Introduction
Dietary shifts by herbivorous insects can have important consequences in both natural and agricultural systems (Graves & Shapiro, Reference Graves and Shapiro2003; Scriber, Reference Scriber2010). From an evolutionary perspective, a shift to a new host may mediate the process of speciation, especially if adaptation to plants with divergent traits reduces gene flow between host ‘races’ (Michel et al., Reference Michel, Sim, Powell, Taylor, Nosil and Feder2010; Craig & Itami, Reference Craig and Itami2011; Downey & Nice, Reference Downey and Nice2011). Over longer time scales, successive shifts to dissimilar hosts may account for much of the contemporary diversity of phytophagous taxa (Janz et al., Reference Janz, Nylin and Wahlberg2006; Fordyce, Reference Fordyce2010). Colonization of a new host can also have short-term, economic consequences. New insect ‘biotypes’ are sometimes found infesting a crop that was previously considered an unsuitable or poor host (Lushai et al., Reference Lushai, Markovitch and Loxdale2002; Downie, Reference Downie2010), and insects introduced for the biological control of an invasive weed may unexpectedly attack a non-target, native plant as well (Olivieri et al., Reference Olivieri, Singer, Magalhães, Courtiol, Dubois, Carbonell, Justy, Beldade, Parmesan and Michalakis2008).
Successful colonization of a novel host will depend in part on whether the insect population possesses the requisite amount of genetic and phenotypic variation (Garcia-Robledo & Horvitz, Reference Garcia-Robledo and Horvitz2011). Some host-range expansions require little genetic change because the insect is fortuitously well-adapted to the newly encountered plant (Agosta, Reference Agosta2006; Singer et al., Reference Singer, Wee, Hawkins, Butcher and Tilmon2008; Van Asch et al., Reference Van Asch, Julkunen-Tiito and Visser2010). In other cases, a change in diet breadth is accompanied by modification of key behavioural, physiological or morphological traits that gradually improve survival and reproduction on the novel host (Matzkin et al., Reference Matzkin, Watts, Bitler, Machado and Markow2006; Forister et al., Reference Forister, Ehmer and Futuyma2007; Dworkin & Jones, Reference Dworkin and Jones2009). Few studies have documented such changes, however, in part because host-range expansions are often not recognized until well after they occurred (Feder & Forbes, Reference Feder, Forbes and Tilmon2008; Futuyma, Reference Futuyma and Tilmon2008; Matsubayashi et al., Reference Matsubayashi, Ohshima and Nosil2010). Genetic changes associated with colonization of a new host can be investigated by hybridizing conspecific populations (or closely related species) already associated with different hosts (Sheck & Gould, Reference Sheck and Gould1995; Keese, Reference Keese1996), but such ‘wide’ crosses may not accurately reflect genetic mechanisms underlying early stages of adaptation (Xue et al., Reference Xue, Magalhães, Li and Yang2009; Midamegbe et al., Reference Midamegbe, Vitalis, Malausa, Delava, Cros-Arteil and Streiff2011). Some differences between long-established host races could represent local adaptation to environmental conditions other than the host plant (Coyne & Orr, Reference Coyne and Orr2004).
Selection experiments provide a useful tool for investigating the processes by which insects adapt to new food plants. Under quasi-natural selection, replicate selection lines can be switched to a novel host; and, in contrast to artificial selection, there is no conscious selection for any particular character (Fry, Reference Fry2003; Garland & Rose, Reference Garland and Rose2009). Lines on both the ancestral and novel hosts are maintained in the same controlled environments, and various performance traits can be measured after only a few generations (Agrawal, Reference Agrawal2000; Magalhães et al., Reference Magalhães, Fayard, Janssen, Carbonell and Olivieri2007). If selection lines have diverged in their ability to use the novel host, they can be hybridized to determine the underlying genetic basis (Tucić & Šešlija, Reference Tucić and Šešlija2007). In this study, we performed crosses to examine the inheritance of increased acceptance of a novel host by the seed beetle Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae: Bruchinae).
Callosobruchus beetles have long served as a model organism for examining the evolution of host ranges (Wasserman & Futuyma, Reference Wasserman and Futuyma1981; Messina & Slade, Reference Messina and Slade1997; Messina, Reference Messina2004a; Fricke & Arnqvist, Reference Fricke and Arnqvist2007; Rova & Björklund, Reference Rova and Björklund2011). This insect has infested human stores of grain legumes for thousands of years, so that laboratory conditions provide a reasonable approximation of its ‘natural’ environment. Typical hosts of C. maculatus in storage belong to the legume tribe Phaseoleae, especially the genus Vigna Savi. We have been investigating the rate of beetle adaptation to a marginal host, lentil (Lens culinaris Medikus), which is instead a member of the tribe Fabeae (Choi et al., Reference Choi, Mun, Kim, Zhu, Baek, Mudge, Roe, Ellis, Doyle, Kiss, Young and Cook2004).
Lentil is a very poor host for most C. maculatus populations, but populations able to develop in lentil seeds are found occasionally (Wasserman, Reference Wasserman1986; Credland, Reference Credland1987, Reference Credland, Fujii, Gatehouse, Johnson, Mitchell and Yoshida1990). For an Asian-origin population associated with mung bean (Vigna radiata [L.] Wilczek), larval survival to adult emergence was initially found to be about 1% in lentil, and the lifetime fecundities of females provided only with lentil seeds were less than a third of those provided with mung beans (Messina et al., Reference Messina, Mendenhall and Jones2009a,b). Moreover, about 25% of females appeared not to recognize lentil as a potential host, i.e. they laid no eggs. Nevertheless, three, independent, quasi-natural selection experiments (Fry, Reference Fry2003) eventually yielded self-sustaining populations on lentil after each population underwent a severe bottleneck. These experiments mimicked a scenario in which beetles in seed stores encounter an abundance of lentil seeds after an ancestral host is no longer available. In fewer than 20 generations, larval survival in all three experiments rose from about 1% to >85% in the three lentil-adapted lines (Messina et al., Reference Messina, Mendenhall and Jones2009a). Acceptance of lentil by egg-laying females increased two- to three-fold in the lentil-adapted lines (Messina & Jones, Reference Messina and Jones2009; Messina et al., Reference Messina, Jones, Mendenhall and Muller2009b).
Hybridization of lentil-adapted and mung-bean lines indicated that the rapid increase in larval survival in lentil was mediated by both additive and non-additive genetic components (Messina & Jones, Reference Messina and Jones2011). Larvae from backcrosses to the lentil line emerged at a rate consistent with purely additive inheritance, whereas larvae produced from backcrosses to the mung-bean line survived much at a lower rate than would be predicted by simple additivity. Crosses were performed here to determine the mode of inheritance of host acceptance. An artificial-selection experiment suggested that genes influencing greater acceptance of lentil for oviposition are distinct from those causing improved larval performance (Messina et al. Reference Messina, Jones, Mendenhall and Muller2009b; see also Fox, Reference Fox1993).
We used no-choice (single-host) conditions to assay host acceptance by parental and hybrid lines rather than choice (paired-host) conditions. For C. maculatus, the two protocols can yield different conclusions regarding the genetics of host use (Chiu & Messina, Reference Chiu and Messina1994; Messina & Slade, Reference Messina and Slade1997; Messina, Reference Messina2004b). No-choice arenas provide a more realistic assessment of the likelihood of exploiting a novel host and are more likely to mimic beetles that encounter abundant seeds of a novel host in human stores of grain legumes (Messina & Slade, 1996). As Futuyma (Reference Futuyma and Tilmon2008) noted, insects in natural populations are unlikely to make simultaneous comparisons of multiple hosts, and comparative choice tests typically do not distinguish between greater acceptance of a preferred plant and greater aversion to a disfavoured plant (Martel & Boivin, Reference Martel and Boivin2011).
Materials and methods
Source population and selection lines
Females of C. maculatus attach eggs singly to the surfaces of legume seeds. Hatching larvae burrow into the seed directly beneath the oviposition site and complete development within a single seed. Adults emerge from circular exit holes in the seeds, and commence mating and oviposition within several hours after emergence. All lines used here were derived from a population that was established from infested mung beans in Tirunelveli, India (Messina, Reference Messina1991; Mitchell, Reference Mitchell1991). This population has been kept on mung beans in the laboratory for >200 generations and yet has maintained genetic variation for several fitness-related traits (Messina, Reference Messina2004a, and references therein).
Three lentil-adapted lines (hereafter, L1–3) were established independently and sequentially as described by Messina et al. (Reference Messina, Mendenhall and Jones2009a,Reference Messina, Jones, Mendenhall and Mullerb). In brief, >2000 (for the L1 line) or >4000 (for L2 and L3 lines) adults from the Asian population were added to either 750 g or 1500 g of lentil seeds (≈12,000 or 25,000 seeds). Because initial survival in lentil was about 1%, each line underwent a severe bottleneck. Larval survival rose rapidly and had exceeded 90% at the time of the current study (Messina & Jones, Reference Messina and Jones2011). New generations of stock cultures of both the lentil-adapted lines and the ancestral, mung-bean (hereafter, M) line were formed by adding 1500–2000 adults to about 750 g of lentil or mung bean. Cultures were maintained and experiments were conducted in a growth chamber at 24°C and constant light.
Line crosses
Crosses were performed between the three L lines and the M line after each L line had spent 30 generations on lentil. Because the L lines were not formed simultaneously (Messina et al., Reference Messina, Mendenhall and Jones2009a), the three sets of crosses were performed at different times and hence were subjected to separate analyses. For each set, we first isolated 650–900 infested lentil seeds or mung beans from stock cultures in 4-ml vials. To form hybrids, we collected from the isolated seeds 150–200 unmated, newly emerged females and an equal number of males from the opposite line. Females and males were added to a culture jar containing ≈750 g of mung beans. Stock cultures of both parental lines (L and M) were also initiated on mung beans at the same time, which meant that the L line was reverted to mung beans for a generation. Thus, we produced four lines (two parental lines plus the two reciprocal-hybrid lines) for assays of host acceptance, and test females in all lines developed in the same host (mung bean).
As hybrid and parental beetles began to emerge as adults, each culture was sieved to remove all previously emerged adults. Newly emerged adults were then collected within an hour after sieving. Such a brief post-emergence period ensured that all test females had no egg-laying experience. For the cross between the L1 and M lines, each newly emerged pair was placed in a 60-mm Petri dish containing approximately 100 lentil seeds or mung beans (N=100 pairs per treatment on lentil and 50 pairs per treatment on mung bean). For the crosses between the L2 or L3 lines and the M lines, host acceptance was assayed on lentil only. Newly emerged parental or hybrid pairs were again placed in dishes with ≈100 lentil seeds, with 75 pairs per treatment. Host acceptance was estimated as the total number of eggs laid after six days, by which time most females had laid their lifetime complement of eggs.
For the L1×M cross, egg counts were analyzed with two-way analysis of variance, with the fixed effects of cross type (four levels) and oviposition host (lentil or mung bean). This analysis was followed with a one-way ANOVA that estimated the effect of cross type only for females provided with lentil seeds. One-way ANOVA was also used to examine the effect of cross type in crosses between the L2 or L3 lines and the M line. After each ANOVA, Tukey's HSD tests were used for post-hoc comparisons of means (Wilkinson et al., Reference Wilkinson, Blank and Gruber1996). Egg counts were square-root transformed in all analyses to better conform to assumptions of ANOVA. In addition to analyzing mean egg number, host acceptance was also estimated as the percentage of females that laid at least one egg on lentil during the six-day exposure. Statistical analyses were not applied to these percentages because they were not strictly independent of the egg-count data.
Timing of host acceptance
A final experiment in this study compared host acceptance by parental and hybrid females soon after adult emergence. This experiment follows previous work showing that L and M females differ in the timing of acceptance of lentil, as well as lifetime fecundity on lentil (Messina et al., Reference Messina, Jones, Mendenhall and Muller2009b). Pairs of parental and hybrid beetles were collected within an hour of adult emergence as described above. Each pair was first placed in an empty 60-mm dish for 12 h to allow mating. Females were then transferred to dishes containing about 100 lentil seeds. For each cross type, half of the females were removed after 24 h. The remaining females were allowed to oviposit a further 48 h and thus were exposed to seeds for a total of 72 h. The experiment included eight treatments (four cross types× two exposure periods), with 27–29 females per treatment. Since egg numbers are expected to be much greater among females allowed to oviposit for 72 h than among females allowed to oviposit for 24 h, exposure period was not used as a main effect in ANOVA. Instead, the effect of cross type was analyzed separately for each exposure period, and Tukey's HSD test was again used as a conservative test of differences between pairs of means.
Results
Line crosses
When females from crosses between the L1 and M lines were provided mung bean or lentil, the number of eggs laid over six days was influenced by both cross type (F 3, 592=11.0, P<0.001) and test host (F 1, 592=597.0, P<0.001), as well as a significant cross type×host interaction (F 3, 592=8.6, P<0.001). As expected, there was little variation in egg number on mung bean, but L1 females laid more than twice as many eggs on lentil as M females did (fig. 1). Hybrid females from both reciprocal crosses laid an intermediate number of eggs on lentil (fig. 1). Post-hoc mean comparisons indicated that the mean number of eggs laid by hybrids on lentil was significantly greater than the number laid by M-line (P≤0.01) and was either significantly lower than the number laid by L1 females (P<0.001 for L1 vs. L1♀×M♂) or nearly so (P=0.06 for L1 vs. M♀×L1♂). Egg counts on lentil did not differ between the two types of hybrids (P=0.23). Hybrids were intermediate with respect to the percentage of females laying at least one egg on lentil, but they resembled L1 females more than M females (table 1).
Table 1. Percentages of parental and hybrid C. maculatus females laying at least one egg on lentil seeds over six days.
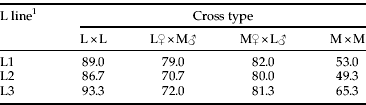
1 Each L line had spent 30 generations on lentil and all test females developed in the ancestral host, mung bean. N=75–100 females per cross type.

Fig. 1. Mean number of eggs (+SE) laid on mung bean or lentil by C. maculatus females from crosses between the L1 and M lines. The L1 line had spent 30 generations on lentil and all test females developed in the ancestral host, mung bean. Dashed line, midparent value; N=50 (on mung bean) or 100 (on lentil) females per cross type.
Crosses between the M line and the L2 or L3 lines also suggested additive inheritance of acceptance of lentil (fig. 2). In the L2×M crosses, egg counts again depended on cross type (F 3, 296=10.9, P<0.001), and post-hoc comparisons indicated that the mean number of eggs laid by L2 females exceeded the means in the other three cross types (P≤0.04). In turn, hybrids from the M♀×L2♂ cross laid more eggs than did M-line females (P<0.02), but the difference in mean egg number between L2♀×M♂ and M females was marginally non-significant (P=0.10). The number of eggs laid on lentil by hybrids did not depend on cross direction (P=0.89), and the percentage of hybrids laying at least one egg was again intermediate to those in the two parental lines (table 1).

Fig. 2. Mean number of eggs (+SE) laid on lentil by C. maculatus females from crosses between the L2 or L3 lines and the M line. The two L lines had spent 30 generations on lentil and all test females developed in the ancestral host, mung bean. Dashed lines, midparent value; N=75 females per cross type.
Acceptance of lentil also depended on cross type in crosses between the L3 and M lines (F 3, 296=17.5, P<0.001). In this case, mean egg number was completely consistent with additive inheritance (fig. 2). Post-hoc comparisons indicated that females from the four cross types fell into three statistically distinct groups: L3>L3♀×M♂=M♀×L3♂>M (P≤0.03 for significant differences between each parental line and each hybrid line, P=0.99 for the comparison of the two reciprocal hybrids). For both reciprocal crosses, the percentages of females that laid at least one egg were close to the midparent value of 79% (table 1).
Timing of host acceptance
During the first 24 h of exposure to lentil seeds after the 12-h mating period, L1 females laid more than three times as many eggs as M females (fig. 3). However, mean egg number among hybrids was not intermediate, as it was in the six-day assay. Instead, both types of hybrids appeared to be reluctant to lay eggs on lentil during the 24-h period and, therefore, resembled M females (fig. 3). Egg number varied significantly among cross types (F 3, 111=4.5, P<0.01), and post-hoc comparisons confirmed that the four groups fell into only two statistical subsets: L1>M♀×L1♂=M=L1♀×M♂. Although the mean number of eggs laid by hybrids in 24 h resembled the mean for the M line, the percentage of females accepting lentil at all, i.e. laying ≥1 egg, was more nearly intermediate, particularly among L1♀×M♂ females (table 2).
Table 2. Percentages of parental and hybrid C. maculatus females laying at least one egg on lentil seeds over 24 or 72 h1.
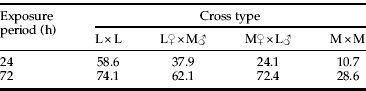
1 The L1 line had spent 50 generations on lentil and all test females developed in the ancestral host, mung bean. N=27–29 females per treatment.

Fig. 3. Mean number of eggs (+SE) laid on lentil by C. maculatus females from crosses between the L1 and M line. Seeds were provided for 24 or 72 h. The L1 line had spent 50 generations on lentil and all test females developed in the ancestral host, mung bean. N=27–29 females per treatment (▪, 24 h; □, 72 h).
Mean egg number also varied among cross types when females were allowed to oviposit on lentil for 72 h (F 3, 109=4.5, P=0.001). In contrast to the pattern observed after 24 h, hybrid behaviour after 72 h on lentil resembled the behaviour of L1 females (fig. 3). Mean egg counts of L1 females and both types of hybrid females were significantly greater than the mean for the M line (all P values <0.02), but there were no differences among the L1 and hybrid lines (all P values >0.85). Despite the three-fold difference in the time available for oviposition, M-line females provided lentil for 72 h laid a similar number of eggs as those on lentil for 24 h (fig. 3). Consequently, hybrid females from the L1♀×M♂ cross laid more than five times as many eggs as M females after 72 h. Percentages of females laying at least one egg also reflected the similar oviposition behaviour of hybrid and L1 females (table 2).
Discussion
Relatively few studies have used crosses to examine variation in host use among conspecific insect populations (Drès & Mallet, Reference Drès and Mallet2002; Futuyma, Reference Futuyma and Tilmon2008). The genetic mechanisms underlying such variation are of interest because they influence both the feasibility of host-mediated speciation and the likelihood of new pest biotypes (Matsubayashi et al., Reference Matsubayashi, Ohshima and Nosil2010). Available data suggest wide variation among insect species in the relative contributions of dominance, additivity and epistasis, as well as in the number and kinds of loci involved in host choice (Fox et al., Reference Fox, Stillwell, Amarillo-S, Czesak and Messina2004; Dworkin & Jones, Reference Dworkin and Jones2009; Xue et al., Reference Xue, Magalhães, Li and Yang2009; Michel et al., Reference Michel, Sim, Powell, Taylor, Nosil and Feder2010). For example, an effect of sex-linked genes on oviposition has been detected mainly among the Lepidoptera, in which there is female heterogamy (Janz, Reference Janz1998; Berenbaum & Feeny, Reference Berenbaum, Feeny and Tilmon2008).
This study examined the genetic basis of an experimental increase in host acceptance under controlled environmental conditions. Crosses between the three L lines and the M line suggested that alleles influencing oviposition on lentil are autosomal and inherited additively, with no evidence of cytoplasmic effects. Hybrid oviposition in the initial L1×M cross implied a possible paternal effect (fig. 1), but the effect of cross direction was not significant and no such trend was observed in the other crosses (fig. 2). We cannot determine the number of loci involved in host acceptance, but the apparent additive expression of relevant alleles produced substantially greater oviposition on lentil after <10 generations of quasi-natural selection (Messina et al., Reference Messina, Jones, Mendenhall and Muller2009b). Alleles promoting increased oviposition on the novel host (lentil) did not simultaneously decrease acceptance of the ancestral host (fig. 1 and Messina et al., Reference Messina, Jones, Mendenhall and Muller2009b), as is assumed in some models of speciation via host shifts (Feder & Forbes, Reference Feder, Forbes and Tilmon2008). A greater genetic predisposition to lay eggs on lentil also appeared to be host-specific, i.e. it could not be explained by a simple reduction in the threshold for accepting any type of seed (Messina & Jones, Reference Messina and Jones2009).
Although the long-term assays consistently indicated intermediate host acceptance by hybrids, our results also raise two caveats. Females from the L and M lines differed not only in lifetime fecundity on lentil; M-line females also commenced oviposition later (Messina et al., Reference Messina, Jones, Mendenhall and Muller2009b). When lentil acceptance was assayed soon after adult emergence, hybrid females were as reluctant to lay eggs on lentil as M females (fig. 3). Consideration of this assay alone, therefore, would suggest dominance toward the M-line parent. In contrast, the number of eggs laid by hybrid females after three days might produce the opposite conclusion. Estimating the inheritance of oviposition behaviour, therefore, may be highly sensitive to experimental protocol. It is also conceivable that the patterns observed in this study would be quite different if ancestral and novel hosts had been presented simultaneously in choice tests (Chiu & Messina, Reference Chiu and Messina1994; Fox et al., Reference Fox, Stillwell, Amarillo-S, Czesak and Messina2004).
A second implication of this study is that inheritance patterns observed from hybridization of divergent populations or related species need not reflect specific genetic changes within newly adapted populations (Coyne & Orr, Reference Coyne and Orr2004; Henniges-Janssen et al., Reference Henniges-Janssen, Schöfk, Reineke, Heckel and Groot2010). Messina & Slade (Reference Messina and Slade1997) examined the inheritance of host acceptance in crosses between the same Asian population studied here and a population from West Africa. In contrast to the additivity suggested by the present study, the oviposition behavior of both F1 and F2 hybrids was consistent with directional dominance toward the Asian population. Populations that have not shared fairly recent common ancestry may accumulate different genetic architectures with respect to host-use traits (Bieri & Kawecki, Reference Bieri and Kawecki2003). Even replicate selection lines may adapt to novel environments through somewhat different genetic mechanisms (Fox et al., Reference Fox, Wagner, Cline, Thomas and Messina2009). It, therefore, may be difficult to draw general conclusions about the means by which herbivorous insects adapt to new food plants.
More extensive crossing designs can be used to detect potential non-additive contributions to host acceptance in C. maculatus (Tucić & Šešlija, Reference Tucić and Šešlija2007; Fox et al., Reference Fox, Wagner, Cline, Thomas and Messina2009). In assays of larval performance, backcross data showed that the striking difference in survival between the L and M lines (1% vs. >90%) was influenced by both additive and non-additive genetic components (Messina & Jones, Reference Messina and Jones2011). Divergent selection lines produced by experimental evolution can also be subjected to a variety of genomic tools to identify specific genes and alleles involved in colonization of a novel host (Burke et al., Reference Burke, Dunham, Shahrestani, Thornton, Rose and Long2010; Stapley et al., Reference Stapley, Reger, Feulner, Smadja, Galindo, Ekblom, Bennison, Ball, Beckerman and Slate2010). To date, only a few insects have been subjected to genomic analyses with respect to adaptive changes in diet breadth (Matzkin et al., Reference Matzkin, Watts, Bitler, Machado and Markow2006; Berenbaum & Feeny, Reference Berenbaum, Feeny and Tilmon2008; Midamegbe et al., Reference Midamegbe, Vitalis, Malausa, Delava, Cros-Arteil and Streiff2011). Microarray analyses should make it feasible to understand the molecular basis of adaptation to novel legumes in C. maculatus (Chi et al., Reference Chi, Salzman, Balfe, Ahn, Sun, Moon, Yun, Lee, Higgins, Pittendrigh, Murdock and Zhu-Salzman2009).
Multiple studies have demonstrated that C. maculatus populations possess ample standing-genetic variation for rapid adaptation to novel hosts (Wasserman & Futuyma, Reference Wasserman and Futuyma1981; Fricke & Arnqvist, Reference Fricke and Arnqvist2007; Rova & Björklund, Reference Rova and Björklund2011). How such variation persists is unclear, particularly since it appears to be maintained even in populations that have been reared under uniform laboratory conditions for many generations (Messina, Reference Messina2004a). The mass-selection experiments that produced lentil-adapted lines in this study may not accurately mimic host shifts in natural beetle populations because a large number of founders was needed to establish self-sustaining populations on the new host (Messina et al., Reference Messina, Mendenhall and Jones2009a,Reference Messina, Jones, Mendenhall and Mullerb). Nevertheless, populations of C. maculatus are likely to encounter novel hosts repeatedly as a result of frequent human transport of infested seeds, and colonization of such hosts by a smaller number of beetles may similarly produce a new ‘biotype.’ It is perhaps not surprising that populations of this now-cosmopolitan insect show substantial differences in their ability to attack particular legume hosts (Wasserman, Reference Wasserman1986; Credland, Reference Credland, Fujii, Gatehouse, Johnson, Mitchell and Yoshida1990). Comprehensive genetic analyses of beetle populations and selection lines can serve as a model for understanding the evolution of diet breadth in pest insects.
Acknowledgements
We thank C. Christensen for technical assistance. This work was supported by the Utah Agricultural Experiment Station (paper no. 8340).







