowledge: (1) in a systematic review published in 2006, including data from the United States, Australia, and Italy, in which the rate was 0.5 PVCR-BSI per 1,000 PVC days Reference Maki, Kluger and Crnich12 and (2) in a study published in 2018, including data of pediatric and neonatal ICUs from Australia, in which the rate was 0.67 PVCR-BSIs per 1, 000 PVC days. Reference Worth, Daley, Spelman, Bull, Brett and Richards15
Although a systematic review was published in 2019 by the Alliance for Vascular Access Teaching and Research (AVATAR) group on PVCR-BSI rates, the studies included did not report PVC days as denominators of PVCR-BSIs rates, and for that reason such data were not comparable with our study. Reference Ray-Barruel, Xu, Marsh, Cooke and Rickard24 This systematic review by AVATAR included studies in which PVCR-BSI rates were presented as follows Reference Ray-Barruel, Xu, Marsh, Cooke and Rickard24 : Australia (0.39 PVCR-BSI per 10,000 occupied bed days), Reference Rhodes, Cheng and McLellan25 Germany (3.04 PVCR-BSI per 1,000 patient days), Reference Salm, Schwab, Geffers, Gastmeier and Piening26 Spain (1.17 PIVC-BSI per 10,000 patient days Reference Saliba, Hornero and Cuervo27 and 0.05 PIVC per 1,000 patient days Reference Freixas, Bella, Limón, Pujol, Almirante and Gudiol28 ), and the United States (0.0150 PVCR-BSI per 100 patient days Reference DeVries, Valentine and Mancos29 and 0.57 PIVCR-BSI per 1,000 patient days Reference Duncan, Warden, Bernatchez and Morse30 ). In different studies, the risk of acquiring BSI was not as high if PVCs were used instead of central lines. Reference Miliani, Taravella and Thillard31-Reference McKinley, Davidson, Broome, Schenk and Safdar33
In our ICUs, the pooled mean of the distribution of crude mortality amounted to 18% of PVCR-BSIs cases, compared with 6.67% mortality of with PVC patients that were not infected. In recent studies from Spain and Japan, the mortality rates attributable to PVCR-BSI were 13.2% and 12.9%, respectively. Reference Saliba, Hornero and Cuervo27,Reference Sato, Nakamura and Fujita34
The excess LOS of patients with PVCR-BSI in our study was 51% higher than in patients without PVCR-BSI; in the previously cited study from Japan, patients who had acquired PVCR-BSI required a longer duration of antibiotic treatment than patients without PVCR-BSI (33.5 vs 15.8 days; P = .004). Reference Sato, Nakamura and Fujita34
The microorganism profile of PVCR-BSI in our ICUs showed a predominance of gram-negative bacteria (58%): Escherichia coli (16%), Klebsiella spp (11%), Pseudomonas aeruginosa (6%), Enterobacter spp (4%), and others (20%) including Serratia marcescens. Within the 42% of gram-positive bacteria, the predominant species were coagulase-negative staphylococci (CoNS) and Staphylococcus aureus (12%).
This finding contrasts starkly with those from industrialized countries, in which gram-positive pathogens were the predominant cause of PVCR-BSI. Reference Elsayed and Laupland35 In a recent study conducted in Japan, the causative pathogens were gram positive in 58% of cases and gram negative in 35.8%. Reference Sato, Nakamura and Fujita34 The higher percentages of gram-positive pathogens in our ICUs may indicate that lack of adequate catheter and hub care, inadequate hand hygiene technique, or lack of compliance with hand hygiene in resource-limited settings.
The predominance of gram-positive pathogens causing PVCR-BSI in industrialized countries has been reported in a wide range of studies. Staphylococcus aureus PVCR-BSI has been identified in industrialized countries as a serious condition that can influence prognosis. Reference Saliba, Hornero and Cuervo27,Reference Sato, Nakamura and Fujita34,Reference Austin, Sullivan, Whittier, Lowy and Uhlemann36 No data showing microorganisms profile for PVCR-BSI from representative studies from other resource-limited countries are available.
The most prevalent PVCR-BSI pathogens identified (Escherichia coli, Klebsiella spp, and Staphylococcus aureus) presented considerable resistance rates. The resistance of Pseudomonas aeruginosa to fluoroquinolones (ie, ciprofloxacin, levofloxacin, moxifloxacin, or ofloxacin) was 26.93%; resistance to amikacin was 25.00%; and resistance to IPM or MEM was 25.93%. All of these rates were <43.48%, the resistance found in patients with CLAB in the last international INICC report of 45 countries. Reference Rosenthal, Bat-Erdene and Gupta23 Also in this previous report, the resistance of Acinetobacter baumannii to IPM or MEM was 63.15% in patients with PVCR-BSI versus 73.44% in patients with CLAB. The resistance of Klebsiella pneumonia to ceftriaxone or ceftazidime was 75.00% in patients with PVCR-BSI versus 67.54% in patients with CLAB, and the resistance to IPM or MEM or ertapenem was 40.35% in patients with PVCR-BSI versus 36.1% in patients with CLAB. The resistance of Escherichia coli to CRO or CAZ was 56.99% in patients with PVCR-BSI versus 52.94% in patients with CLAB. Reference Rosenthal, Bat-Erdene and Gupta23
Regarding gram-positive bacteria, in our study, resistance of Staphylococcus aureus to oxacillin was 53.66%, which is similar to the 49% resistance reported in another study in India Reference Dalai, Padhi, Padhi and Parida37 and to resistance rates found in patients with CLAB in the last international INICC Report. Reference Rosenthal, Bat-Erdene and Gupta23 Enterococcus faecalis was 100% sensitive to vancomycin, which is also similar to the findings of a study conducted in India in which PVCR-BSI Enterococcus spp were 100% sensitive to vancomycin. Reference Dalai, Padhi, Padhi and Parida37
The implementation of PVC insertion and maintenance bundles to decrease PVCR-BSI rates is common in industrialized countries. Reference Ray-Barruel, Xu, Marsh, Cooke and Rickard24,Reference Saliba, Hornero and Cuervo27 To reduce the hospitalized patient’s risk of infection, PVCR-BSI surveillance by number of device days is essential because it effectively characterizes the threatening situation created by PVCR-BSIs. This method must be followed by the implementation of multifaceted and surveillance programs aimed at PVCR-BSI prevention and control. Likewise, it is important to address the burden of antimicrobial resistance and to report susceptibility to antimicrobials of PVCR-BSI-associated pathogens in order to take effective measures to prevent resistant strains from being transmitted. Reference Ray-Barruel, Xu, Marsh, Cooke and Rickard24,Reference Saliba, Hornero and Cuervo27
In this study, the INICC focused on ICU data; that is, the healthcare setting in which patient safety is most seriously threatened due to their critical condition and exposure to invasive devices. Reference Rosenthal, Maki and Salomao38 Throughout the past 19 years, INICC has undertaken a global effort in the 6 WHO regions to respond to the burden of HAIs, and the INICC has achieved extremely successful results by increasing hand hygiene compliance and by improving compliance with infection control bundles and interventions, as described in several INICC publications. Reference Rosenthal, Alvarez-Moreno and Villamil-Gomez39-Reference Rosenthal, Duenas and Sobreyra-Oropeza45 The primary application of these data is to serve as a guide for the implementation of prevention strategies and other quality improvement efforts for the reduction of PVCR-BSI rates and their related adverse events to the minimum possible level.
This study has several limitations. The purpose of this study was to obtain updated data on PVCR-BSI, device utilization, bacterial resistance, LOS, and mortality of patients with and without PVCR-BSI in adult and pediatric ICUs, but it does not provide insights regarding the impact of INICC interventions, such as the implementation of the INICC multidimensional approach and ISOS. Reference Rosenthal17,Reference Rosenthal, Maki and Graves46 The impact of the adoption of such resources is to be published in prospective, interventional studies at hospitals that have participated in the INICC over a considerable period. Reference Rosenthal, Ramachandran and Villamil-Gomez41,Reference Rosenthal, Rodriguez-Calderon and Rodriguez-Ferrer44,Reference Rosenthal, Duenas and Sobreyra-Oropeza45,Reference Rosenthal, Desse and Maurizi47-Reference Rosenthal, Todi and Alvarez-Moreno61,Reference Rosenthal, Rodrigues and Alvarez-Moreno40,Reference Rosenthal, Ramachandran and Duenas62,Reference Rosenthal, Maki and Rodrigues43 Second, our study was limited by the fact that benchmarking with CDC-NSHN, or other institutions, was not possible because PVCR-BSI rates are not reported to such institutions nor are they determined by PVC days. 63,64 Third, due to the low economic resources of our ICUs, culture orders and processing may have been less than ideal, which likely influenced the rates of PVCR-BSI, and the number of patients for whom blood cultures should have been performed but were not is unknown because these data were not registered. Fourth, we did not obtain data on the illness severity score at patient admission to the ICU, which is likely associated with crude mortality. Finally, we have not presented data on trends over time for this 6-year study.
In conclusion, we have presented the only available comprehensive data from limited-resource countries showing PVCR-BSIs per 1,000 PVC days, and benchmarking of our findings was limited to comparison with the results of 2 studies from industrialized countries: a systematic review with data from the United States, Australia, and Italy published in 2006 Reference Maki, Kluger and Crnich12 and a prospective study from Australia. Reference Worth, Daley, Spelman, Bull, Brett and Richards15 Our PVCR-BSI rates were much higher than those derived from the data available from the mentioned industrialized countries. Therefore, it is evident that PVCR-BSIs in ICUs from resource-limited countries represent a challenge to patient safety. PVCR-BSI systematic surveillance and prevention programs, including antibiotic resistance reports, should be widely implemented to reduce the incidence of PVCR-BSI and its adverse-related events worldwide.
Acknowledgments
The authors thank the many healthcare professionals at each member hospital who assisted with the conduct of surveillance in their hospital; Mariano Vilar and Débora López Burgardt, who work at INICC headquarters in Buenos Aires; the INICC Country Directors and Secretaries (Haifaa Hassan Al-Mousa, Hail Alabdaley, Altaf Ahmed, Carlos A. Álvarez-Moreno, Anucha Apisarnthanarak, Bijie Hu, Hakan Leblebicioglu, Yatin Mehta, Toshihiro Mitsuda, and Lul Raka,); and the INICC Advisory Board (Carla J. Alvarado, Nicholas Graves, William R. Jarvis, Patricia Lynch, Dennis Maki, Toshihiro Mitsuda, Russell N. Olmsted, William Rutala, Syed Sattar, and Wing Hong Seto), who so generously supported this unique international infection control network.
Financial support
The funding for the activities carried out at INICC headquarters were provided by the corresponding author, Victor D. Rosenthal, and the Foundation to Fight against Nosocomial Infections.
Conflicts of interest
All authors report no conflicts of interest related to this article.
Appendix with remaining co-authors
Argentina: Desse, J. E.; Pérez, I.; Viegas, M.; Spadaro, M.L. ; Montanini, A; Ocampo, A.V.; Maurizi, D.M.; Rios-Aguilera, A.M.; Fainstein, D.E.; Cermesoni, R.; Alda, E.; Chaparro, G. J.; Golschmid, D.; Cabrera, R.; Bianchi, A. C.; Vimercati, J.; Rodríguez-del-Valle, M. C.; Domínguez, C.V.; Saul, P.A.; Chediack, V.; Stagnaro, J.P.; Alvarez, G.; Benchetrit, G.; Caridi, M.; Sztokhamer, D.; Bourlot, I.; García, M.; Arregui, N.V.; Romani, A.; Marcos, L.S.; Botta, P.; Ramasco, L.; Olivieri, M.S.; Juarez, P.D.; Gallardo, P.F.; Brito, M.P.; Cardena, L.P.
Bahrain: Saeed, N.K.; Abdul-Aziz, S.; ALSayegh, S.; Humood, M.Z., Mohamed-Ali, K.; Swar, S., Magray, T.A.S.;
Brazil: Alves-De-Oliveira, A.; Vasconcelos-Carneiro, A.P.; Dos Anjos-Lima, J.; Pinto-Coelho, K. H.; Maciel-Canuto, M. L.; Rocha-Batista, M.X.; Moreira, T.; Rodrigues-Amarilo, N.; Lima-de-Barros, T.M.; Aguiar-Portela, T.B.; Sugette-de-Aguiar, T.; Serpa-Maia, F.I.; Fernandes-Alves-de-Lima, L.; Teixeira-Josino, L.A.; Sampaio-Bezerra, M.; Furtado-Maia, R.C.; Romário-Mendes, A.; De-Souza-Kuchenbecker, R.; Pires-Dos-Santos, R.; Salomao, R.; Maretti-da-Silva, M.A.; Blecher, S.; Villins, M.; Servolo-Medeiros, E.A.; da-Silva-Escudero; D.V.; Andrade-Oliveira-Reis, M.; Laia, D.; Takeda, C.; Azevedo-Ferreira-Lima, D.; Do-Nascimento, S.C.; Olszewski, J.; Tenorio, M.T.; Silva-Lemos, A.C.; Cardoso, D.M.; Correa-Barbosa, M.A.; Assunção-Ponte, G.; Faheina, J.; Aguiar Leitao, F,; Brito-Aguiar –Portela, T.
Bulgaria: Kostadinov, E.D.; Dicheva, V. J.; Petrov, M. M.
China: Guo, C.; Yu, H.; Liu, T.; Song, G.; Wang, C.; Ye, G.
Colombia: Álvarez-Moreno, C.; Barahona-Guzman, N.; Lagares-Guzman, A.; Rodriguez-Ferrer, M.; Valbuena, R.; Suárez, F.; Torres, P.; Mojica-Carreño , B.E.; Garcia-Laverde, G.; Gomez-Nieto, K.; Avila-Acosta, C.; Raigoza-Martinez, W.; Linares, C.; Rodriguez-Pena, J.; Gualtero-Trujillo, S.L.; Sarmiento, S.S.J.; Gamba-Moreno, L.J.; Ariza-Ayala, B.E.; González-Rubio, P.A.; Valderrama-Márquez, I.; Cañas-Giraldo, L.M.; Marin-Tobar, D.A.; Luis Marino Otela-Baicue, A.; Martinez, A.; Gallardo-Castro, J.A.; Vargas-Palomino, A.; Villamil-Gomez, W.; Cuervo-Millan, F.
Costa Rica: Muñoz-Gutierrez, G.A.; Arguello-Ruiz, A.; Zuniga-Chavarria, M.A.; Maroto-Vargas, L.; Valverde-Hernández, M.; Solano-Chinchilla, A.; Calvo-Hernandez, I.; Chavarria-Ugalde, O.
Dominican Republic: Tolari, G.; Rojas-Fermin, R.A.; Diaz-Rodriguez, C.V.; Huascar, S.; Ortiz, M.
Ecuador: Valencia, F.; Pelaez, C.; Gonzalez-Flores, H.A.; Bovera, M. M.; Alquinga, N.; Santacruz, G.; Jara, E.; Delgado, V.; Unigarro, L.; Garcia, M. F.; Figueroa, V.; Marin, K.; Jara, F.; Silva-Guayasa, L.G.
Egypt: Bayani, V.; Ahmed, S.A.; Alansary, A.M.; Hassan, A.R.; Abdullorziz-Ghazi, I.; Abdel-Halim, M.M.; El-Fattah, M.A.; Abdelaziz-Yousef, R.H.; Hala, A.; Abdelhady, K.M.; Ahmed-Fouad, H.; Mounir-Agha, H.; Hamza, H.S.; Salah, Z.; Abdel-Aziz, D.M.; Ibrahim, S.B.; Helal, A.M.; AbdelMassih, A.F.; Reham-Mahmoud, A.; Elawady, B.; El-sherif, R.H.; Fattah-Radwan, Y.A.; Abdel-Mawla, T.S.; Kamal-Elden, N.M.; Abdelhamid, Y.; Fouda, R.; Mohammed-Hassan, D.; Mansour, M.
El Salvador: Lilian De-Jesus-Machuca, L.; Bran-de-Casares, C.
India: Sengupta, S.; Karmakar, A.; Raj, S.; Roy, I.; Mukherjee, S.; Bej, Mm.; Mukherjee, P.; Baidya, S.; Durell, A.; Mandal, S.; Durga, P.; Sengupta, S.; Giri, A.; Kharbanda, M.; Purkayasta, S.K.; Sinchan; Tabhat, S.; Mahangare, S.; Patwardhan, S.; Dmhicu; Mahale, N.; Upadhyay, N.A.; Triwad, G.; Shaikh, N.; Bhujbal, S.; Dominic, S.; Shingte, V.; Shri, A.; Shrivastava A.M. ; Biswas, S.K.; Divatia, J.V.; Padmini, B.; Saranya, S.; Sharma, S.; Sarma, S. ; Rodrigues, C.; Khanna, G.; Dwivedy, A.; Sriram, A.; Eappen, J.; Binu, S.; Shetty, S.; Thomas, V.; Shah, S.; Singhal, T.; Kothari, V.; Narain, R.; Poojary, A.; Patil, P.; Kukreja, S.; Sheeba, J.; Todi, S.K.; Chabukswar, S.; Bhattacharyya, M.; Ramachandran, B.; Ramakrishnan, N.; Purkayasta, S.K.; Sakle, A.S.; Kumar, S.; Warrier, A.R.; Kavathekar, M.S.; Sahu, S.; Mubarak, A.; Modi, N.; Jaggi, N.; Gita, N.; Bedanta, S.; Mishra; Sahu, S.; Jawadwala, B.; Zala, D.; Zompa, T.; Mathur, P.; Nirkhiwale, S.; Vadi, S.; Singh, S.; Agarwal, M.; Sen, N.; Karlekar, A.; Punia, D.P.; Kumar, S.; Gopinath, R.; Nair, P.K.; Chakravarthy, M.; Sandhu, K.; Kambam, C.; Mohanty, S.K.; Varaiya, A.; Pandya, N.; Vaibhavi, R.; Subhedar, M. R.; Vanajakshi, Singla, D.; Patel, M.; Bhakta, A.; Krupanandan, R.; Ranganathan, L.; Mani, A.K.; Rajagopal, S.; Abraham, B.K.; Venkatraman, R.; Devaprasad, D.; Sinchan, Tabhat, S.; Pillai, H.; Divekar, D.G.; Suryawanshi, M.V.; Rajalakshmi, A.; Kantroo, V.; Kansal, S.; Chawla, R.; Chawla, A.; Bhamare, S.; Thorat, S.; Sarda, O.; Nadimpalli, P.; Sahoo, P.; Mohanty, N.; Misra, S.; Ray, B.; Patel, M.H.; Gokul, D.; Aggarwal, C.; Pawar, N.K.; Kardekar, S.N.; Tamboli, A.S.; Manked, A.; Khety, A.; Sharma, S.; Sarma, S.; Subodh, K.; Roy, I.; Mukherjee, S.; Bej, M.; Mukherjee, P.; Baidya, S.; Durell, A.; Mandal, S.; Paul, D.; Sengupta, S.; Giri, A.; Gehlot, S.G.; Bhattacharya, S.; Sasidharan, A.; Agarwal, A.; Palaniswamy, V.; Sharma, P.; Selvaraj; Saurabh; Agarwal, M.; Soni, D.K.; Gopalakrishnan, R.; Blessymole, S.; Khanna, D.K.; Chacko, F.; Gokul, B.N.; Sukanya, R.; Pushparaj, L.; Thejasvini; Rangaswamy, S.; Delhi, S.; Garg, A.; Ekta; Lakhe, M.; Sharma, C.B.; Singh, G.; Kaur, A.; Rautaraya, B.; Basarkar, S.; Mohapatra, S.; Mohapatra, S.; Mishra, B.K.; Sengupta, S.; Karmakar, A.; Raj, S.; Dubal, P.S.; Raphel, A.K.O.; Bandyopadhyay, R.; Mendos, A.; Sharma, C.B.; Ekta; Kambam, C.; Chhabra, K.D.; Khanna, S.
Iran: Masjedi, M.; Maghsudi, B.; Sabetian, G.; Sanaei, A.; Yousefipour, A.; Nikandish, R.; Sanaei, A.; Shafiee E.; Paydar, S.; Khalili, H.A.; Moradi, A.; Sadeghi, P.; Bolandparvaz, S.
Jordan: Mubarak, S.; Makhlouf, M.; Awwad, M.; Ayyad, O.; Shaweesh, A.A.; Khader, M.M.; Alghazawi, A.; Hussien, N.; Alruzzieh, M.
Kingdom of Saudi Arabia: Mohamed, Y.K.; ALazhary, M.; Abdul Aziz, O.A.; Alazmi, M.; Mendoza, J.; De Vera, P.A.; Rillorta, A.S.; Mildred de Guzman, Girvan, M.; Torres, M.; Alzahrani, N.; Alfaraj, S.; Gopal, U.; Manuel, M.G.; Alshehri, R.; Lessing, L.; Alzoman, H.; Abdrahiem, J., Adballah, H.; Thankachan, J.; Gomaa, H.; Asad, T.; AL-Alawi, M.; Al-Abdullah, N.A.; Demaisip, N.L.; Laungayan-Cortez, E.; Cabato, A.F.; Gonzales, J.M.; Al Raey, M.A.; Al-Darani, S.A.; Aziz, M.R.; Al-Manea, B.; Samy, E.; AlDalaton, M.; Alaliany, M.J.; Alabdely, H.M.; Helali, N.J.; Sindayen, G.; Malificio, A.A.; Al-Dossari, H.B.; Kelany, A.; Algethami, A.G.; Mohamed, D.; Yanne, L.; Tan, A.; Babu, S.; Abduljabbar, S.M.; Al-Zaydani, M.A.; Ahmed, H.; Al Jarie, A.; Al-Qathani, A.S.M.; Al-Alkami, H.Y.; AlDalaton, M.; Alih, S.J.B.; Alaliany, M.J.; Gasmin-Aromin, R.; Balon-Ubalde, E.; Diab, H.H.; Kader, N.A.; Hassan-Assiry, I.Y.; Kelany, A.; Albeladi, E.; Aboushoushah, S.; Qushmaq, N.; Fernandez, J.; Hussain, W.M.; Rajavel, R.D.; Bukhari, S.Z.; Rushdi, H.; Turkistani, A.A.; Mushtaq, J.J.; Bohlega, E.; Simon, S.; Damlig, E.; Elsherbini, S.G.; Abraham, S.; Kaid, E.; Al-Attas, A.; Hawsawi, G.; Hussein, B.; Esam, B.; Caminade, Y.; Santos, A.J.; Abdulwahab, M.H.; Aldossary, A.H.; Al-Suliman, S.; AlTalib, A.A.; Albaghly, N.; HaqlreMia, M.E., Kaid, E.; Altowerqi, R.; Ghalilah, K.M.; Alradady, M.; Al-Qatri, A.; Chaouali, M; Shyrine, E.L.; Philipose, J.; Raees, M.; AbdulKhalik, N.S.; Madco, M.; Acostan, C.; Safwat, R.; Halwani, M.; Abdul-Aal, N.A.H.; Thomas, A.; Abdulatif, S.M.; Ali-Karrar, M.A.; Al-Gosn, N.; Al-Hindi, A.A.; Jaha, R.N.; AlQahtani, S.N.; Ayugat, E.P.; Al-Hussain, M.I.; Aldossary, A.; Al-Suliman, S.; Al-Talib, A.A.; Albaghly, N.; Haqlre-Mia, M.E.; Briones, S.; Krishnan, R.; Tabassum, K.; Alharbi, L.; Madani, A.; Al-Hindi, A.A.; Al-Gethamy, M.A.; Alamri, D.M.
Kosovo: Spahija, G.; Gashi, A.
Kuwait: Kurian, A.; George, S.M.; Mohamed, A.M.; Ramapurath, R.J.; Varghese, S.T.; Abdo, N.M.
Foda-Salama, M.; Al-Mousa, H.H.; Omar, A.A.; Salama, M.F.; Toleb, M.; Khamis, S.
Lebanon: Kanj, S.S.; Zahreddine, N.K.; Kanafani, Z.; Kardas, T.; Ahmadieh, R.; Hammoud, Z.; Zeid, I.; Al-Souheil, A.; Ayash, H.; Mahfouz, T.
Macedonia: Mitrev, Z.; Bogoevska-Miteva, Z.; Jankovska, K.; Guroska, S.T.
Malaysia: Ng, C.; Hoon, Y.M.; Hasan, M.S.; Othman-Jailani, M.I.; Hadi-Jamaluddin, M.F.; Othman, A.A.; Zainol, H.; Wan-Yusoff, W.N.; Gan, C.S.; Lum, L.C.S.; Ling, C.S.; Aziz, F.A.; Zhazali, R.; Abud-Wahab, M.R.; Cheng, T.S.; Elghuwael, I.M.; Wan-Mat, W.R.; Abd-Rahman, R.; Mohamad-Zaini, R.H.; Omar, M.
Mexico: Perez-Gomez, H.R.; Kasten-Monges, M.; Esparza-Ahumada, S.; Rodriguez-Noriega, E.; Gonzalez-Diaz, E.; Mayoral-Pardo, D.; Cerero-Gudino, A.; Altuzar-Figueroa, M.A.; Perez-Cruz, J.; Escobar-Vazquez, M.; Aragon, D.M.L.; Coronado-Magana, H.; Mijangos-Mendez, J.C.; Corona-Jimenez, F.; Aguirre-Avalos, G.; Ramirez, M.; Gomez, M.E.; Lozano, M.; Mercado, V.N.; Zamudio-Lugo, I.; Gomez-Gonzalez, C.J.; Miranda-Novales, M.G.; Villegas-Mota, I.; Reyes-Garcia, C.; Ramirez-Morales, M.K.; Sanchez-Rivas, M.; Cureno-Diaz, M.A.; Matias-Tellez, B.; Gonzalez-Martinez, J.; Juarez-Vargas, R.; Pastor-Salinas, O.; Gutierrez-Munoz, V.H.; Conde-Mercado, J.M.; Bruno-Carrasco, G.; Martin Antonio Manrique; Monroy-Colin, V.A.; Cruz-Rivera, Z.; Rodriguez-Pacheco, J.; Cruz, N.L.; Hernandez-Chena, B.E.; Denicia Caleco, J.A.; Leyva-Medellin, E.E.; Salamanca-Meneses, A.; Cosio-Moran, C.; Ruiz-Rendon, R.; Aguilar-Angel, L.A.; Sanchez-Vargas, M.; Mares-Morales, R.C.; Fernandez-Alvarez, L.C.; Castillo-Cruz, B.V.; Gonzalez-Ma, M.R.; Zavala-Ramír, M.C.; Rivera-Reyna, L.; del-Moral-Rossete, L.G.; Lopez-Rubio, C.; Valadez-de-Alba, M.; Miranda-Novales, M.G.; Irma Zamudio-Lugo, I.; Gomez-Gonzalez, C.J.; Hector Torres Hernandez, H.T.; Sobreyra-Oropeza, M.
Mongolia: Bat-Erdene, A.; Chuluunchimeg, K. H.; Baatar, O.; Batkhuu, B.; Ariyasuren, Z.; Bayasgalan, G.; Baigalmaa, S.; Uyanga, T.S.; Suvderdene, P.; Enkhtsetseg, D.; Suvd-Erdene, D.; Chimedtseye, E.; Bilguun, G.; Tuvshinbayar, M.; Dorj, M.; Khajidmaa, T.; Batjargal, G.; Naranpurev, M.; Bat-Erdene, A.; Bolormaa, T.; Battsetseg, T.; Batsuren, Ch.; Batsaikhan, N.; Tsolmon, B.; Saranbaatar, A.; Natsagnyam, P.; Nyamdawa, O.
Morocco: Madani, N.; Abouqal, R.; Zeggwagh, A. A.; Berechid, K.; Dendane, TP.;
Nepal: Koirala, A.; Giri, R.; Sainju, S.; Acharya, S.P.
Pakistan: Paul, N.; Parveen, A.; Raza, A.; Nizamuddin, S.; Sultan, F.; Imran; Sajjad, R.; Khan, M.; Sana, F.; Tayyab, N.; Ahmed, A.; Zaman, G.; Khan, I.; Khurram, F.; Hussain, A.; Zahra, F.T.; Imtiaz, A.; Daud, N.; Sarwar, M.; Roop, Z.; Yusuf, S.; Hanif, F.; Shumaila; Zeb, J.; Ali, S.R.; Demas, S.; Ariff, S.; Riaz, A.; Hussain, A.S.
Palestine: Kanaan, A.; Jeetawi, R.
Panama: Castaño, E. G.; Moreno-Castillo, Lara, L.; García-Mayorca, E., Rojas-Bonilla, M.I. ; Ballinas Aquino, J.M.
Peru: Prudencio-Leon, W. E.; Castillo-Bravo, L. I.; Aibar-Yaranga, K. F.; Marquez-Mondalgo, V. A.; Mueras-Quevedo, J.; Meza-Borja, C.; Flor, J.L.; Fernandez-Camacho, Y.M.; Castaneda-Sabogal, A.; Ramirez, E.; La-Hoz-Vergara, C.E.; Cuellar, L.E.; Velandres, M.C.; Atencio-Espinoza, T.
Philippines: Mendoza, M. T.; Javellana, O. P.; Tajanlangit, A.N.L.; Navoa-Ng, J. A.; Sg-Buenaflor, M. C.; Berba, R.; Labro, E.; Carma, R.; Dy, A.M.P.; Fortin, J.D.; Cesar, J.L.; Bonifacio, B.S.; Llames, M.J.P.; Gata, H.L.B.; Tamayo, A.S.; Calupit, H.K.E.; Catcho, V.V.; Bergosa, L.D.; Abuy, M.T.B.; Dayapera, K.M.
Poland: Barteczko-Grajek, B.; Rojek, S.; Szczesny, A.; Domanska, M.; Gawor, M.; Piwoda, M.; Rydz-Lutrzykowska, J.; Grudzinska, M.; Kolat-Brodecka, P.; Smiechowicz, K.; Tamowicz, B.; Mikstacki, A.
Russia: Kretov, V.; Shalapuda, V.; Molkov, A.; Puzanov, S.; Utkin, I.; Tchekulaev, A.; Tulupova, V.
Serbia: Vasiljevic, S.; Nikolic, L.; Ristic, G.; Eremija, J.; Kojovic, J.; Lekic, D.; Simic, A.
Slovakia: Hlinkova, S.; Lesnakova, A.
Thailand: Khuenkaew, Y.; Iamngamsupha, J.; Siriyakorn, N.; Prasanthai, V.
Tunisia: Borgi, A.; Bouziri, A.
Turkey: Tuncer, G.E.; Bulut, C.; Hatipoglu, C.A.; Sebnem, F.E.; Kaya, A.; Ersoz, G.; Kuyucu, N.; Karacorlu, S.; Gorenek, L.; Erdem, H.; Yildizdas, D.; Horoz, O.O.; Guclu, E.; Kaya, G.; Karabay, O.; Altindis, M.; Oztoprak, N.; Sahip, Y.; Uzun, C.; Erben, N.; Usluer, G.; Ozgunes, I.; Ozcelik, M.; Ceyda, B.M.; Oral, M.; Unal, N.; Cigdem, Y.G.; Bayar, M.K.; Bermede, O.; Saygili, S.; Yesiler, I.; Memikoglu, O.; Oncul, A.; Ozdemir, D.; Geyik, M.F.; Erdogan, S.Y.; Dilek, A.; Esen, S.; Turgut, H.; Sungurtekin, H.; Ugurcan, D.; Yarar, V.; Bilir, Y.; Bayram, N.; Devrim, I.; Agin, H.; Ceylan, G.; Yasar, N.; Oruc, Y.; Ramazanoglu, A.; Turhan, O.; Cengiz, M.; Yalcin, A.N.; Dursun, O.; Gunasan, P.; Kaya, S.; Senol, G.; Gululu, A.; Arman, D.; Gelebek, Y.; Zengin, H.
United Arab Emirates: Al-Rahma, H.; Annamma, P.; El-Houfi, A.
Venezuela: Vidal, H.; Perez, F.; D-Empaire, G.; Ruiz, Y.; Hernandez, D.; Aponte, D.; Salinas, E.; Vidal, H.R.; Navarrete, N.; Vargas, R.; Sanchez, E.; Guzman-Siritt, M.E.; Orozco, N.; Montes-Bravo, L.; Duran-Gil-De-Anez, Z.
Vietnam: Ngo Quy, C.; Thu, T.A.; Nguyet, L.T.T.; Hang, T.T.T.; Hanh, T.T.M.;







