Introduction
Fruit flies (Diptera: Tephritidae) are globally distributed, picture-winged flies of variable size. With >4000 species described, the family ranks among the most diverse groups of true flies (White & Elson-Harris, Reference White and Elson-Harris1992; Thompson, Reference Thompson1999). Most are phytophagous, with larvae developing in the seed-bearing organs of plants. Although commonly named ‘fruit flies,’ larval development can take place in other parts of host plants besides fruits, including flowers and stems. About 35% of fruit fly species attack soft fruits, including many commercially important ones (White & Elson-Harris, Reference White and Elson-Harris1992).
Several tephritids are critically important as fruit crop pests (Thompson, Reference Thompson1999). Economic impacts can be enormous, and control or eradication requires substantial budgets. For example, Dowell & Wange (Reference Dowell, Wange, Mangel, Carey and Plant1986) stated that establishment of major fruit fly threats to the Californian fruit industry would cause crop losses of US $910M yearly, and an eradication program would cost US $290M. Annual losses in the eastern Mediterranean (Israel, Palestinian Territories, Jordan) linked to fruit fly infestations are estimated at US $192M (Enkerlin & Mumford, Reference Enkerlin and Mumford1997). Indirect losses resulting from quarantine restrictions imposed by importing countries to prevent entry and establishment of unwanted fruit fly species can also be enormous. Most economically important fruit fly pests belong to four genera: Anastrepha Schiner (New World Tropics), Bactrocera Macquart, Ceratitis MacLeay and Dacus Fabricius (Old World Tropics).
In recent decades, several Bactrocera species have been introduced accidentally in other parts of the world with established fruit industries in spite of quarantine procedures, often with major economic consequences. For example, the papaya fruit fly (B. papayae Drew & Hancock), introduced in Australia in 1995, led to a major blockade of papaya exports from northern Queensland and major losses to local growers in 1995–1998. Only through an eradication program, costing US $32.5M, could the pest be eradicated and commercial trade restored (Cantrell et al., Reference Cantrell, Chadwick and Cahill2002). The carambola fruit fly (B. carambolae Drew & Hancock), introduced into Suriname, has lead to drastic export reductions in the region, threatening the US $1M annual export from Guyana to neighboring Caribbean countries (USDA/APHIS, 2000).
Bactrocera invadens, a species native to Asia, was recorded for the first time on the African mainland in 2003 (Lux et al., Reference Lux, Copeland, White, Manrakhan and Billah2003) and has already become a pest species of major concern to fruit growers. Here, we develop correlative ecological niche models (ENMs) for this species, which can be projected geographically to estimate the global distributional potential of the species (Peterson, Reference Peterson2003). ENMs are based on digital geospatial data layers and how they correlate with known occurrences of the species in its region of origin. We develop ENM predictions of invasive potential and test them quantitatively in Africa to measure the predictive power of the methodology for anticipating the species' global potential distribution.
Invasion history and economic impact of Batrocera invadens
In 2003, an unknown Bactrocera species was found in Kenya (Lux et al., Reference Lux, Copeland, White, Manrakhan and Billah2003). Taxonomic expertise showed that it was a member of the B. dorsalis complex, an Asian complex including several pest species (Drew & Hancock, Reference Drew and Hancock1994). Identical specimens from earlier surveys in Sri Lanka were initially classified as aberrant forms of B. dorsalis (Hendel) but eventually were re-identified as B. invadens (Drew et al., Reference Drew, Tsuruta and White2005).
Immediately subsequent to its discovery in Kenya, the species was recorded in several countries on the African mainland (Mwatawala et al., Reference Mwatawala, White, Maerere, Senkondo and De Meyer2004, Drew et al., Reference Drew, Tsuruta and White2005). It is now known to occur in tropical Africa from Senegal to Mozambique, as well as in the Comoro Islands in the Indian Ocean (De Meyer et al., Reference De Meyer, Mohamed and White2007). The native range, known so far, ranges from Sri Lanka to southern India (Drew et al., Reference Drew, Tsuruta and White2005; Sithanantham et al., Reference Sithanantham, Selvaraj and Boopathi2006) with some isolated records from Bhutan (Drew et al., Reference Drew, Romig and Dorji2007). It is not clear whether Bhutan should be considered as part of the native range. The B. dorsalis species complex comprises several morphologically very similar taxa (Drew et al., Reference Drew, Raghu and Halcoop2008). Other representatives of this complex occur in the same region (e.g. B. dorsalis and B. kandiensis: Drew & Hancock Reference Drew and Hancock1994). The native range of B. invadens is likely larger than currently assumed, since specimens may be misidentified as other representatives of the complex (see, for example, records for B. dorsalis distribution by Stephens et al., Reference Stephens, Kriticos and Leriche2007). Therefore, the Bhutan records are considered here as part of the native range.
This invasive species has major economic impacts, ranking among the most devastating pests of local horticultural products, particularly mango (Pouilles-Duplaix, Reference Pouilles-Duplaix2007). Research in West (Vayssières et al., Reference Vayssières, Goergen, Lokossou, Dossa and Akponon2005) and East Africa (Ekesi et al., Reference Ekesi, Nderitu and Rwomushana2006; Mwatawala et al., Reference Mwatawala, De Meyer, Makundi and Maerere2006a,Reference Mwatawala, De Meyer, Makundi and Maerereb; Rwomushana et al., Reference Rwomushana, Ekesi, Gordon and Ogol2008) has demonstrated that it can become dominant in mango monocultures. In Benin, >60% losses due to fruit flies were recorded on main mango cultivars of economic interest in the second half of the mango season (Vayssières, Reference Vayssières2007a), and phytosanitary pressure lead to uprooting mango plantations in one area (Borgou) in this country (Vayssières, Reference Vayssières2007b). Native pest species, such as the mango fruit fly (Ceratitis cosyra (Walker)), appear to be outcompeted by this invasive species, although pre-invasion data are largely lacking. In addition, B. invadens is polyphagous in nature and has been reported from 44 different hosts belonging to 23 plant families (De Meyer et al., Reference De Meyer, Mohamed and White2007).
The timing and exact pathway of invasion by B. invadens into Africa are not known. An intensive 1999–2004 sampling program (Copeland et al., Reference Copeland, Wharton, Luke, De Meyer, Lux, Zenz, Machera and Okumu2006) examined ~4000 fruit samples (~980,000 pieces of fruit) from 882 plant taxa and 116 plant families from coastal and western Kenya, and from the Central Highlands. However, not until March 2003 was B. invadens collected in the coastal region (Lux et al., Reference Lux, Copeland, White, Manrakhan and Billah2003). Fruit flies were sampled intensively in commercial mango orchards across coastal Guinea in West Africa in 1992–1996 (Vayssières & Kalabane, Reference Vayssières and Kalabane2000) and Mali in 2000 (Vayssières et al., Reference Vayssières, Sanogo and Noussourou2004) but did not detect B. invadens; the first B. invadens specimens in that part of the African mainland were not detected until June 2004 (Drew et al., Reference Drew, Tsuruta and White2005). This species' presence in these countries before 2000 is, therefore, unlikely. Unfortunately, no similar studies were conducted at that time elsewhere in Africa where the fly currently occurs. That the first specimens were from the East African coast may indicate that the species' port of entry was the East African coast, although clear proof is lacking. A brief outbreak of a methyl eugenol-responding species in Mauritius in 1996, attributed to B. dorsalis (White et al., Reference White, De Meyer, Stonehouse, Price and Seewooruthun2001), may actually have been B. invadens. The available non-teneral sample was recently re-examined, but results were inconclusive (White, Reference White2006). In Asia, the earliest specimens date to 1993 in Sri Lanka (Drew et al., Reference Drew, Tsuruta and White2005), 2000 for Bhutan (Drew et al., Reference Drew, Romig and Dorji2007) and 2005 for India (Sithanantham et al., Reference Sithanantham, Selvaraj and Boopathi2006). However, given likely confusion with B. dorsalis, careful revision of all Bactrocera material from that region is needed.
Material and methods
Occurrence data
Native-range distributional data for B. invadens were derived from surveys in Sri Lanka during 1993–1996 (Tsuruta, unpublised data) and from the literature (Sithanantham et al., Reference Sithanantham, Selvaraj and Boopathi2006). Records from Bhutan were drawn from Drew et al. (Reference Drew, Romig and Dorji2007). Sources for non-native (i.e. non-Asian) distributional data are summarized in table 1, resulting from independent surveys conducted by the authors in different parts of Africa, supplemented by published records (Drew et al., Reference Drew, Tsuruta and White2005; White, Reference White2006). All records are based upon specimens clearly identified as B. invadens and differentiated from other taxa within the B. dorsalis complex. All, bar the records from southern India, were based on specimens for which identification was confirmed by taxonomic experts. After removal of duplicate records, 34 native and 192 non-native records could be referenced to reasonably precise (i.e. to within 10 km) sites. This list is exhaustive, in the sense that it comprises all distributional data currently published, as well as extensive unpublished data made available for this study. The non-native data enable quantitative tests of the predictive ability of the ecological niche models regarding the geographic potential of the species.
Table 1. Distribution records for Bactrocera invadens with georeferences in decimal degrees. A, non-native records; O, native records.

For georeferencing, when possible, we used coordinates from specimen labels. When such information was lacking, however, we extracted coordinates from electronic gazetteers, like GeoNet (http://earth-info.nga.mil/gns/html/index.html), or from specialized locality databases available in some institutions for their collections. Records were plotted on maps and inspected visually to detect obvious errors; peripheral records were investigated individually.
Only occurrence data originating from the species' native distribution were used to generate ENMs. Since no evidence indicates recent range expansion by B. invadens in Asia, and given that model predictions with and without the Bhutanese records differed only slightly, we present here only results from models based on distributional data, including the Bhutanese records (see above).
Environmental data
Raster geospatial data sets used to characterize environments across the native distributional area and worldwide consisted of ‘bioclimatic’ variables interpolated at 1 km spatial resolution (Hijmans et al., Reference Hijmans, Cameron, Parra, Jones and Jarvis2005). Particular variables used included annual mean temperature, mean diurnal range, maximum temperature of warmest month, minimum temperature of coldest month, annual precipitation and precipitation of the wettest and driest months. These particular climate dimensions were chosen to represent environmental dimensions relevant to distributions and survival of small arthropods, in particular fruit flies (Fletcher, Reference Fletcher, Robinson and Hooper1989; Vargas et al., Reference Vargas, Chang, Komura and Kawamoto1987; Vera et al., Reference Vera, Rodriguez, Segura, Cladera and Sutherst2002). No vegetation or land cover data layers were used owing to the heterogenous nature of habitats, including man-made horticultural environments that can potentially be occupied by these species. Although host range can provide useful information with regard to species recognition in Bactrocera (Drew, Reference Drew2004; Drew et al., Reference Drew, Raghu and Halcoop2008), this information remains incomplete for B. invadens, particularly as regards the native range. In addition, as the majority of point localities used in this study are derived from para-pheromone trapping surveys, they do not comprise host data.
Ecological niche modeling (ENM)
Our approach is based on the idea of modeling species' ecological niches, which are considered to constitute long-term stable constraints on species' potential geographic distributions (Peterson et al., Reference Peterson, Soberón and Sánchez-Cordero1999; Peterson, Reference Peterson2003; Raxworthy et al., Reference Raxworthy, Martínez-Meyer, Horning, Nussbaum, Schneider, Ortega-Huerta and Peterson2003; Martínez-Meyer et al., Reference Martínez-Meyer, Peterson and Hargrove2004; Wiens & Graham, Reference Wiens and Graham2005). Niche shifts have recently been reported for some species (Broennimann et al., Reference Broennimann, Treier, Müller-Schärer, Thuiller, Peterson and Guisan2007; Fitzpatrick et al., Reference Fitzpatrick, Weltzin, Sanders and Dunn2007; Steiner et al., Reference Steiner, Schlick-Steiner, Van der Wal, Reuther, Christian, Stauffer, Suarez, Williams and Crozier2008), but niche shifts over short evolutionary time frames remain controversial (Peterson & Nakazawa, Reference Peterson and Nakazawa2008). Ecological niches are herein defined as the set of conditions under which a species is able to maintain populations without immigration (Grinnell, Reference Grinnell1917, Reference Grinnell1924). This condition is assumed here although the species is an extraordinary poorly known one, in particular in its native range in South Asia. As such, distinguishing source and sink populations is not conducted since it would require a level of data richness not presently possible. Several avenues of research have demonstrated accurate predictions of invasive species' potential distributions (Peterson & Vieglais, Reference Peterson and Vieglais2001; Welk et al., Reference Welk, Schubert and Hoffmann2002; Peterson, Reference Peterson2003; Morrison et al., Reference Morrison, Porter, Daniels and Korzukhin2004; Thuiller et al., Reference Thuiller, Richardson, Pysek, Midgley, Hughes and Rouget2005; De Meyer et al., Reference De Meyer, Robertson, Peterson and Mansell2008). Our approach consisted of four steps: (i) model ecological niche requirements based on known native-range occurrences of the species; (ii) test the accuracy of the native range predictions by splitting the dataset into a training and testing set; (iii) test the accuracy of non-native range predictions (trained using all native records) using all available African distributional records; and (iv) project the niche model globally to identify areas putatively susceptible to invasion. The global projection was based on a niche model trained using all the native range records. Other studies have used the software package CLIMEX to describe potential distributions of invasive fruit fly species (e.g. Yonow & Sutherst, Reference Yonow and Sutherst1998; Sutherst et al., Reference Sutherst, Collyer and Yonow2000; Vera et al., Reference Vera, Rodriguez, Segura, Cladera and Sutherst2002; Stephens et al., Reference Stephens, Kriticos and Leriche2007). CLIMEX differs from correlative ENM techniques in that it simulates mechanisms considered to limit geographical distributions of species in relation to climate (Sutherst, Reference Sutherst2003; Stephens et al., Reference Stephens, Kriticos and Leriche2007).
We used two correlative ENM techniques to estimate the potential distribution of this species, a genetic algorithm (GARP: Stockwell & Peters, Reference Stockwell and Peters1999) and a maximum entropy method (Maxent: Phillips et al., Reference Phillips, Anderson and Schapire2006), both on default settings. These two techniques provided contrasting results in recent comparisons of niche modeling techniques (Elith et al., Reference Elith, Graham, Anderson, Dudík, Ferrier, Guisan, Hijmans, Huettmann, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberón, Williams, Wisz and Zimmermann2006; Peterson et al., Reference Peterson, Papeş and Eaton2007, Reference Peterson, Papeş and Soberón2008). GARP is an evolutionary-computing approach to discovery of nonrandom associations between occurrences and raster GIS data layers that describe potentially relevant aspects of ecological landscapes. As GARP has been used widely (Peterson, Reference Peterson2001, Reference Peterson2005; Anderson et al., Reference Anderson, Gomez-Laverde and Peterson2002, Reference Anderson, Lew and Peterson2003; Stockwell & Peterson, Reference Stockwell and Peterson2002), we do not present detailed descriptions of the methodology herein. In general, all analyses were run on default settings, and the best-subsets procedure (Anderson et al., Reference Anderson, Lew and Peterson2003; Rice et al., Reference Rice, Martınez-Meyer and Peterson2003) was used to choose a subset of models for further consideration, which were then summed to produce a single grid summarizing model agreement in predicting presence vs. absence. This grid was converted to a binary prediction of presence vs. absence by choosing the lowest threshold at which the species was known to occur (Pearson et al., Reference Pearson, Raxworthy, Nakamura and Peterson2007). The result was a set of binary grids summarizing the geographic extents of the environmental niche calculated by GARP for the species.
Maxent estimates the ecological niche of a species by determining the distribution of maximum entropy, subject to the constraint that the expected value of each environmental variable (or functions of these) under this estimated distribution matches its empirical average (Phillips et al., Reference Phillips, Anderson and Schapire2006). Maxent makes use of presence records and a set of background values (pseudoabsences) drawn from the entire study region. We used default parameters in Maxent (version 1.3.0) to produce models: feature selection automatic, regularization multiplier at unity, maximum iterations 500, convergence threshold 10−5 and random test percentage at zero. The result is a set of probabilities that sum to unity across the entire study area; to make values more manageable, these suitability indices are usually presented as logistic transformations of cumulative probabilities (Phillips et al., Reference Phillips, Anderson and Schapire2006), with values ranging 0–100 (low to high suitability).
Spatial predictions of presence and absence can include two types of error, omission (predicted absence in areas of actual presence) and commission (predicted presence in areas of actual absence: Fielding & Bell, Reference Fielding and Bell1997). Because GARP is a random-walk procedure, it does not produce unique solutions; consequently, we followed best-practices approaches to identifying optimal subsets of resulting replicate models (Anderson et al., Reference Anderson, Lew and Peterson2003). In particular, we developed 100 replicate models; of these models, we retained the 20 with lowest extrinsic omission error rates and then retained the ten models with intermediate extrinsic commission error (i.e. we discarded the ten models with area predicted present showing greatest deviations from the overall median area predicted present across all low-omission models). This ‘best subset’ of models was summed pixel by pixel to produce final predictions of potential distributions in the form of grids with values ranging from 0 (all models agree in predicting absence) to 10 (all models agree in predicting presence). Since the two modeling techniques produce different sorts of output with very different frequency distributions, correct choice of thresholds becomes critical in interpreting the resulting maps (Peterson et al., Reference Peterson, Papeş and Eaton2007). As such, we used the lowest training presence threshold approach (LTPT) of Pearson et al. (Reference Pearson, Raxworthy, Nakamura and Peterson2007); specifically, we inspected the native-range occurrence information relative to the raw outputs from GARP and Maxent. We determined the lowest predictive level at which any training presence point was predicted and used that level as a minimum criterion for prediction of presence (vs. absence) in non-native regions.
Model testing
To evaluate the model predictions, we offer two sets of tests. First, we developed initial models across the native range region based on a subset of available data, in which ten randomly chosen points were set aside (for testing) prior to model development; this procedure was repeated twice, with different random subsamples. Statistical significance of these predictions was assessed using the cumulative binomial probability approach described below. Second, we assessed the predictive ability in Africa (using African records) for a model that was calibrated using all records from the native region. Given the rather crude resolution of this initial exploration, we assumed that different invaded-range occurrences were independent, neglecting possible effects of spatial autocorrelation. Because our goal was predicting global invasive potential, we tested model predictivity with the null hypothesis that the observed coincidence between prediction and test points was no better than chance expectations.
The most common mode of evaluating niche models in recent literature is via the area under the curve in a receiver operating characteristic (ROC) analysis (e.g. Elith et al., Reference Elith, Graham, Anderson, Dudík, Ferrier, Guisan, Hijmans, Huettmann, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberón, Williams, Wisz and Zimmermann2006). ROC analysis, however, is not appropriate to the present situation for two reasons: (i) ROCs require absence data, which are not available in the present case; and (ii) ROCs weight type 1 and type 2 errors equally, but the focus on invasive potential would weight omission error more heavily than commission error (Soberón & Peterson, Reference Peterson2005; Peterson et al., Reference Peterson, Papeş and Soberón2008). However, we use an adaptation of the ROC curve approach as a means of assessing predictive ability visually, plotting omission on an inverse scale (=‘sensitivity’) against proportion of area predicted present (an estimator of 1–specificity: Phillips et al., Reference Phillips, Anderson and Schapire2006, Peterson et al., Reference Peterson, Papeş and Soberón2008).
Models were tested using binomial tests that incorporate dimensions of correct prediction of both presences (based on success in predicting independent test data) and absences (based on proportion of the area predicted present, which is taken as the probability of a success). Given that B. invadens as yet has only invaded Africa broadly, the universe of testing was taken as Africa (including Madagascar and the Comoro Islands) south of 18°N. Models were tested at the LTPT threshold described above.
Results
Figure 1 shows the known distributional information for B. invadens from its native range (Asia) and non-native distributional areas (Africa and the Indian Ocean). The projections of the two ENMs for the native range (fig. 2) were similar; both indicate Sri Lanka and southern India as highly suitable. GARP predicted higher suitability in coastal regions (particularly the east coast) and the Ganges Delta in Bangladesh, while Maxent indicated suitability more restricted to isolated pockets in these parts when high threshold values are taken into account only. When lower thresholds were included in Maxent, the predicted areas were more similar between the two methods (fig. 2); we note that the LTPT for Maxent was 0.027 out of 100, whereas for GARP it was 8 out of 10. Testing model predictions by the two algorithms based on two separate random subsets, predictions from both models were significantly (P<0.05) better than random expectations. For example, in one of the random subsamplings, the GARP model predicted 11.5% of the area present, but managed to predict 9 of 10 independent test points correctly; similarly, the Maxent model predicted 14.7% of the area present, but predicted all ten test points correctly, the associated binomial probabilities were both lower than 10−9. The training and testing sets may not be completely independent as the native range occurrence records are clustered in a small region; however, model predictions were also tested with records from the invaded range in Africa (see below).

Fig. 1. Distribution records for B. invadens. Native records in India (Ind), Sri-Lanka (Sri) and Bhutan (Bhu). Non-native records in Africa.

Fig. 2. Predicted distribution of Bactrocera invadens in its native range in Asia, using genetic algorithm for rule-set prediction (GARP) and maximum entropy method (Maxent). White, predicted absence, as indicated by the LTPT thresholding; shades of grey indicate higher levels of prediction (chosen arbitrarily), with black the highest strength for predicted presence.
Projecting niche models to Africa and Madagascar (fig. 3) again yielded similar predictions between the two methods, with Maxent again appearing more conservative. Both models predicted high suitability in the Equatorial rain forest belt and the East African coastal regions. The GARP model predicted higher suitability in areas farther removed from the coast, particularly in Ivory Coast in the west, and Tanzania and Mozambique in the east. Also, the latitudinal limits identified by GARP predictions were broader, especially southwards, with high suitability being predicted for much of the Angolan and Mozambican coastlines; these differences were less dramatic once lower thresholds were considered in Maxent. The same tendencies are observed in global projections (fig. 4); GARP predicted somewhat broader potential distributional areas in tropical South America and Southeast Asia (particularly Thailand, Cambodia and Vietnam). The only areas where Maxent indicated broader potential distributional areas than GARP are in parts of Borneo, Papua New Guinea and the western Amazon.

Fig. 3. Predicted distribution of Bactrocera invadens in Africa and Madagascar, using genetic algorithm for rule-set prediction (GARP) and maximum entropy method (Maxent). White, predicted absence, as indicated by the LTPT thresholding; shades of grey indicate higher levels of prediction (chosen arbitrarily), with black the highest strength for predicted presence.

Fig. 4. Predicted distribution of Bactrocera invadens globally, using genetic algorithm for rule-set prediction (GARP) and maximum entropy method (Maxent). White, predicted absence, as indicated by the LTPT thresholding; shades of grey indicate higher levels of prediction (chosen arbitrarily), with black the highest strength for predicted presence.
We used the non-native populations of B. invadens in Africa as a means of testing model predictivity regarding suitable areas for the species globally. Omission error was minimal, 3 of 192 invaded-range test points were excluded from model predictions in each case. In both cases, model predictions were considerably better than expectations under random (null) models (binomial tests, both P<10−14), indicating that both approaches offer significant predictivity regarding the global potential distribution of the species. Inspecting ROC plots for the two model predictions based on independent testing data on a landscape distant from that where the models were trained, it is clear that the two models are similar in performance. Maxent appears to perform better at middle-level omission values, while GARP appears to perform better at lower omission values (fig. 5).
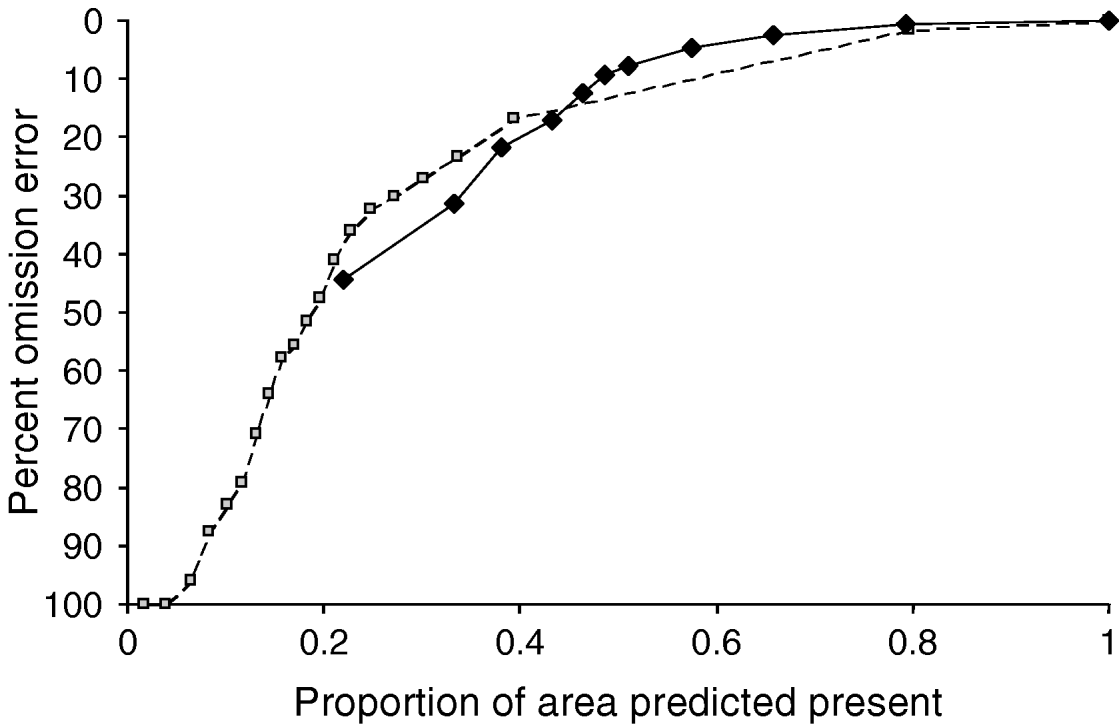
Fig. 5. Comparison of accumulation of predictive ability vs. proportion of area (Africa) predicted present in genetic algorithm for rule-set prediction (GARP) and maximum entropy method (Maxent) models (![]() , Maxent; –◆–, GARP).
, Maxent; –◆–, GARP).
Discussion
Models in ecological dimensions
The two niche modeling algorithms employed in this study present a similar overall picture, although Maxent is somewhat more conservative. Comparing with the updated Köppen-Geiger climate classification (Kottek et al., Reference Kottek, Grieser, Beck, Rudolf and Rubel2006), most suitable areas identified by our models fall within the Equatorial climate categories (minimum temperatures ≥18°C), especially Af (Equatorial rainforest, fully humid) and Am (Equatorial monsoon). The GARP model also assigns high suitability to a large part of the Aw (Equatorial savannah with dry winter) climate class.
This result suggests that B. invadens prefers hot and humid environments. Annual precipitation must be high, although it does not have to be continuous. Equatorial monsoon type climate (Am) is defined as a climate with a short dry season, but with still sufficient moisture to keep the soil humid throughout the year. Equatorial savannah climate type has a distinct dry period with driest-month precipitation of <60 mm. Continuous presence of B. invadens in Af amd Am climates is not as-yet supported by field data, for lack of field studies, but presence in Aw climates is now amply demonstrated. Mwatawala et al. (Reference Mwatawala, De Meyer, Makundi and Maerere2006b) trapped B. invadens in orchards in the Morogoro region of central Tanzania continuously for 61 weeks in 2004–2005. Morogoro is situated in the transition zone between bimodal and unimodal rainfall belts in Tanzania with a distinct dry season; B. invadens is present year-round, although populations increase dramatically during the rainy season. Similar observations were made in Benin, in areas also demonstrating fly activity during a clear dry season (Vayssières, Reference Vayssières2004; Vayssières et al., Reference Vayssières, Goergen, Lokossou, Dossa and Akponon2005).
Stephens et al. (Reference Stephens, Kriticos and Leriche2007) developed a model for the closely related B. dorsalis using a different approach (CLIMEX). The optimal climate suitability for Africa, identified in that study, corresponds reasonably well with optimal conditions for B. invadens, although some marked differences are evident. The CLIMEX model for B. dorsalis predicts optimal suitability further south along the South African coast (representing a warm temperate climate type, fully humid, with hot summers), while parts of the interior of Tanzania and northern Mozambique and parts of Nigeria were rated as less suitable. Non-native populations of B. dorsalis in Hawaii, have been rated to prefer humid areas (Vargas et al., Reference Vargas, Stark and Nishida1989, Reference Vargas, Stark and Nishida1990); hence, the climatic optimal conditions for the two species likely overlap broadly. Studies on niche partitioning in areas where both taxa occur, however, are lacking.
Model predictivity
Despite the fact that the great majority of known occurrences fall within predicted areas, some isolated occurrences of B. invadens in other ecological situations are known. Observations show that the species can occur in lowland moist and dry savannah in western Africa, the Sudan and Zambia, which present climates with longer dry periods and hot conditions during part of the year. Some of these occurrences may correspond to anthropogenic microclimates (see, e.g. Coetzee, Reference Coetzee2004). For example, the B. invadens collecting sites in the Sudan (fig. 1) are irrigation schemes along the Blue Nile River; although situated in low-rainfall savannah habitat, these irrigated areas are typically very humid and partly under cultivation, with suitable host plants such as mango, citrus, guava and banana. However, such is not the case for the other sites in Zambia and West Africa.
These discrepancies can be caused by two factors, incomplete sampling in the native region or actual niche differentiation in the non-native populations. It is plausible that the currently available native-range occurrence data are incomplete (cf. above). Bactrocera invadens might then have a much broader ecological niche in its native range. We should also take into consideration that these particular habitat types (lowland wet and dry savannah) are not present in the native distributional area, so the modeling algorithms have been presented with incomplete data on the species' distributional potential in such habitats; regions with similar climate conditions are found in central and northern India, but B. invadens records are not available from these regions. A more thorough inventory for the species in its native region, or at least detailed inspection and re-evaluation of Bactrocera records from the region, might present additional information that could improve the models. Currently, however, such information is not available.
In case of niche differentiation in invaded regions, two elements are known to cause exotic species to expand beyond their predicted climate envelope. It may result from adaptive changes in the fundamental niche of the species or changes in the realized niche (i.e. fundamental niche constrained by biotic interactions) (Broennimann et al., Reference Broennimann, Treier, Müller-Schärer, Thuiller, Peterson and Guisan2007). Given the short time span between detection of the invasion and the observation of presence beyond the predicted range, the likelihood that evolutionary change has occurred that might have affected the fundamental niche of the species seems unlikely. More likely, release from biotic constraints like enemy release (Colautti et al., Reference Colautti, Ricciardi, Grigorovich and MacIsaac2004) has an effect on the realized niche of B. invadens. As such, caution should be taken with regard to the boundaries of the models presented here, since these isolated records indicate some potential for the taxon to occur outside them. The fly's abundance in these areas is unclear for lack of continuous trapping data.
Potential threat of B. invadens outside its native range
Given the apparent rapid spread of B. invadens across Africa, and its impact on local horticulture, the risk of this species being introduced, establishing and invading other regions of the world should be considered. Our models indicate regions of the world that are climatically suitable for the species, but they do not indicate regions that will necessarily become invaded by the species. For a species to invade in a new region, it must overcome a series of challenges (Richardson & van Wilgen, Reference Richardson and van Wilgen2004; De Meyer et al., Reference De Meyer, Robertson, Peterson and Mansell2008). Richardson & van Wilgen (Reference Richardson and van Wilgen2004) listed six barriers that a species has to overcome to become invasive in a new region. Our analyses are only able to assess one of them, the likelihood of the species surviving in the new region. Regions highly suitable for the species, as indicated by the models, are more likely to be invaded than regions that have a low suitability. In Africa, for example, most of West Africa, Central Africa and Madagascar, and parts of East Africa, are indicated as highly suitable by the models. Large regions of the Neotropics are also indicated as being suitable, as is most of Southeast Asia. A comprehensive assessment of invasion risk for this species for various parts of the world will require that other barriers be assessed (Thuiller et al., Reference Thuiller, Richardson, Pysek, Midgley, Hughes and Rouget2005), which will require better knowledge of the species' basic biology and natural history.
As we have not explored all of the invasion challenges that non-native species face, our maps should not be interpreted as maps of invasion risk or likelihood of establishment. However, a region presenting suitable climatic conditions for the species is likely more vulnerable than one presenting unsuitable conditions. Regions highlighted as highly suitable by the models include areas already invaded by the species, giving some confidence in the models. Although the species has invaded several parts of Africa, we cannot be certain about risk of individuals being introduced to other regions (e.g. Neotropics or Southeast Asia), and whether propagule pressure will be sufficient to enable the species to establish there. Insights into propagule pressure can be obtained by examining the volume of trade between regions where the fly currently occurs and those regions that have suitable climate conditions (Thuiller et al., Reference Thuiller, Richardson, Pysek, Midgley, Hughes and Rouget2005).
Another important consideration is whether individuals introduced to these areas can survive the local conditions long enough to breed successfully. An important element in this respect will be interspecific competition with native fruit flies. Most regions identified as being at risk already have established fruit fly faunas, comprising native species and sometimes previously introduced exotics; polyphagous species, infesting diverse fruits that also act as hosts for B. invadens, are already present. Duyck et al. (Reference Duyck, David and Quilici2004) stated that where polyphagous tephritid species have been introduced in areas already occupied by a polyphagous tephritid, interspecific competition has generally resulted in a decrease in numbers and niche shifts of the previously established species, without leading to complete exclusion. Duyck et al. (Reference Duyck, David and Quilici2004, Reference Duyck, David and Quilici2007) assumed that life-history strategy could be a determining factor in this competition.
In Africa, most native polyphagous pests, such as Ceratitis capitata, express r-selected traits. Invasive Bactrocera species, on the other hand, display more K-selected traits. From the case studies presented by Duyck et al. (Reference Duyck, David and Quilici2004, Reference Duyck, David and Quilici2007), K-selected species appear to be better invaders. In the case of B. invadens on the African mainland, some details seem to confirm this hypothesis. Data from Nguruman Rift Valley Province in Kenya show that the principal pest detected in monitoring traps in mango orchards was C. cosyra prior to 2003, but has gradually been replaced by B. invadens since then (S. Ekesi, unpublished data). Although pre-invasion data are lacking, Mwatawala et al. (Reference Mwatawala, De Meyer, Makundi and Maerere2006a,Reference Mwatawala, De Meyer, Makundi and Maerereb) showed that, in Tanzania, B. invadens is the major pest species in hosts such as mangoes, which were initially predominantly infested by native Ceratitis species such as C. cosyra. The latter seems to be displaced in large part by the former. However, abiotic factors may also determine different use of host resources. Vayssières et al. (Reference Vayssières, Goergen, Lokossou, Dossa and Akponon2005), for example, showed that C. cosyra is still dominant during the dry season, but B. invadens dominates during the rainy season, probably reflecting its preference for humid environments. Whether the presence of C. cosyra in the dry season is the result of a shift due to interspecific pressure from the invasive species is, however, not clear for lack of comparative data predating the invasion. A better understanding of both the various biotic and abiotic factors and of the particular interspecific competition mechanisms is needed for a more complete predictive model for invasive fruit flies such as B. invadens.
Acknowledgements
This work was financed in part by the International Atomic Energy Agency (contract 12710). Thanks to various collaborators throughout Africa who provided distributional data from different regions in the continent.








