INTRODUCTION
Wildlife diseases normally only come to our attention if they have a direct effect on humans or domestic animals (e.g. West Nile flavivirus outbreak in humans in northeastern USA; Rappole, Derrickson and Hubalek, 2000), or if an epidemic occurs that causes dramatically increased mortality rates in a wild species (e.g. the 1988 epidemic of phocine distemper morbillivirus in North Sea harbour seals, Phoca vitulina; de Swart et al. 1995). The effects of most wildlife diseases, including arboviruses, are not so obvious, but nevertheless can affect population size and growth rates through modest increases in mortality and morbidity (Anderson and May, 1991; Grenfell and Gulland, 1995). An understanding of these effects requires quantitative analysis of pathogen transmission (Grenfell et al. 2002). Such studies have rarely been undertaken for tick-borne viruses. A notable exception is Louping Ill virus of red grouse vectored by Ixodes ricinus (Hudson et al. 1995).
Great Island Virus (GIV) is a tick-borne member of the genus Orbivirus, family Reoviridae (Bucher-Osmond, 2003). It has 10 segments of double-stranded RNA (dsRNA) packaged in an icosahedral particle. Different strains of the virus are serologically distinct and have unique electrophoretic profiles of their dsRNA. Great Island Virus (GIV) is transmitted to avian hosts by the 3-host hard tick I. uriae (White, 1852) whose biology and more recently spatial genetic structure had been described (Eveleigh and Threlfall, 1975; Barton et al. 1996; McCoy and Tirard, 2002; McCoy, Tirard and Michalakis, 2003). GIV has been found in seabird colonies of both the northern and southern hemispheres, reflecting the bipolar distribution of I. uriae (Nuttall et al. 1981, 1984a; Doherty et al. 1975). However, the impact of GIV on its avian hosts has not been assessed. Indeed it is not known which seabird species actually supports the transmission of GIV. In this paper we identify guillemots as the principal host of the virus and provide evidence for viraemic transmission and also non-viraemic transmission (Jones et al. 1987; Lord and Tabachnik, 2002) between co-feeding ticks. In an accompanying paper (Nunn et al. 2006), we determine the most important parameters affecting GIV transmission rate between ticks and guillemots.
Our study was undertaken at the mixed seabird colony on the Isle of May, Scotland. At this site common guillemot (Uria aalge, Pontoppidan, 1763) and black-legged kittiwake (Rissa tridactyla, Linnaeus, 1758) are the main hosts for I. uriae, since our earlier work showed these two relatively abundant species support far higher numbers of ticks than the other seabird species present on the island (Barton et al. 1996). The study system permits systematic sampling of ticks (sedentary) and birds (nesting) during the breeding season. The longevity and nest site fidelity of the birds also permits the same marked individuals to be recaptured and sampled year-after-year. To determine whether guillemots and/or kittiwakes are infected with GIV and are able to support transmission to ticks, we examined virus infection prevalence in ticks removed from birds and levels of virus-specific neutralizing antibody present in blood.
MATERIALS AND METHODS
Materials
BHK21 cells and Vero cells were a gift from Steve Moss, CEH Oxford. Tissue culture plates (Corning), Eagles Minimum Essential Media (Sigma) and penicillin streptomycin antibiotics (Gibco-BRL) were purchased. Polyacrylamide for gel electrophoresis and all other chemical reagents were obtained from Sigma.
Four serologically distinct GIV strains named Broadhaven virus (BRDV), Colony B North virus (CBNV), Above Maiden virus (AMDV) and Colony virus (COYV) were used for plaque reduction neutralization tests. Filtered, high titre frozen (−70 °C) stocks were derived from BHK-21 cells infected with 0·01 plaque forming units (pfu) per cell. BRDV was a gift from Steve Moss. It was derived from a fourth round plaque pick of FT363 virus. FT363 was isolated from a pool of 10 I. uriae engorged nymphs collected in 1975 from a seabird colony at St Abbs Head, Scotland (Nuttall et al. 1981) which lies 32 km south of the Isle of May. CBNV, AMDV and COYV were each isolated from single adult I. uriae collected on the Isle of May in 1994. Passage 2 stocks of these three viruses were used.
Study site
The study was carried out between 1993 and 1996 at the seabird colony on the Isle of May (56 °11′N, 2 °33′W), Scotland. Approximately 12000 pairs of guillemots and 6–7000 pairs of kittiwakes breed on the island. The two species nest in both discrete and mixed groups.
Capture and sampling of birds and ticks
Breeding kittiwakes and adult breeding and immature pre-breeding guillemots were captured with either a wire crook on the end of a 3 metre long bamboo pole, a monofilament noose fitted to a 5 metre fishing rod or in box traps. Birds not caught incubating eggs or brooding chicks were classified as breeders or pre-breeders on the basis of the presence or absence of a brood patch. Blood samples were taken (under licence) from the foot web (guillemot) or wing (kittiwake) using a sterile 23G needle and pressing a 4·25 cm2 3 MM Whatman filter paper against the wound. Ethanol (80%) was used to clean the wound site before and after sampling. The filter papers were air-dried then stored at −70 °C in sealed bags containing silica gel. In 1995, heparinized capillary blood samples were also taken. Each bird was searched for ticks by one of us (T.R.B.) using both sight and touch for 3 min The search method detects nymphs and adult females but very few larvae. Ticks were removed using ethanol-sterilized forceps and placed in plastic tubes and stored alive at 4 °C ([les ]2 weeks) before being frozen (−70 °C). Before release, all birds were ringed with a unique British Trust for Ornithology serial metal ring and birds in the study area were ringed with unique colour ring combination rings for identification from afar.
Virus prevalence
Virus was isolated, titrated and plaque-purified from individual adult female ticks using BHK21 and Vero tissue culture cells according to the method of Nuttall et al. (1984b). In brief, virus isolation was from whole ticks that were homogenized in tissue culture media. After centrifugation, supernatants were added to cell monolayers that were observed microscopically for 6 days for signs of virus infection. Material containing infectious virus was stored at −70 °C. Red blood cell fractions (20 μl) from the heparinized capillary blood samples collected in 1995 were also used for virus isolation.
Purification, electrophoresis and silver-staining of viral dsRNA
BHK21 cells in a 25 cm2 flask were infected with 0·1 virus particles/cell and harvested 24 h p.i. by pelleting (10000 g, 1 min) and resuspending in 0·18 ml of TRIzol (Gibco-BRL) solution by pipetting. Then 0·02 ml of chloroform was added and the mixture vortexed, chilled on ice for 5 min and microcentrifuged at 16000 g for 15 min. An equal volume of isopropanol was added to the upper colourless phase, which was vortexed, incubated at 4 °C for 15 min and microcentrifuged at 16000 g for 15 min. The pellet was washed with 0·5 ml of 75% ethanol, then dried, resuspended in 12 μl ddH2O and stored at −20 °C. The RNA concentration was estimated by measuring absorbance at 260 nm (1A260 unit of dsRNA is equivalent to about 60 μg/ml).
Five ng purified viral dsRNA per lane was separated on 1 mm thick, 8 cm long upright 10% polyacrylamide slab gels at 42 mA for 4·5 h using a discontinuous Tris-glycine buffer system. Gels were fixed for 20 min in 30 ml of 10% ethanol, 0·5% glacial acetic acid. The fixative was removed and 30 ml of freshly made 0·1% (w/v) silver nitrate solution added. After 1 h the gel was washed 3 times in large volumes of ddH20 then developed by adding 25 ml of 36% sodium hydroxide 1% (v/v) of 37% formaldehyde. The reaction was stopped by extensive washes with distilled water.
Detection of virus-specific neutralizing antibodies
Discs (1·8 cm2) were punched from the filter paper blood samples, and eluted overnight at 4 °C in 320 μl of phosphate-buffered saline, 0·05% Tween 20, 50 μg/ml penicillin/streptomycin (PBST). The protein concentration of the whole and eluted blood was determined by Bradford assay (Bio-Rad Laboratories GmbH). Eluted blood samples were diluted to 10 mg/ml with PBST; no sample was diluted more than 2-fold to achieve this concentration. Vero cells (2×105) in 0·5 ml of cell medium (RPMI 6% foetal bovine serum) were added to each well of a 24-well plate that was then placed in an incubator for 3 h. Meanwhile, 10−1 to 10−3 dilutions of each virus strain were made in PBST. Ten μl of each eluted blood sample was mixed with 10 μl of each virus dilution and incubated at 30 °C for 2 h before adding the entire mixture to a single Vero cell well. Thus the final dilution of virus on the plate was 10−3 to 10−5. Blood samples were each assayed 4 times (duplicate wells on 2 plates). Cells were placed in the incubator for 1 h then overlaid with 0·5 ml of carboxymethylcellulose, and incubated for 4 days before fixing with formal saline and staining with crystal violet. Results were expressed as average fold decrease in virus titre relative to controls. The controls used were incubation of virus for 2 h in (1) PBST or (2) chicken blood eluted from filter paper. Samples that decreased virus titre more than 20-fold were regarded as seropositive. The cut-off point was 6 standard errors higher than the average value for chicken negative control samples. A 10-fold cut-off point (ca. 90% plaque reduction) for seropositivity is commonly used in plaque reduction neutralization tests. However, its use increases the risk of false positive results, and has no effect on the overall conclusions of this work.
Statistical analysis
All data sets comprised unique individuals. Statistical analyses were performed using generalised linear modelling techniques (GLM; McCullagh and Nelder, 1989). In each analysis, the initial model, including all the explanatory variables (date, site, tick feeding stage and number/host, bird species and breeding status) was fitted and examined. Models were simplified taking account of previous analyses until the minimal adequate model was found. Normal model checking procedures were employed and appropriate transformations applied. Boxcox analysis indicated that the most appropriate transformation to normalize the neutralizing antibody data was y=(decrease in pfu)−y2.
RESULTS
Virus prevalence in ticks and birds
Virus prevalence data were obtained from 37 female ticks feeding on 23 breeding guillemots and 106 female ticks feeding on 37 breeding kittiwakes. There was no difference in the mean infection prevalence of virus in feeding female ticks removed from breeding kittiwakes and guillemots (Table 1; χ12≈0·38, P=0·54). However, GIV was only isolated from kittiwake ticks at sites that also contained breeding guillemots (Table 1). Virus prevalence did not show a significant year effect (χ12≈0·91, P=0·34). Only 9 nymphs and no larvae were found on the kittiwakes that were captured so no attempt was made to analyse their infection prevalence. We were able to compare only on-host ticks from the two bird species because we did not find off-host (or questing ticks) in kittiwake nests. Virus prevalence data for off-host ticks in guillemot colonies are reported in the accompanying paper (Nunn et al. 2005).
Table 1. Mean virus prevalence in female ticks removed from breeding kittiwakes and guillemots inhabiting mixed colonies (present) or discrete colonies >50 m apart (absent) (The number of ticks (Ntick) and birds (Nbird) examined are shown.)

Few infected ticks were found feeding on guillemots so data for pre-breeding and breeding guillemots we pooled in subsequent analyses to increase infected tick sample size (no pre-breeding kittiwakes were caught). Virus prevalence was positively associated with the number of female ticks feeding on guillemots (F1,72=4·0, P=0·049). Furthermore, among guillemots that had 2 or more female ticks, if 1 female was infected with virus, in most cases (6 out of 10 birds) all the other females on that bird were also infected (filled symbols, Fig 1A). Remarkably, all the infected ticks feeding on the same bird were infected with the same virus assessed by examination of viral RNA profiles (Fig. 1B). This did not appear to be due to contamination otherwise we would expect at least 1 tick to have a relatively high virus titre (the source of contamination) and the others from the same bird to have low, or undetectable (i.e. <10 pfu), titres. However, at least 2 female ticks, from 7 of 10 guillemots with multiple ticks, had measurable virus titres (data from 4 of these birds are shown in Table 2).

Fig. 1. (A) Association between number of female ticks on guillemot (filled circles: black breeders, grey pre-breeders) and breeding kittiwake (open triangles) and the number of these ticks infected with GIV. Linear regression lines are shown. R2 values: guillemot 0·598, P=0·052; kittiwake 0·107, P=0·664. (B) Typical double-stranded RNA profiles (segment numbered at right) of GIV strains present in infected ticks obtained from a breeding guillemot (B1), 2 pre-breeding guillemots (PB1-2) and 2 breeding kittiwakes (K1-2).
Table 2. Details of GIV infection for guillemot whose blood was tested for virus and which had 1 or more infected ticks when captured (<10 plaque forming units (pfu) indicates virus was undetectable by plaque assay. Asterisked birds were assayed for virus-specific neutralizing antibodies.)

The situation for kittiwakes (breeding alongside guillemots) contrasted markedly with that of guillemots. In most cases (5 of 7), only 1 of 2 or more female ticks co-feeding on kittiwakes was infected (open triangles, Fig. 1A). Furthermore, in both cases where 2 kittiwake ticks were infected, each tick harboured a different virus strain (Fig. 1B). A simple comparison of virus amplification in female ticks feeding on guillemots and kittiwakes is given by the number of infected ticks on birds divided by the number of birds that had infected ticks. That is: 28/10=2·8 for guillemots and 9/7=1·3 for kittiwakes (data in Fig. 1A).
Of 27 pre-breeding guillemots examined, blood from only 1 bird yielded virus in tissue culture; no ticks were found on this bird. Virus was not isolated from the blood of 27 breeding guillemots, or 16 kittiwakes breeding alongside guillemots. Eight guillemots (2 breeders and 6 pre-breeders) from which capillary blood samples were taken had 1 or more virus-infected female ticks (n=17) feeding on them (Table 2). None of the 8 blood samples yielded virus in tissue culture. Four of these guillemots had 2 or more ticks feeding on them (Table 2). Twelve of the 13 ticks from the 4 birds were infected and, as described above, ticks from the same bird were infected with the same virus. Detection of virus in the feeding ticks but not in the blood taken from the same bird shows that feeding ticks are not equivalent to blood samples. Thus sampling and comparing feeding adult ticks (which may have been infected as larvae, nymphs or adults) is the most sensitive way to look for differences in virus infection prevalence between kittiwakes and guillemots.
Virus-specific neutralizing antibodies
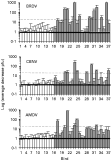
The average protein concentration of whole blood taken from 8 guillemots was 151·4 mg/ml (range 132·1–176·4 mg/ml). The final concentration of the eluted blood sample (10 mg/ml) and virus is therefore equivalent to a serum dilution of about 1[ratio ]30. Data were obtained on neutralizing antibodies present in blood samples from 23 breeding guillemots and 16 breeding kittiwakes. All 16 kittiwake blood samples were from birds breeding adjacent to guillemots of which 29% had 1 or more GIV infected female ticks feeding on them when captured (Table 1). Breeding guillemots had significantly higher levels of GIV neutralizing antibody than breeding kittiwakes against all 3 virus strains that were tested (BRDV F2,37=32·8, P<0·001; CBNV F2,37=20·94, P<0·001; AMDV F2,37=15·63, P<0·001). Kittiwake had insignificant levels of neutralizing antibody comparable to controls (PBST or eluted chicken blood) (Fig. 2). BRDV, CBNV and AMDV were neutralized by 44%, 35% and 22% of the guillemot blood samples respectively (Fig. 3). Twenty-six percent of the guillemot blood samples neutralized 2 or 3 of the strains, 35% 1 strain and 39% none of the 3 strains tested. Only 10 of the blood samples were tested against COYV so data for this virus are not shown in Fig. 3. No detectable neutralizing antibodies against any of the virus strains were present in samples from 3 pre-breeding guillemot that yielded infected ticks but had no virus detectable in their blood (Table 2).

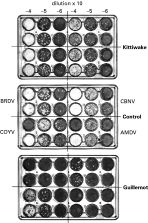
Fig. 2. Typical plaque reduction neutralization result for breeding kittiwake and guillemot. As indicated for the control (adult chicken blood), 1 virus strain was assayed in each quarter of the plate. Two wells on the control plate (COYV-4 and AMDV-4) had no virus added to them. These are results for birds 12 (kittiwake) and 20 (guillemot) in Fig. 3.

Fig. 3. Neutralization of 3 GIV strains by blood from breeding kittiwakes (white) and breeding guillemots (grey). All kittiwake samples were from birds inhabiting colonies immediately adjacent to guillemot colonies. Lower solid line indicates point of no increase or decrease compared to control. Upper dotted line indicates cut-off point for seropositivity (see main text for explanation).
DISCUSSION
Both guillemots and kittiwakes attend breeding colonies as pre-breeders for at least 1 but usually 2–4 years before they recruit to the breeding population (Porter and Coulson, 1987; Harris, Halley and Swann, 1994). During this time they are exposed to parasitism by GIV infected ticks and, if susceptible to virus infection, a proportion of both species will be immune to GIV by the time they become breeders. In June, more female ticks feed on kittiwakes than on guillemots (Barton et al. 1996) and therefore a higher percentage of kittiwakes living alongside guillemots have 1 or more infected ticks feeding on them at this time of year. Despite this, neutralizing antibodies against GIVs were not detected in blood samples taken from breeding kittiwakes parasitized by infected ticks. The infected adult ticks feeding on kittiwakes were presumably infected, by feeding on guillemots, during an earlier life stage. This is supported by the observation that infected ticks on the same kittiwake contained different virus strains. In contrast to kittiwakes, 61% of the breeding guillemots had high titre ([ges ]1[ratio ]30) antibodies that neutralized one or more of the virus strains by [ges ]95%, demonstrating past infection by GIV. All 3 GIV strains infect guillemots. However, differences in the percentage of birds that were immune to each strain suggests there may be temporal changes in strain abundance.
Virus infection prevalence in ticks feeding on birds also supports the hypothesis that guillemots, but not kittiwakes, are infected by GIV. No GIV infected ticks were present in colonies inhabited solely by kittiwake, whereas in isolated guillemot colonies as well as mixed kittiwake/guillemot colonies, a similar percentage of female ticks were infected with GIV. GIVs have also been isolated from ticks feeding exclusively on guillemots on Great Saltee Island, Eire (Nuttall et al. 1984a). Guillemots can develop a detectable viraemia since GIV was isolated directly from the blood of one pre-breeding guillemot during this study. The number of kittiwake blood samples tested for virus is too small (n=16) to conclude that they never develop a viraemia, although previous studies report the isolation of GIVs from tissues and blood of auks (including guillemots and Atlantic puffins, Fratercula arctica) but not kittiwakes (Nuttall, 1985). GIVs that can infect kittiwakes may exist but show no cross-neutralization with the strains we tested by virus neutralization assay. However, the most parsimonious explanation of our results is that guillemots, but not kittiwakes, are susceptible to GIV infection. It is an open question whether the presence of kittiwakes increases (by supporting greater numbers of I. uriae) or reduces (not productive hosts for GIV) the rate of virus transmission, but the steep decline in breeding kittiwake numbers on the island between 1993 and the present day (S.W., personal communication) may well have affected GIV transmission rates in mixed kittiwake/guillemot colonies. By contrast to kittiwakes, the number of breeding guillemots has remained fairly constant and data from 2004–2005 indicate virus prevalence and mean number and distribution of ticks (n=148) feeding on guillemots (n=189) are similar to the values observed between 1993 and 1996 (M.A.N., unpublished data).
Horizontal transmission to uninfected ticks requires that they either feed on a viraemic host or co-feed with infected ticks (Jones et al. 1987). During co-feeding (or non-systemic) transmission, tick-borne viruses are transmitted between ticks, usually feeding in close proximity, in the absence of detectable viraemia (Labuda et al. 1997). In our system, kittiwakes do not seem to support virus transmission, whereas transmission to female ticks feeding on guillemots appears to be highly efficient. Given the present data we can ask whether the transmission between ticks feeding on guillemot is via a viraemic or non-viraemic co-feeding mechanism. It seems likely that viraemic transmission occurs since GIV was isolated directly from guillemot blood and an engorged female tick found off-host in the box traps used to capture pre-breeding guillemots contained 820000 plaque-forming units of virus which is 30 times more virus than any other titrated tick (M.A.N., unpublished data) suggesting it had fed on a viraemic host. Two other observations indicate that co-feeding transmission may also occur. First, the positive association between virus prevalence and the number of female ticks feeding on adult guillemots is most readily explained by a co-feeding mechanism. Under viraemic transmission, virus prevalence and number of feeding ticks should be independent unless some guillemots have consistently higher tick burdens than others, or ticks prefer to feed on viraemic birds. Second, 4 guillemots with multiple ticks had no detectable viraemia but all the ticks on each of the birds were infected with the same virus strain. Notably, more than 95% of female ticks are clustered on the head and neck of guillemots (T.R.B., unpublished observation) and proximity appears to increase the efficiency of co-feeding transmission (Labuda et al. 1997).
Thus GIV transmission probably occurs via both viraemic and non-viraemic routes. Experimental verification of these routes in wild guillemots is ongoing. The experimental observation that co-feeding transmission can occur on hosts that have neutralizing antibody to a virus (Jones et al. 1997; Labuda et al. 1997) is particularly significant for GIV, since it may often encounter conditions where its sedentary tick vector feeds exclusively on breeding guillemots whose high levels of virus neutralizing antibody may prevent viraemic but not non-systemic transmission. Co-feeding transmission also appears to have a critical role in the survival of other arboviruses. It is required for the persistence of louping ill virus (Hudson et al. 1995; Jones et al. 1997; Norman et al. 2004) and tick-borne encephalitis virus (Randolph, Gern and Nuttall, 1996; Randolph et al. 1999) in nature, under conditions where they would otherwise become locally extinct. Non-systemic transmission has now also been demonstrated for insect-borne arboviruses (Lord and Tabachnik, 2002) and may be a general feature of arbovirus transmission. If co-feeding transmission proves widespread, it has profound consequences for arbovirus control since non-viraemic (but co-feeding competent) hosts may be difficult to identify and vaccination may not prevent virus amplification by non-viraemic transmission.
We would like to thank Chris Wernham for assistance in the field and Scottish Natural Heritage for permission to work on the Isle of May National Nature Reserve. This work was funded by the Natural Environment Research Council.