INTRODUCTION
Nitrogen (N) is an essential nutrient for plant growth. Plants can take up both ammonium (NH4 +–N) and nitrate (NO3 −–N) as their N source (Marschner, Reference Marschner1995). A multitude of studies have shown that most plants prefer NO3 −–N to NH4 +–N (Britto and Kronzucker, Reference Britto and Kronzucker2002; Marschner, Reference Marschner1995). With the exception of wetland species such as rice, plants supplied mainly with NO3 −–N typically grow larger and senesce later, while plants supplied with high NH4 +–N develop smaller leaves, grow smaller and yield less (Britto and Kronzucker, Reference Britto and Kronzucker2002; Gerendás et al., Reference Gerendás, Zhu, Bendixen, Ratcliffe and Sattelmacher1997; Marschner, Reference Marschner1995).
Plant growth and productivity depend on photosynthesis. Plant photosynthesis is profoundly influenced not only by external factors, including light, temperature and CO2 concentration, but also by internal forces such as chlorophyll content, enzyme activity and stomatal conductance (Hopkins and Hüner, Reference Hopkins and Hüner2009). As we have known, chlorophyll absorbs sunlight and uses its energy for plant photosynthesis, and ribulose-1, 5–bisphosphate carboxylase/oxygenase (Rubisco) is a crucial enzyme involved in the fixation of CO2 in photosynthesis. Nitrogen is an important constituent of chlorophyll as well as enzymes and proteins involved in photosynthesis. Up to 70–80% of the total leaf N is present in chloroplasts (Makino and Osmond, Reference Makino and Osmond1991), with 20 to 30% of the total leaf N contained within the protein Rubisco (Evans and Seemann, Reference Evans, Seemann and Briggs1989; Kumar et al., Reference Kumar, Parry, Mitchell, Ahmad, Abrol, Foyer and Noctor2002; Makino, Reference Makino2003). Thus, N limitation often leads to lowered Rubisco activity and photosynthesis (Evans, Reference Evans1989). Accordingly, photosynthesis is positively and strongly dependent on N supply (Cechin and de Fátima Fumis, Reference Cechin and Fumis2004; Evans, Reference Evans1989; Pasquini and Santiago, Reference Pasquini and Santiago2012; Toth et al., Reference Toth, Meszaros, Veres and Nagy2002). Moreover, plant photosynthesis has been shown to be higher when supplied with NO3 −–N instead of NH4 +–N (Guo et al., Reference Guo, Liu and Shi2006; Valentine et al., Reference Valentine, Osborne and Mitchell2002). Plant stomatal movements control gas (CO2 and oxygen) exchange and transpiration rates, thus influencing photosynthesis. Research has shown that the presence of NH4 +–N reduces the stomatal conductance as compared with NO3 −–N (Hawkins and Lewis, Reference Hawkins and Lewis1993). In addition, it has also been demonstrated that a nitrate transporter (atNRT1.1) functions in stomatal opening (Guo et al., Reference Guo, Young and Crawford2003). Although the detrimental effects of NH4 +–N on plant growth, chlorophyll and photosynthesis have been documented (Britto and Kronzucker, Reference Britto and Kronzucker2002; Britto et al., Reference Britto, Siddiqi, Glass and Kronzucker2001; Gerendás et al., Reference Gerendás, Zhu, Bendixen, Ratcliffe and Sattelmacher1997), recent research has revealed that NH4 +–N at low concentrations can be beneficial to plants (Azarmi and Esmaeilpour, Reference Azarmi and Esmaeilpour2010; Kotsiras et al., Reference Kotsiras, Olympios, Drosopoulos and Passam2002; Tabatabaei et al., Reference Tabatabaei, Yusefi and Hajiloo2008). For example, Azarmi and Esmaeilpour (Reference Azarmi and Esmaeilpour2010) observed that cucumber grown in a 25:75 NH4 +–N/NO3 −–N solution had the highest total fruit yield. Strawberry has also been found to produce the highest vegetative growth and fruit yield even at 50:50 NH4 +–N/NO3 −–N (Tabatabaei et al., Reference Tabatabaei, Yusefi and Hajiloo2008). These studies imply that different plant species may have different optimal NH4 +–N/NO3 −–N ratios.
Applying NO3 −–N-form fertilizers often increases vegetable plant yield, but it may cause NO3 − accumulation within the plant, especially in leafy vegetables, which may be detrimental to human and animal health (Santamaria, Reference Santamaria2006; Umar and Iqbal, Reference Umar and Iqbal2007). Such applications may also lead to water pollution (Burow et al., Reference Burow, Nolan, Rupert and Dubrovsky2010) and increased trace gas emissions (van Groenigen et al., Reference van Groenigen, Velthof, Oenema, van Groenigen and van Kessel2010). Several studies have shown that combining NO3 −–N with small amounts of NH4 +–N as a N source can increase plant growth and dry biomass (Azarmi and Esmaeilpour, Reference Azarmi and Esmaeilpour2010; Claussen, Reference Claussen2002; Tabatabaei et al., Reference Tabatabaei, Fatemi and Fallahi2006, Reference Tabatabaei, Yusefi and Hajiloo2008) and decrease NO3 −–N in leaf tissues (Kotsiras et al., Reference Kotsiras, Olympios, Drosopoulos and Passam2002; Wang et al., Reference Wang, Zhou, Dong, Shen and Putheti2009).
Therefore, the objective of this study was to examine the influence of different NH4 +–N/NO3 −–N ratios on chlorophyll content, stomatal conductance, Rubisco activity and net photosynthetic rate, dry biomass and NO3 −–N accumulation in spinach grown hydroponically.
MATERIALS AND METHODS
Experiment
Spinach (Spinacia oleracea L., cv. Yinchuandayuan) seeds were surface-sterilized with 50 °C water for 30 min, washed thoroughly and soaked in deionized water for 12 h, then sown in a nursery bed prepared with clean moist vermiculite in a greenhouse under natural photoperiod and day/night temperatures of 25 °C/15 °C. Thirty days after sowing, 30 seedlings of uniform size and vigour with four leaves were transplanted to each container and allowed to grow.
Five combinations of NH4 +–N/NO3 −–N percentage N ratios were used: 0:100 (control), 25:75, 50:50, 75:25 and 100:0. There were three replicates for each treatment. Complete randomized arrangement was used. Each container was given 12 L of full strength Hoagland solution. The concentrations of nutrients in the solution were 12 mmol N L−1, 1 mmol P L−1, 6 mmol·K L−1, 5 mmol Ca L−1, 2 mmol·Mg L−1, 0.50 mg B L−1, 2.8 mg Fe L−1, 0.50 mg Mn L−1, 0.05 mg Zn L−1, 0.02 mg Cu L−1 and 0.09 mg Mo L−1. A nitrification inhibitor, C2H4N4 (dicyandiamide, 7 μmol L−1), was added to each container to prevent nitrification. During the experiment, the solution was aerated for 30 min twice per day with an electronic inflator, and renewed every 10 days. The pH of the solution was monitored daily and kept at 6.50 by adding sufficient 0.5 mol L−1 HCl or NaOH.
Sampling and harvest
On the 36th day after transplanting, net photosynthetic rate and stomatal conductance of spinach leaves were measured in situ, and five measured plants were immediately removed from each container, rinsed with distilled water, blotted with paper towels and then divided into roots and aboveground part (consisting of leaf blades and petioles). Half of the leaves were used for chlorophyll extraction and the other half was kept at −80 °C for the Rubisco activity determination. To measure spinach biomass, another five plants were harvested from each container following the above cleaning and separation processes, dried at 65 °C and weighed.
Measurements
Chlorophyll content:
According to the method described by Ritchie (Reference Ritchie2006), 0.5 g of fresh spinach leaves (ground to <2 mm) were put into a 50 mL Erlenmeyer flask containing 20 mL of extraction solvent (ethanol:acetone:water = 4.5:4.5:1). The flask was sealed airtight and placed in dark for 24 h. One milliliter of the extracts was placed in a clean tube, and 4 mL fresh extraction solvent was then added. After thorough shaking, the absorbance was recorded at 645 nm and 663 nm on a spectrophotometer (SPECTRUM 756 UV, Shanghai Spectral Instrument Co., Shanghai, China). Chlorophyll concentrations in the extraction solution were then estimated using the Arnon equations (Arnon, Reference Arnon1949), and the leaf chlorophyll content was calculated according to the relative dilution quotient.
Rubisco activity:
The Rubisco activity was determined with a method modified from the method of Cheng and Fuchigami (Reference Cheng and Fuchigami2000). Samples (about 2.0 g each) stored at −80 °C were immediately homogenized with Rubisco extractant on an ice bath, filtered through four layers of gauze and centrifuged for 10 min at 15,000 × g. The supernatant was collected as a crude enzyme extract and frozen at 0 °C until further use.
The crude enzyme extract of 50 μL was pippetted into a semi-micro cuvette with 900 μL Rubisco determination solution and homogenized. Using distilled water as a blank and setting the absorbance value to zero at 340 nm on a UV-2450PC spectrometer (Shimadzu, Japan), the absorbance values of the samples were recorded for 0–40 s after addition of 50-μL 5-mmol L−1 RuBP. The initial Rubisco activity was calculated using the absolute descending value of the absorbance from 0 to 40 s.
The total Rubisco activity was measured as follows: The crude enzyme extracts and enzyme determination solution were kept at 30 °C for heat preservation and activation for 15 min before mixing with 50-μL 5-mmol L−1 RuBP. The subsequent steps were the same as the measurement of the initial Rubisco activity.
The Rubisco activation state refers to the ratio of the initial Rubisco activity to the total Rubisco activity (Cheng and Fuchigami, Reference Cheng and Fuchigami2000).
Net photosynthetic rate and stomatal conductance:
Net photosynthetic rate and stomatal conductance of the uppermost, fully expanded leaves were measured using a Li–Cor 6400 (LiCor, Lincoln, NE, USA). The leaf chamber was attached to one leaf for measurement at ambient CO2 (380 ± 10 mmol CO2 mol−1 air, PPFD of 1500 mmol m−2·s−1); leaf temperature was controlled at 25 ± 3 °C, and relative humidity in the leaf chamber was roughly 45% throughout the measurements.
Plant NO3 −–N:
Fresh samples (2.0 g) of the aboveground spinach were ground in 20-mL deionized water in an agate mortar, decolorized with activated carbon and then filtered to prevent chlorophyll pigment interference. The filtrate was used to measure NO3 −–N concentration on a continuous-flow autoanalyser (Autoanalyzer 3, Bran + Luebbe GmbH, Germany; Wang et al., Reference Wang, Zhou, Dong, Shen and Putheti2009).
Statistical analysis
Experimental data were statistically analysed with single factor (ratios of NH4 +–N/NO3 −–N) analysis of variance (ANOVA) for completely randomized design using Statistical Analysis Software (SAS) version 9.1 for Windows. The least significant difference (LSD) method was used to separate the treatment means.
RESULTS
Chlorophyll content
Spinach leaf chlorophyll content differed significantly (p < 0.05) among treatments (Table 1). Chlorophyll a, chlorophyll b and total chlorophyll in spinach leaves decreased with increasing NH4 +–N/NO3ˉ–N ratios. A positive correlation existed between NO3ˉ–N in the solution and chlorophyll a (Chla = 0.6120 + 0.0770x, r = 0.9767; p < 0.01), chlorophyll b (Chlb = 0.2060 + 0.0307x, r = 0.9830; p < 0.01) and total chlorophyll (Chlt = 0.8160 + 0.1080x, r = 0.9797; p < 0.01). Compared with the control (0:100), chlorophyll a, chlorophyll b and total chlorophyll from the 25:75 treatment significantly decreased by 25.5%, 17.2% and 23.3% respectively. The chlorophyll a/chlorophyll b ratio showed little difference among the treatments except in the 100:0 treatment, which had a significantly higher chlorophyll a/chlorophyll b ratio than others.
Table 1. Chlorophyll content in spinach leaves at different NH4 +–N/NO3ˉ–N ratios.
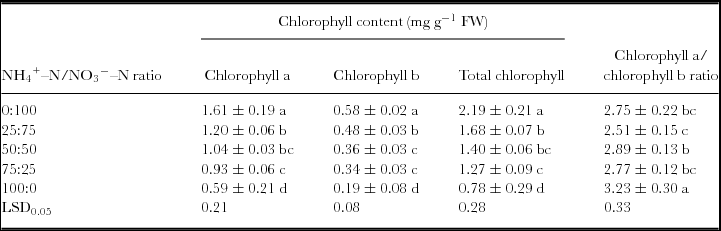
Values within a column followed by the same letter do not differ significantly at p < 0.05. Mean ± SE of mean values (n = 3).
Stomatal conductance
Stomatal conductance declined as NH4 +–N/NO3 −–N ratios increased (Table 2). Conductance was 3.1, 9.6, 10.1, and 18.2% lower in the 25:75, 50:50, 75:25 and 100:0 treatments, respectively, than in the control. However, there were no differences among the 0:100, 25:75 or 50:50 NH4 +–N/NO3 −–N ratio treatments.
Table 2. Net photosynthetic rate and stomatal conductance of spinach leaves at different NH4 +–N/NO3 −–N ratio treatments.
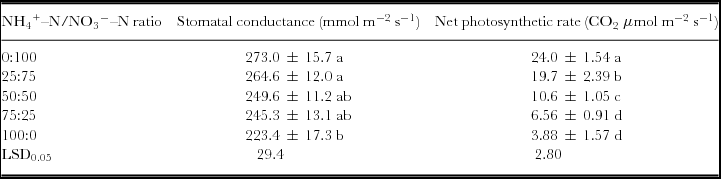
Values within a column followed by the same letter do not differ significantly at p < 0.05. Mean ± SE of mean values (n = 3).
Rubisco activity
The initial Rubisco activity dropped with increasing NH4 +–N/NO3 −–N ratios and significantly differed (p < 0.05) from the control (0:100) in the 50:50, 75:25 and 100:0 treatments (Figure 1A). However, there were no significant differences in the total Rubisco activity among the treatments. The Rubisco activation state was only significantly lower than the control (0:100) in the 75:25 and 100:0 treatments (Figure 1B).

Figure 1. (a) Rubisco activity, and (b) activation state in spinach leaves under different NH4 +–N/NO3ˉ–N ratios. The bars represent ± SE of mean values. Data points with different letters indicate significant difference at p < 0.05.
Net photosynthetic rate
The net photosynthetic rate of spinach leaves was reduced in the presence of NH4 +–N (Table 2). It was 82.1, 44.1, 27.3 and 16.2% of the control for the 25:75, 50:50, 75:25 and 100:0 treatments respectively. Significant differences in the net photosynthetic rate were found among all NH4 +–N/NO3 −–N ratio treatments.
Dry matter yield and NO3 −–N accumulation
Spinach dry matter yield decreased as NH4 +–N/NO3 −–N ratios increased (Figure 2A). However, there was no significant difference in the aboveground dry weights between 0:100 and 25:75 treatments. Dry matter yield of spinach had higher correlation with net photosynthetic rate and the initial Rubisco activity (Table 3).

Figure 2. (a) Relative dry biomass, and (b) nitrate concentration of spinach as influenced by NH4 +–N/NO3ˉ–N ratios in hydroponic culture. The bars represent ±SE of mean values. Data points with different letters indicate significant difference at p < 0.05.
Table 3. Correlation coefficients between dry matter yield (DMY), nitrate content (NIT), chlorophyll content (CHL), stomatal conductance (SC), initial Rubisco activity (IRA), Rubisco activation state (RAS) and net photosynthetic rate (NPR) of spinach leaves.
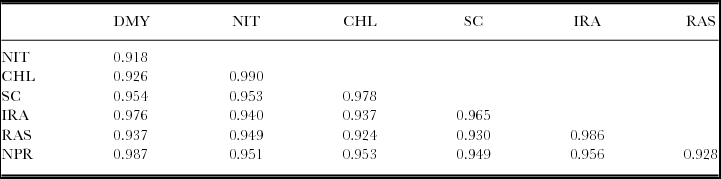
Critical values for correlation coefficient significance are 0.878 at p = 0.05 and 0.959 at p = 0.01. n = 5.
Plant nitrate concentrations decreased with increasing NH4 +–N/NO3 −–N ratios (Figure 2B). Biomass NO3 −–N concentrations were significantly positively correlated with the proportion of the total N supplied as NO3 −–N (r = 0.983**), chlorophyll contents, stomatal conductance and other measurements (Table 3). Plant NO3 −–N concentration was reduced by 38% at the 25:75 treatment in comparison with the 0:100 treatment.
DISCUSSION
Our experimental data showed that higher proportions of NH4 +–N in the N supply to spinach significantly reduced the chlorophyll content, stomatal conductance, Rubisco activity and net photosynthetic rate and thus dry matter yield (Table 3), but no statistical differences in dry matter yield were found between the 0:100 and 25:75 NH4 +–N/NO3 −–N ratios, implying that increasing the NO3 −–N proportion of the total N above 75% does not result in higher biomass accumulation. Previous reports have shown that plants supplied with NH4 +–N as the sole or major N source develop smaller leaves (Walch-Liu et al., Reference Walch-Liu, Neumann, Bangerth and Engels2000) and grow more slowly (Claussen and Lenz, Reference Claussen and Lenz1999; Guo et al., Reference Guo, Zhou, Shen and Zhang2007). Conversely, increased plant growth and yield have been reported when plants were fertilized mainly with NO3 −–N (25:75 NH4 +–N to NO3 −–N; Azarmi and Esmaeilpour, Reference Azarmi and Esmaeilpour2010; Chen et al., Reference Chen, Liu, Gai, Zhu, Yang and Wei2009; Tabatabaei et al., Reference Tabatabaei, Fatemi and Fallahi2006). Several causes and/or mechanisms have been proposed to explain ammonium's (NH4 +) toxicity to plants. For instance, NH4 + reduces cell division and elongation (Walch-Liu et al., Reference Walch-Liu, Neumann, Bangerth and Engels2000), resulting in smaller plants. High external NH4 + may break down the regulation of its influx into root cells, leading to an elevated efflux of the influxed NH4 +, a 40% increase in energetic cost of root cells, and thus to a decline in growth (Britto et al., Reference Britto, Siddiqi, Glass and Kronzucker2001). It has been also demonstrated that NH4 + at higher concentrations can uncouple electron transport from photophosphorylation, thus reducing photosynthetic rates (Peltier and Thibault, Reference Peltier and Thibault1983). The presence of a dual-affinity nitrate transporter gene (atNRT1.1) in guard cells has demonstrated an important role of NO3 −–N in regulating stomatal opening and thus photosynthesis and growth (Guo et al., Reference Guo, Young and Crawford2003). We infer that lowered photosynthesis at high levels of NH4 +–N could also be due to a reduction in light absorption resulting from lower chlorophyll concentrations (Table 1), smaller leaf size (data not shown) and reduced stomatal conductance (Table 2). Indeed, high NH4 +–N has been shown to reduce stomatal conductance (Hawkins and Lewis, Reference Hawkins and Lewis1993; Lopes and Araus, Reference Lopes and Araus2006) as well as to suppress plant uptake of potassium (K+), an important regulator of stomatal movement (Outlaw Jr., Reference Outlaw1983; Wang et al., Reference Wang, Li and Zhang2003). The combination of both elevated NH4 +–N supply and diminished NO3 −–N reduced photosynthesis and yield.
The current results showed no statistical differences in dry matter yield between the 0:100 and 25:75 NH4 +–N/NO3 −–N ratios. In a preliminary experiment with different concentrations of NO3 −–N alone, it was found that spinach biomass significantly declined (18% less) when NO3 −–N concentration was lowered from 12 mM to 9 mM. Therefore, replacing this deficit in NO3 −–N with small amounts of NH4 +–N can actually maintain plant biomass. In combination, our plant biomass and net photosynthetic rate results also imply that dark respiration under NH4 +–N nutrition might be more efficient than under NO3 −–N nutrition because plant dry matter accumulation is the balance between photosynthetic gains and respiratory losses. Bloom et al. (Reference Bloom, Sukrapanna and Warner1992) found that barley used 23% of root carbon catabolism for N absorption and assimilation under NO3 −–N nutrition and only 14% under NH4 + nutrition. Barker et al. (Reference Barker, Volk and Jackson1965) also observed that excised bean leaves assimilated an average of 46% of the absorbed NH4 + into organic compounds, compared with 28% of the absorbed NO3 −. Therefore, plants supplied with NH4 + might increase the efficiency of carbon catabolism, although NH4 + usually inhibited plant photosynthesis. Thus, plant dry biomass may not necessarily decline under low NH4 + nutrition.
Our study also showed that partial replacement of NO3 −–N with NH4 +–N markedly reduced the NO3 −–N content in spinach without significantly decreasing biomass at the 25:75 NH4 +–N/NO3 −–N ratio. This may be pertinent to both vegetable production and environmental quality concerns. Although NO3 −–N is naturally present in soils, water and plants, excess NO3 −–N in plants (particularly in leafy vegetables) may harm human health (Chan, Reference Chan2011; Santamaria, Reference Santamaria2006). To protect consumers, several countries have already established maximum NO3 −–N levels in vegetables (Santamaria, Reference Santamaria2006). Measures to reduce the NO3 −–N content in vegetable plants have been proposed, including genotype selection, balanced fertilization, altered nitrogen forms and organic farming (Umar and Iqbal, Reference Umar and Iqbal2007). The current experiment highlighted that applying 25% of N nutrition as NH4 +–N could reduce NO3 −–N concentrations in spinach by 38% without influencing spinach biomass production (Figure 2). Furthermore, reduction in NO3 −–N fertilizer usage may lower soil NO3 − levels, thereby mitigating not only water contamination of NO3 −–N via leaching (Burow et al., Reference Burow, Nolan, Rupert and Dubrovsky2010) but also N2O emission to the atmosphere via denitrification (Gollany et al., Reference Gollany, Molina, Allmars, Layese, Baker and Cheng2004; Goulding, Reference Goulding2000). However, NH4 +–N fertilizer should be applied in combination with a nitrification inhibitor as NH4 + can quickly convert into NO3 − (Di et al., Reference Di, Cameron, Shen, Winefield, O’Callaghan, Bowatte and He2009).
In conclusion, higher NH4 +–N/NO3 −–N ratios decreased the chlorophyll content, net photosynthetic rate, stomatal conductance and the Rubisco activity of spinach leaves. However, no statistical differences in plant biomass were found between the 0:100 and 25:75 NH4 +–N/NO3 −–N ratios. Nitrate concentrations in spinach leaves were reduced by 38% at the 25:75 NH4 +–N/NO3 −–N treatment compared with the 0:100 treatment. These findings suggest that an appropriate combination of NO3 −–N and NH4 +–N would not impact spinach yield while greatly reducing NO3 −–N content in this leafy vegetable.
Acknowledgements
This study was supported in part by the Science-Technology Project of An’hui Province, China (No: 1101C0603046) and the Transformation Program in Agricultural Scientific and Technological Achievements of An’hui Province, China (No: 10140306017). The authors would like to thank the two anonymous reviewers for their valuable suggestions on improving the manuscript.







