Introduction
Herbivory is a strong selective pressure in plants (Coley & Aide Reference Coley, Aide, Price, Lewinsohn, Fernandes and Benson1991, Coley & Barone Reference Coley and Barone1996). To reduce the effect of herbivores, plants offer food or nesting sites to recruit insect defenders such as ants, which are natural predators of herbivorous arthropods (Del-Claro et al. Reference Del-Claro, Berto and Reu1996, Heil Reference Heil2008, Mithöfer & Boland Reference Mithöfer and Boland2012, Oliveira & Freitas Reference Oliveira and Freitas2004). In this sense, some myrmecophytic plants possess specialised structures that facilitate ant nesting, called domatia. In turn, ants provide with nutrients and defence against herbivores (Beattie Reference Beattie1985, Fiala & Maschwitz Reference Fiala and Maschwitz1992, Mayer et al. Reference Mayer, Frederickson, McKey and Blatrix2014). These hollow cavities are in many cases spontaneously produced by plants (Davidson Reference Davidson1993) and sometimes are induced by ants (Edwards et al. Reference Edwards, Frederickson, Shepard and Yu2009).
It has been shown that defensive traits can differ between sexual forms (Cornelissen & Stiling Reference Cornelissen and Stiling2005). However, evidence involving sex-related differences in indirect defences mediated by ants is limited and is mainly focused on extrafloral nectaries. For example, Beaumont et al. (Reference Beaumont, Mackay and Whalen2016) found a greater abundance of ants and herbivores in males than on female plants of Adriana quadripartita. Sandoval-Molina (Reference Sandoval-Molina2018) reported more ants foraging on extrafloral nectaries of female plants than in male plants in a dioecious population of Opuntia robusta, but in a trioecious population male plants had more ants than females and hermaphrodites. In the same study, he found that females were slightly more damaged than males in the dioecious population, while in the trioecious population males were equally damaged as females, but hermaphrodites were less damaged than the other sexes. However, nothing is known about intersexual differences in domatia traits and how they may change the outcome of ant–plant interactions.
One explanation for sex-related differences in defensive traits is the resource allocation related to the sexual expression. Eckhart and Seger (Reference Eckhart, Seger, Vuorisalo and Mutikainen1999) suggested that sex-related differences in sexual reproduction should influence plant growth and anti-herbivore defences. The resource-competition hypothesis (RCH) explains that the competition for resources between the reproduction, growth and defence is stronger in female plants than in males or hermaphrodites (Delph Reference Delph, Geber, Dawson and Delph1999, Herms & Mattson Reference Herms, Mattson, Baranchikov, Matison, Hain and Payne1991, Lloyd & Webb Reference Lloyd and Webb1977). This is because female plants invest more resources in reproduction (e.g., fruits and seeds) than the other sexes, as a result their growth is slower and invests less energy in plant defences, sustaining greater tissue damage (Herms & Mattson Reference Herms, Mattson, Baranchikov, Matison, Hain and Payne1991). For this reason, females tend to have larger leaves than other sexual forms, increasing their photosynthetic rate to compensate for their resources requirements, as Nicotra et al. (Reference Nicotra, Chazdon and Montgomery2003) reported for Siparuna grandiflora.
Changes in domatia size in response to biotic and abiotic factors have been reported in literature, suggesting that domatia are an adaptive phenotypic plasticity trait (Kokolo et al. Reference Kokolo, Attéké Nkoulémbéné, Ibrahim, M’Batchi and Blatrix2020). In some domatia-bearing plant species, herbivory was shown to change domatia size; even simulated herbivory is able to change domatium size at branch level (Young et al. Reference Young, Stanton and Christian2003). For example, in Cordia nodosa, plants under herbivore pressure had larger domatia than plants excluded from herbivores (Frederickson et al. Reference Frederickson, Ravenscraft, Arcila Hernández, Booth, Astudillo and Miller2013). In Cinnamomum camphora, the position of the domatia varied according to the venation of the leaf ( Reference Nishida, Naiki and Nishida Nishida et al. 2005 ).
Literature on domatia and ant species inhabiting the genus Myriocarpa (Urticaceae) is scarce. Monro (Reference Monro2009b) mentioned that M. longipes Liebm. has domatia, but he did not provide any information on its location, position and ant species that live inside. Longino (Reference Longino2009, Reference Longino2007) reported three species of ants nesting in the domatia of Myriocarpa plants, Pheidole rhinoceros, Pheidole walkeri and Azteca brevis. Previously, M. longipes was reported as a dioecious plant (Steinmann Reference Steinmann2005), but we identified a gynodioecious population (hermaphrodites and females) in Los Tuxtlas Research Station, Veracruz, Mexico (see supplementary materials for details, S1 Figure 1). This sexual dimorphism provides an excellent opportunity to examine sex-related differences in domatia size, position and the outcome of ant–plant interactions. To our knowledge, there are no previous studies in any plant species that explore the differences related to plant sex in domatia traits and their potential effect reducing herbivory.
Here, we conducted a field survey to analyse differences in domatia size, position, ant species inhabiting, leaf area and herbivory between plants with different sexual phenotypes. We hypothesise the existence of sex-related differences in defensive traits, where female plants are expected to be less defended due to differences in domatia traits, such as size, number of domatia and position, exhibiting more herbivore damage than hermaphrodite plants. Thus, we tested the following question: 1) Do the size and position of domatia promote their greater occupancy by ants on female plants than on hermaphrodite plants? 2) Are the leaves of female individuals more damaged than those of hermaphrodite individuals? In addressing the above, this study builds towards understanding the effect of plant sex on the outcome of ant–plant interactions.
Methods
Study site and plant species
We carried out our observations in different years, between 2016 and 2019 at the Los Tuxtlas Research Station, Veracruz, Mexico (18°30′N and 95°03′W), a reserve operated by the Universidad Nacional Autónoma de México (UNAM). It is located in the southeast of the state of Veracruz, Mexico, and is surrounded by extensive lowland plains. Dominant vegetation in this region is tropical evergreen forest (Rzedowski Reference Rzedowski1986), comprising 940 species of vascular plants, divided into 545 genera and 137 families (Ibarra-Manríquez et al. Reference Ibarra-Manríquez, Martínez-Ramos, Dirzo, Nunez-Farfan, Gonzalez-Soriano, Dirzo and Vogt1997). For a more detailed description of Los Tuxtlas, see González Soriano et al. (Reference González Soriano, Dirzo and Vogt1997). Our work was conducted along the main access roads of the research station, on a transect of approx. 700 m of length where plants grow naturally in partially opened areas of the understory. We selected and GPS-marked 20 individuals of Myriocarpa longipes (8 hermaphrodites and 12 females), of similar height and with visible and fresh inflorescences. The selected plants were at a minimum distance of 5 m from each other along the road. We determined the sex of the plant using descriptions provided by Monro (Reference Monro2009a). Until now, this plant species was described as dioecious or monoecious (Steinmann Reference Steinmann2005). However, we found a gynodioecious population growing in the study site.
Myriocarpa longipes (Urticaceae) is a small tree (8–12 m height) distributed from Mexico to South America (Colombia, Bolivia, Brazil). It has ovate to elliptical leaves, 12–55 cm long and 6.5–23 cm wide. It can be found in disturbed, undisturbed and secondary evergreen wet forest, from sea level to 2400 m (Monro Reference Monro2009a, WFO 2020). This plant species has domatia on its branches inhabited by ants (Monro Reference Monro2009b). Domatia in M. longipes are hollow structures in branches; its inner wall is lined with dead pith tissue that forms a spongy layer that is removed by the ants when they are inhabited (M. Sandoval-Molina, pers. obs.).
Ant species composition and domatia measurements
To determine the composition of ant species associated with domatia, from the 20 previously marked plants of M. longipes, we selected only 5 plants (3 hermaphrodites and 2 females). Using an extendable pole pruner, we removed two branches of approximately the same length (∼2 m) from each plant, cutting at the bifurcation base of the main stem. We destructively sampled domatia using a knife and collected ants inhabiting each domatium and were preserved in 70° ethanol in Eppendorf tubes individually tagged. Then, ants were mounted and identified with the aid of taxonomic keys (Fisher et al. Reference Fisher, Cover, Kirsch, Kane and Nobile2007, Mackay & Mackay Reference Mackay and Mackay1989). We corroborated taxonomic identification using the ant species list of Los Tuxtlas Biological Station, Veracruz, Mexico (project ADMAC), and with the assistance of the entomological collection IEXA at the Instituto de Ecologia, A.C., Xalapa, México, where specimens were deposited.
Domatia position was classified into three categories: basal, middle and apical, according to their position along the branches. To explore differences in domatia size between sexual forms, we used a caliper to measure the length and width of the thickest section of the branch where the domatium is located (supplementary materials, S1 Figure 2). We also measured the thickness of the solid part of the stem, from the outside of the stem to the beginning of the domatium. With these measurements, we estimated the cross-sectional area of the hollow part of the stem and the stem area surrounding the domatium.
Leaf area and herbivory
To analyse the differences in leaf area and herbivory between female and hermaphrodite plants, on the 20 plants previously marked, we randomly removed between 3 and 10 leaves per plant. To control leaf age, we selected only fully expanded leaves, excluding the youngest leaves at the top of the branches. We analysed the percentage of leaf area removed by herbivores using the BioLeaf app (Machado et al. Reference Machado, Orue, Arruda, Santos, Sarath and Goncalves2016). This software estimates the percentage of removed tissue in relation to the total leaf area. To measure leaf area between females and hermaphrodites, we selected three leaves with no evident signs of herbivory from each plant and were photographed using a smartphone camera. All the photographs were taken at the same distance (approx. 50 cm) in a 90° angle, using a wooden structure built by us. Fully expanded leaves were placed in a millimeter paper as background and were photographed. We estimated the total area of the leaves using the LeafArea package in R that allows to analyse several photographs simultaneously in ImageJ software (Katabuchi Reference Katabuchi2015, R Core Team 2020).
Statistical analysis
To test for differences in cross-sectional domatia area between sexes, we performed a Wilcoxon test; for the stem area surrounding the domatium, we used a t-test. In addition, we carried out a Pearson Chi-squared to test for association between domatia position and sex, also between ant species and sex, and finally between domatia position and sex. Due to the small frequencies for some factors in the contingency tables (some observations < 5), p-values were calculated by a Monte Carlo simulation, using 5000 replicates.
To test for differences in herbivory, we fitted a generalised linear model (GLM) with gamma distribution and ‘log’ as a link function. We used the arcsine transformed percentage of herbivory as response variable (N = 95), this transformation helped to normalise the data and improved the normal distribution of the model residuals. To test for differences in leaf area, we fitted a GLM with Gaussian distribution and ‘identity’ as link function, using the leaf area as response variable (N = 60). In both models, plant sex was used as explicative variable. Model goodness of fit was checked by inspection of residuals using the DHARMa package (Hartig Reference Hartig2018), which uses a simulation-based approach to create readily interpretable scaled residuals from fitted models. Comparisons were done with post-hoc Tukey’s test using the emmeans package (Lenth Reference Lenth2019). Plots were made using the ggpubr package (Kassambara Reference Kassambara2019) and ggplot (Wickham Reference Wickham2016). We made all statistical analysis using R (R Core Team 2020, RStudioTeam 2020).
Results
We found that domatia were distributed along the branches of M. longipes of both sexes, but domatia inhabited by ants were restricted to middle position in females. We did not find differences in the number of ants-occupied domatia between females and hermaphrodites (X 2 = 0.28, df = 1, p = 0.59). We found that domatia occupied by ants are not commonly distributed along the branches (X 2 = 6.14, df = 2, p = 0.05), inhabited domatia were more frequently found in the middle section of the stem (N = 9) than at the base (N = 2) or top (N = 3) of the branches. Domatia were found in both young and old branches and occupied more than one stem internode; the longitude of domatia varied between 10 and 25 cm. The hole entrance to domatium occurred where leaves had previously grown at the base of the petiole (Fig. 1a, b). We found that domatia were hollow inside and were occupied by ants and found other taxonomic groups living inside domatia cavities, such as beetle larvae, myriapods and salamanders (Bolitoglossa sp.). Plants hosted eight ant species, with adults and larvae (Fig. 1c to h). Five species were found in female plants and six in hermaphrodites (Table 1): Pheidole sp. (Wheeler, 1938) (Fig. 1c), Cephalotes scutulatus (Smith, 1867) (Fig. 1d), Camponotus sp. (Mayr, 1861) (Fig. 1e), Pachycondyla sp. (Smith, 1858) (Fig. 1f), Camponotus atriceps (Smith, 1858) (Fig. 1g), Dolichoderus lutosus (Smith, 1858) (Fig. 1h), Dolichoderus bispinosus (Olivier, 1792) and Azteca sp. (Forel,1878). We found that ant species could co-inhabit the same plant, even on the same branch. We found two or more ant species living in different domatium on the same branch of the same plant.

Figure 1. Field observations of ant–plant interactions in Myriocarpa longipes. (a) The ants enter the domatia through the hole at the base of the petiole, where the leaves have previously grown. (b) Portion of a stem showing the cross-section of the domatia. Dissection of the stem where the domatia are located, showing workers and brood of different ant species: (c) Pheidole sp., (d) Cephalotes scutulatus, (e) Camponotus sp., (f) Pachycondyla sp., (g) Camponotus atriceps and (h) Dolichoderus lutosus. Some domatia show melanised fungi inside.
Table 1. Ant species composition according to the position of the domatia between female and hermaphrodite plants of Myriocarpa longipes. In female plants, ant-inhabited domatia were restricted to the middle position of the branches; no ant species were found in domatia at the base and apical positions.
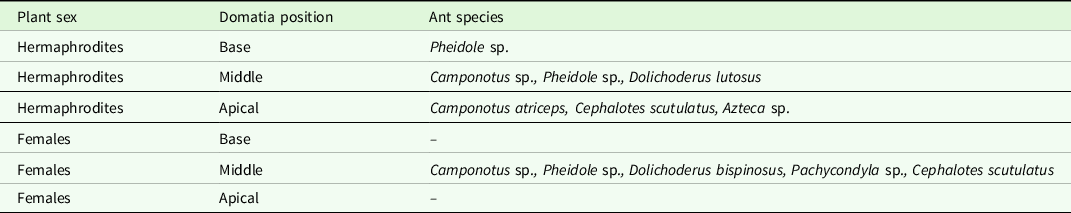
Table 2. Model statistics for the percentage of herbivory and leaf area between female and hermaphrodite plants of Myriocarpa longipes. We showed results from generalised linear models with gamma and Gaussian error distribution. Bold numbers represent significant p-values <0.05.

Herbivory and domatia morphology
We found a significant difference in percentage of herbivory between sexes, female leaves were more damaged than hermaphrodites (t = 2.00, df = 93, p = 0.04; Fig. 2a; Table 2). We did not find differences in leaf area between female and hermaphrodite plants (supplementary materials, S1 Figure 3; Table 2).
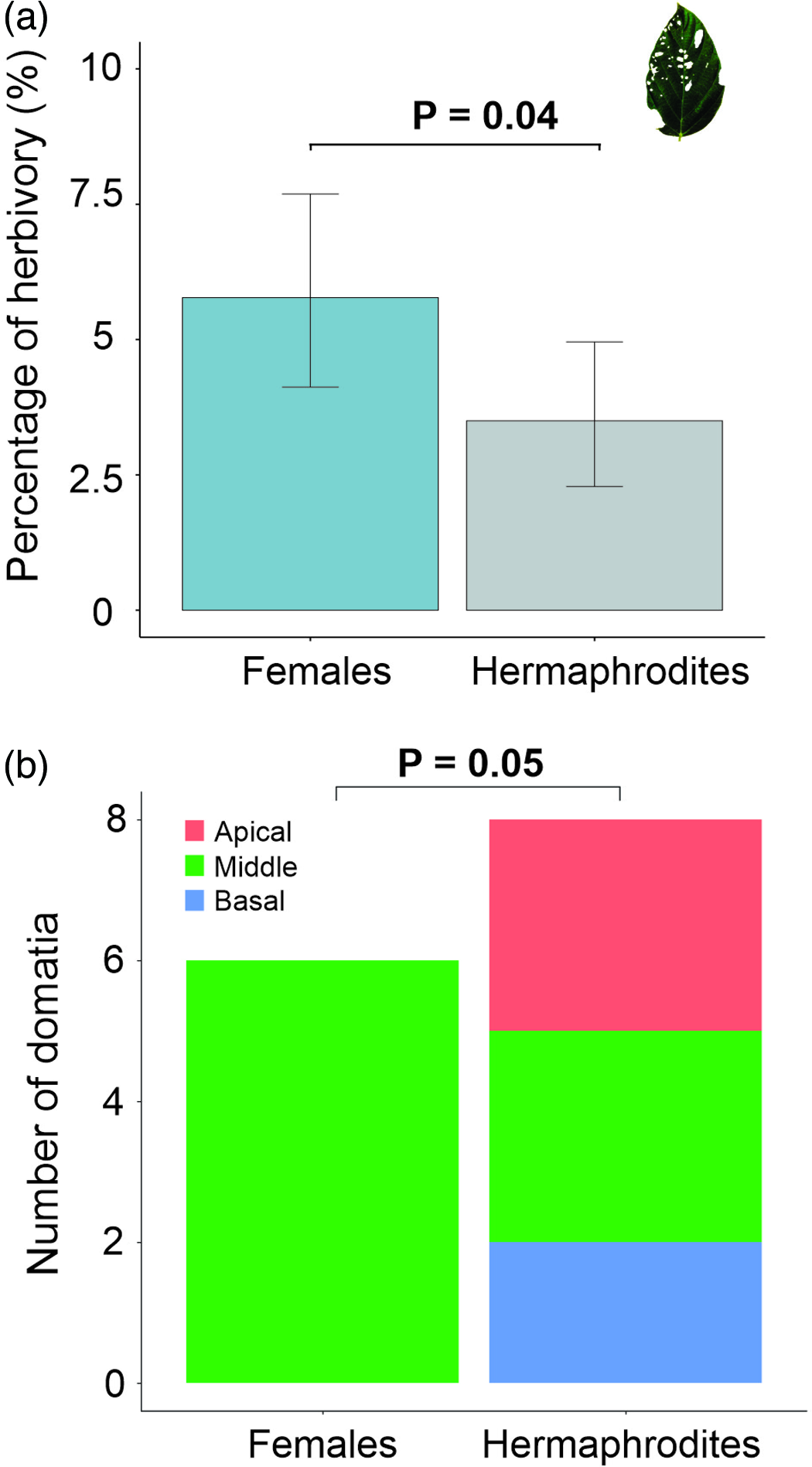
Figure 2. Percentage of herbivory and number of domatia of Myriocarpa longipes. (a) Differences in leaf damage percentage between females and hermaphrodites. (b) Number of domatia inhabited by ants on branches between different sexual forms. The p-value for the post-hoc contrast and Chi-squared are shown above the bar plots and boxplot, respectively. Error lines represent the 95% confidence intervals.
Regarding domatia measurements considering all positions along the branch (supplementary materials, S1 Figure 4), we did not find differences in the cross-sectional area of the domatium between females (mean ± sd: 0.198 cm2 ± 0.170) and hermaphrodites (mean ± sd: 0.117 cm2 ± 0.117). When we compared only the domatia in the middle position of the branches, the females had slightly larger domatia than the hermaphrodites, but this analysis was not statistically significant. We did not detect differences in the stem area surrounding the domatium. We found a marginally significant association between the position of domatia and plant sex (X 2 = 5.83, df = 2, p = 0.055); domatia in females were positioned mainly in the middle of the branches, while in hermaphrodites domatia were found all along the branches (Table 1; Fig. 2b). We did not find a relationship between domatia position and ant species (X 2 = 16.33, df = 14, p = 0.34), nor between ant species and plant sex (X 2 = 6.85, df = 7, p = 0.68).
Discussion
The present study investigated the intersexual differences in domatia size, position, leaf area and herbivory between sexual forms of M. longipes in a gynodioecious population. According to our predictions, female plants had differences in domatia position and exhibited greater herbivore damage in leaves than hermaphrodites. Literature concerning differences in plant traits related to indirect defence between sexual forms is limited and has been focused on extrafloral nectaries bearing plants (Beaumont et al. Reference Beaumont, Mackay and Whalen2016, Sandoval-Molina Reference Sandoval-Molina2018). To our knowledge, this is the first study to show the existence of sex-related differences in domatia position, and its effect on herbivore damage in M. longipes, a facultative myrmecophyte.
In our study, female plants were less defended, with ant-inhabited domatia restricted to the middle section of the branches and showing higher levels of herbivory than hermaphrodite plants. It has been shown that domatia are a highly plastic trait (Kokolo et al. Reference Kokolo, Attéké Nkoulémbéné, Ibrahim, M’Batchi and Blatrix2020) and may even vary at the branch level in the presence of herbivores (Young et al. Reference Young, Stanton and Christian2003). Probably, as the female plants showed restrictions in the position of their domatia, the ants could not occupy them; as a result, fewer ants inhabited and were unable to defend the plant successfully against herbivores. In contrast, hermaphrodite plants had domatia all along their branches, housing a greater number of ants and reducing the damage produced by herbivores.
Domatia restricted to the middle position in females, similar leaf size and a higher proportion of damaged leaves in female individuals than in hermaphrodites suggest that our results are parallel with the predictions of the RCH. This hypothesis proposes that female plants, as result of its higher investment in reproduction, will allocate less resources to defence production, showing greater herbivore damage than hermaphrodites. This hypothesis would be tested if the reproductive allocation of the female had been greater than in the hermaphrodite individual. Another explanation for the magnitude of damage observed in female plants is a lower cost of vegetative tissue production, following the optimal defence hypothesis, should be less defended because the loss of less energetically valuable tissue implies a lower loss in terms of fitness (McKey Reference McKey, Rosenthal and Janzen1979, Rhoades Reference Rhoades, Rosenthal and Janzen1979).
Female plants probably produced fewer chemical defences in their leaves, showing greater herbivore damage than hermaphrodites. However, little is known about secondary metabolites in Myriocarpa species. Niño et al. (Reference Niño, Correa, Cardona and Mosquera2011) reported that the extract of Myriocarpa stipitata has alkaloids, terpenes, tannins and saponins, being the former related to inhibitory activity against yeast. Literature concerning intersexual differences in chemical defences production is limited (Dziedzic et al. Reference Dziedzic, Szopa, Waligórski, Ekiert and Ślesak2020, Iszkuło et al. Reference Iszkuło, Kosiński and Hajnos2013, Janczur et al. Reference Janczur, González-Camarena, León-Solano, Sandoval-Molina and Jenner2021). There is only one study in a cactus that showed that female plants produce lower concentrations of a secondary metabolite (4-hydroxybenzoic acid) than hermaphrodite plants (Janczur et al. Reference Janczur, González-Camarena, León-Solano, Sandoval-Molina and Jenner2021). The extent of damage observed in our study is likely the result of differences in secondary metabolites between sexual forms, but differences in chemical defences on M. longipes remain unexplored and further studies are required to confirm its effect in decreasing herbivory of plants.
Ant species composition inhabiting domatia could be also an important factor influencing the extent of herbivore damage. It is known that the intensity of herbivory exhibited by myrmecophytic plants depends on size or aggressiveness of its ant associates (Miller Reference Miller2007, Rico-Gray & Thien Reference Rico-Gray and Thien1989). This is because ant species vary in their foraging behaviour, patrolling ability, recruitment efforts and predatory activity, resulting in different levels of herbivory (Del-Claro et al. Reference Del-Claro, Rico-Gray, Torezan-Silingardi, Alves-Silva, Fagundes and Lange2016, Fagundes et al. Reference Fagundes, Dáttilo, Ribeiro, Rico-Gray, Jordano and Del-Claro2017). In our study, we found that ant species diversity varied within and between individuals of M. longipes, even within the same branches, and some ant species co-existed on the same plant. It is likely that ant species composition between sexual forms is affecting the extent of herbivore damage, but it is likely that we did not observe this effect due to our small number of dissected plant individuals (five plants). Further studies should increase the number of plants sampled to disentangle the effects of plant sex on domatia size and ant colonies, as well as its effect at the individual level.
It has been shown that domatia size can change in response to herbivory (Frederickson et al. Reference Frederickson, Ravenscraft, Arcila Hernández, Booth, Astudillo and Miller2013). Our results did not show differences in the cross-sectional domatia area between females and hermaphrodites when we considered all domatia positions. However, when we only considered domatia in the middle position, we found a trend that females may have larger middle domatia than hermaphrodites, but this difference was not statistically significant and also probably related to the small number of plants sampled (see supplementary materials, S1 Figure 4). This result suggests that probably even when females restricted the position of domatia, they responded to higher herbivory increasing domatium size in the middle position to house more ants to counter herbivory, but more studies are needed to confirm this effect.
Finally, when ants fail to defend plants, a penalty could be imposed on them, reducing the growth or survival of domatia (Edwards et al. Reference Edwards, Hassall, Sutherland and Yu2006). It is likely that facultative ants inhabiting domatia of females are inefficient deterring herbivores, so plants are ‘taking’ the decision to penalise them restricting the available spaces for nesting. Even if that is reflected in an increase in the amount of herbivory damage. Another possibility is that if nesting sites are being restricted in the female plants, the species composition of ants inhabiting the domatia should change and colonies of more aggressive ant species will be favoured, influencing the foraging behaviour of other insects, such as herbivores, and modifying the outcome of ant–plant interactions.
Conclusion
This work is consistent with the predictions of the RCH. Female plants exhibited greater herbivore damage to the leaves than hermaphrodites and had ant-inhabited domatia restricted to the middle position of their branches.
The results of this study suggest the existence of sex-related differences in domatia position and herbivory in a gynodioecious population of M. longipes. Few studies in the literature address intersexual differences in plant defences in the context of ant–plant interactions (Beaumont et al. Reference Beaumont, Mackay and Whalen2016, Sandoval-Molina Reference Sandoval-Molina2018). However, until now, nothing was known about domatia plants. On the basis of our findings, we highlight the importance of considering plant sex in ant–plant interactions studies. It is possible that differences in resource allocation related to sexual reproduction influence the outcome of these interactions.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467421000584
Data accessibility
All data are fully available without restriction. All files are available from the Harvard Dataverse database (https://doi.org/10.7910/DVN/RLL0T8).
Acknowledgments
The authors would like to thank Rosamond Ione Coates Lutes (Director of the Los Tuxtlas Research Station, UNAM) and Álvaro Campos Villanueva (Los Tuxtlas Research Station, UNAM) for allowing us to carry out the study in the forest belonging to the Station. We thank to Delfino Hernández Lagunes, Dora Luz Martínez Tlapa and Jorge Valenzuela from IEXA-INECOL entomological collection for helping with ant species identification.
Financial support
CONACyT supported this work (grant number: 180694) to Mariusz Krzysztof Janczur.
Conflict of interest
We have no conflicts of interest to disclose.
Ethical standard
We got the permission of the head of the Los Tuxtlas Research Station to carry out research activities on the lands administered by the Instituto de Biología-UNAM. During the study, we did not affect or involve any endangered species.







