Introduction
Angiostrongylus cantonensis, commonly known as rat lungworm, is a zoonotic parasite that infects various rat species. This parasite was first discovered in China over the past few years and its occurrences have been reported in Southeast Asia, Australia, the Pacific Islands, Caribbean and America (Teem et al., Reference Teem, Qvarnstrom and Bishop2013). The parasite's life cycle involves rats as definitive hosts and mollusks as intermediate hosts, while several large vertebrates are considered accidental or dead-end hosts including domestic animals and humans. Human infection occurs by accidental ingestion of infected raw or undercooked molluscs, or any paratenic host, harbouring the infective L3 larval form of the parasite (Chan et al., Reference Chan, Barratt, Roberts, Lee, Shea, Marriott, Chan, Harkness, Malik, Jones, Aghazadeh, Ellis and Stark2015). Clinical manifestations associated with this disease include nausea, vomiting, neck stiffness, headache and progresses to oeosinophilic meningitis and central nervous system angiostrongyliasis with permanent neurological injury or even death if left untreated (Pien and Pien, Reference Pien and Pien1999).
There are several factors that could influence the spread of A. cantonensis such as animal movement, human behaviour, environmental changes and their interactions. For example, the increased habitat diversification and land use change can facilitate the dispersal of the definitive and intermediate hosts of this parasite (Castillo and Paller, Reference Castillo and Paller2018). In the Philippines, three species of non-native rats including Rattus tanezumi (field rats), Rattus exulans (Pacific rat) and the brown or Norway rat Rattus norvegicus are known definitive hosts of A. cantonensis (Eduardo, Reference Eduardo1991; Tujan el at., Reference Tujan, Fontanilla and Paller2016; Castillo and Paller, Reference Castillo and Paller2018). The Philippine Mount Makiling Forest Reserve (MMFR) was recorded to have high mammal species diversity, including native and non-native rats which are the most diverse group in the area (LEAP, 2005). However, anthropogenic activities such as human settlements, tourism and agricultural activities have brought environmental degradation in this area. This has also led to the change in population dynamics of wildlife including non-native rat species (Macandog et al., Reference Magcale-Macandog, Balon, Engay, Nicopior, Luna and Cruz2011). Currently, there are no studies conducted in MMFR on pathogens involving rats. Thus, this study aimed at investigating the extent of A. cantonensis infection among non-native rats in the Philippine MMFR and its adjacent ecosystems and assesses some risk factors leading to possible zoonotic transmission.
Materials and methods
Study area
MMFR covers a total land area of 4224 hectares and lies between 14°06′ to 14°15′ north latitude and 121°09′ to 121°16′ east longitude. Its adjacent areas feature different terrestrial ecosystems such as agricultural, residential and agro-forest exhibiting different ecological patterns and processes (Fig. 1).
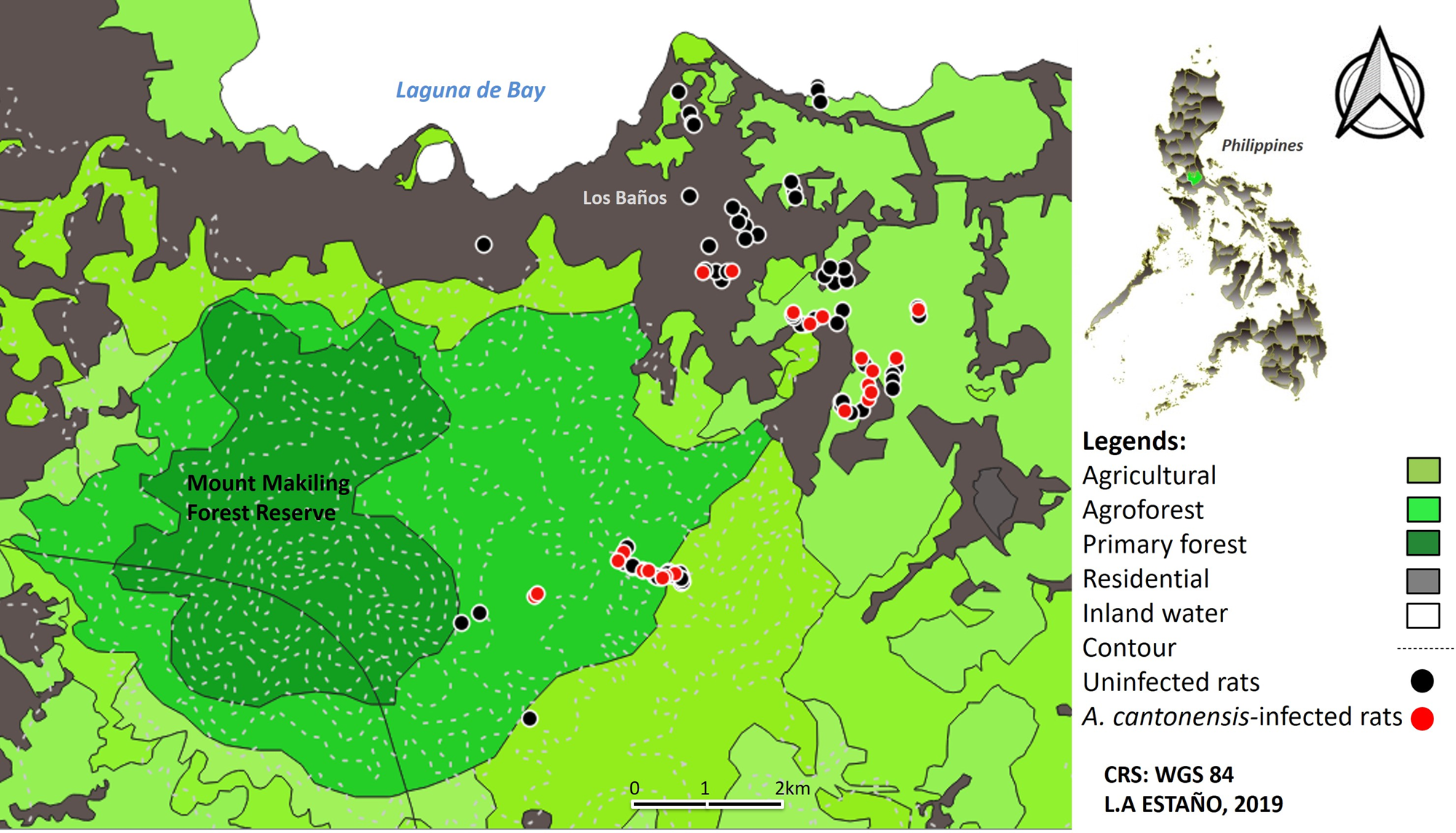
Fig. 1. Land use map and spatial distribution of Angiostrongylus cantonensis collected from non-native rats (Rattus spp.) in Philippine MMFR and adjacent areas.
Sampling design and collection of non-native rat samples
Prior to the collection of samples, the estimated sample size was determined using GPower 3 Statistical Software with a predicted prevalence of 80% and confidence level of 95% (Faul et al., Reference Faul, Erdfelder, Buchner and Lang2009). A total of 90 non-native rat samples of the genus (Rattus spp.) were collected as described in three different sampling sites at 30 rat samples each from residential, agricultural and agro-forest areas in MMFR and adjacent areas. Sampling was done during wet (July–December) and dry seasons (January–June). Ten sampling points from each site were randomly selected and three to five steel wire traps were baited with dried fish and fried coconut coated with peanut butter. The trapped rat samples were then brought to the laboratory and dissected for further examination.
Sample processing
With the approval of the UPLB Institutional Animal Care and Use Committee (IACUC), the rat samples were sedated with 5% isoflurane via drop jar method and were euthanized via cervical dislocation. Morphometrics such as sex, weight, total body length (TBL), fur colour, place of collection was recorded. Rat species were identified using the Synopsis of Philippine Mammal by Heaney et al. (Reference Heaney, Dolar, Balete, Esselstyn, Rickart and Sedlock2010) as a reference and with the aid of a rodent expert in the University. Age classes of rats were classified into juvenile and adult based on the body weight, TBL and reproductive status using mammae formula for female and appearance of testis in male. The rat samples were then dissected, and the heart and lungs examined for adult worms. Subsequently, the worms were collected and preserved in 70% ethanol. The collected adults’ worms were identified as A. cantonensis based on the appearance of copulatory bursa and barber pole for male and female worms, respectively. The heart and liver organs were also artificially digested with pepsin-HCL solution using a hot plate magnetic stirrer (C-MAG IKA, Germany) at 37°C for 1 h. The digested organs were filtered and placed in a Petri dish for microscopic examination of the larvae using a dissecting microscope (Leica, China).
Data analysis
The A. cantonensis prevalence and mean intensity among non-native rats were computed. Differences in prevalence among age, sex, season, habitat and species were analysed using chi-square test of independence. All data were tested for normality of distribution using SPSS v. 20.0 software (IBM Corp., 2011) applying 95% confidence level. Statistical computations were performed using following software's for Windows: Quantitative Parasitology (QP) version 3.0 and Predictive Analysis Software (PASW) version 18.0. The Shapiro–Wilk test was used to determine the normality of the data collected in the study. While Mann–Whitney U-test was used to compare the parasite prevalence between sexes of rats, ages and between dry and wet seasons. In addition, the Kruskal–Wallis test was used to compare the prevalence of parasite infection among sites and rat species. Parasite distribution in the rat host population was also determined using Poulin's discrepancy index (D) with v variance/mean ratio (s 2/m), and k value of negative binomial distribution. Poulin's aggregation index was used to estimate parasite aggregation which examine the value of variance and the mean of helminth parasite distribution, with values ranging from zero (no aggregation) to 1 (theoretical maximum).
Results
This study provides relevant information on A. cantonensis infection of non-native rats in the Philippine MMFR and its adjacent areas. Three species of non-native rats were captured including R. tanezumi, R. norvegicus and R. exulans. The rats were collected in three categorized ecological types of MMFR such as agro-forest, agricultural and residential. Among the rat species, R. tanezumi was ubiquitously found in all types of habitats, while R. norvegicus and R. exulans were only found in residential and agricultural areas, respectively.
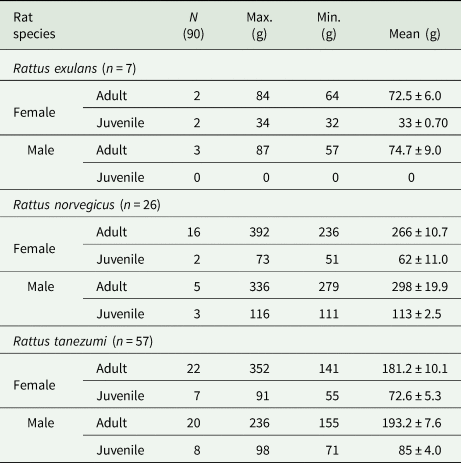
The three species differ remarkedly in adult measurements (Heaney et al., 2010). For R. norvegicus, the juveniles weighed <230 g with TBL of <423 mm, and its adults weighed >230 g with TBL of >423 mm. While the R. tanezumi juveniles weighed <140 g with TBL <350 mm, and its adults weighed >140 g with TBL >350 mm. Moreover, R. exulans juveniles weighed <35 g with TBL <240 mm, and adults weighed >35 g with TBL >240 mm. Moreover, defining appearance of testes in male and vaginal linings in females was also considered in classifying adult rats (Heaney et al., 2010). Classification of age classes of the samples is shown in Table 1.
Table 1. Three species of non-native rats, Rattus spp., collected from Philippine MMFR as categorized based on body weight
Angiostrongylus cantonensis infection of non-native rats in MMFR
Table 2 shows the summary of the 90 rat samples collected and examined for A. cantonensis infection. Results revealed an overall prevalence of 24.4% with a mean intensity of 4 ± 1.3 worms per rat. Among the rat species, R. exulans showed the highest prevalence with 42.9%, followed by R. tanezumi (29.8%), then R. norvegicus (7.7%). Also, mean intensity was observed highest in R. exulans (12 ± 7), followed by R. norvegicus (4 ± 3) and R. tanezumi (2 ± 0.7). Statistical analysis revealed a significant difference in prevalence among the rat species (P = 0.047), however no significant difference was observed in their mean intensities (P = 0.083).
Table 2. Prevalence and mean intensity of Angiostrongylus cantonensis in non-native rats (Rattus spp.) from the Philippine MMFR and adjacent areas (N = 90)
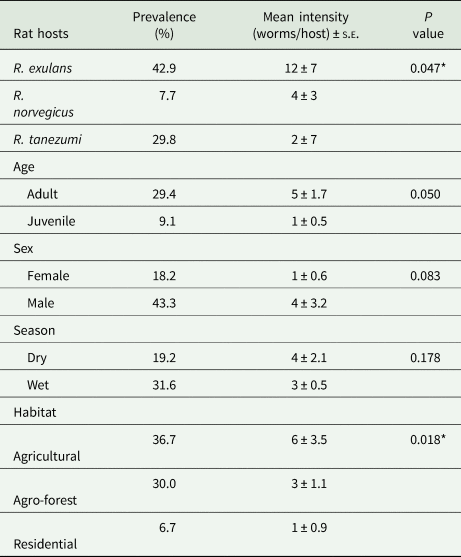
*Significant at P < 0.05.
Results also revealed that there was no significant difference in A. cantonensis prevalence (P = 0.083) and mean intensity (P = 0.063) between male (43.3%; four worms/rat) and female (18.3%; one worm/rat) rats. Among age classes, A. cantonensis prevalence in adult rats was significantly higher (29.4%) than the juvenile rats (9.1%) with a mean intensity of 5 ± 1.7 and 1 ± 0.5 worms/rats, respectively (P = 0.050).
Moreover, statistical analysis revealed no significant difference in the prevalence and mean intensity between dry (19.2%; 4 ± 2.1) and wet season (31.6%; 3 ± 0.5) P = 0.178, P = 0.272. For comparison among habitat types, the highest prevalence was observed in rats from agriculture (36.7%; 6 ± 3.5), followed by agro-forest (30%; 3 ± 1.1), then residential areas (6.71% ± 0.9). Differences of prevalence and mean intensity was found significant at P = 0.018 and P = 0.041, respectively.
Association of rat's total body length and weight with parasite intensity
Pearson's correlation analysis showed that there was a significant positive weak correlation between A. cantonensis parasite infection intensity with the body weight (r = 0.43; P = 0.008) and TBL (r = 0.30; P = 004) of rats, that is, as the rat's body length and weight increase the parasite intensity also increases, albeit moderately (Fig. 2).

Fig. 2. Scatter plot with a line of best fit showing the relationship between parasite intensity and body (A) weight and (B) length of non-native rats (Rattus spp.) in Philippine MMFR.
Distribution pattern of Angiostrongylus cantonensis in non-native rat host population
Poulin's aggregation index was used to estimate parasite aggregation which examines the value of variance and the mean of helminth parasite distribution. Helminth parasites showed an aggregated distribution of helminth parasites with the value of variance significantly greater than the mean (147.24 > 3.54). The resulting variance to mean ratio showed a significant departure from Poisson (random) distribution. Negative binomial distribution parameter was also used to estimate the degree of aggregation. The resulting measurement of aggregation (k) of the negative binomial revealed that helminth parasites are highly aggregated in distribution where the value of k is less than 1 (k = 0.09). Figure 3 shows the frequency distribution of A. cantonensis collected from non-native rats (Rattus spp.) which depicts a highly aggregated distribution.
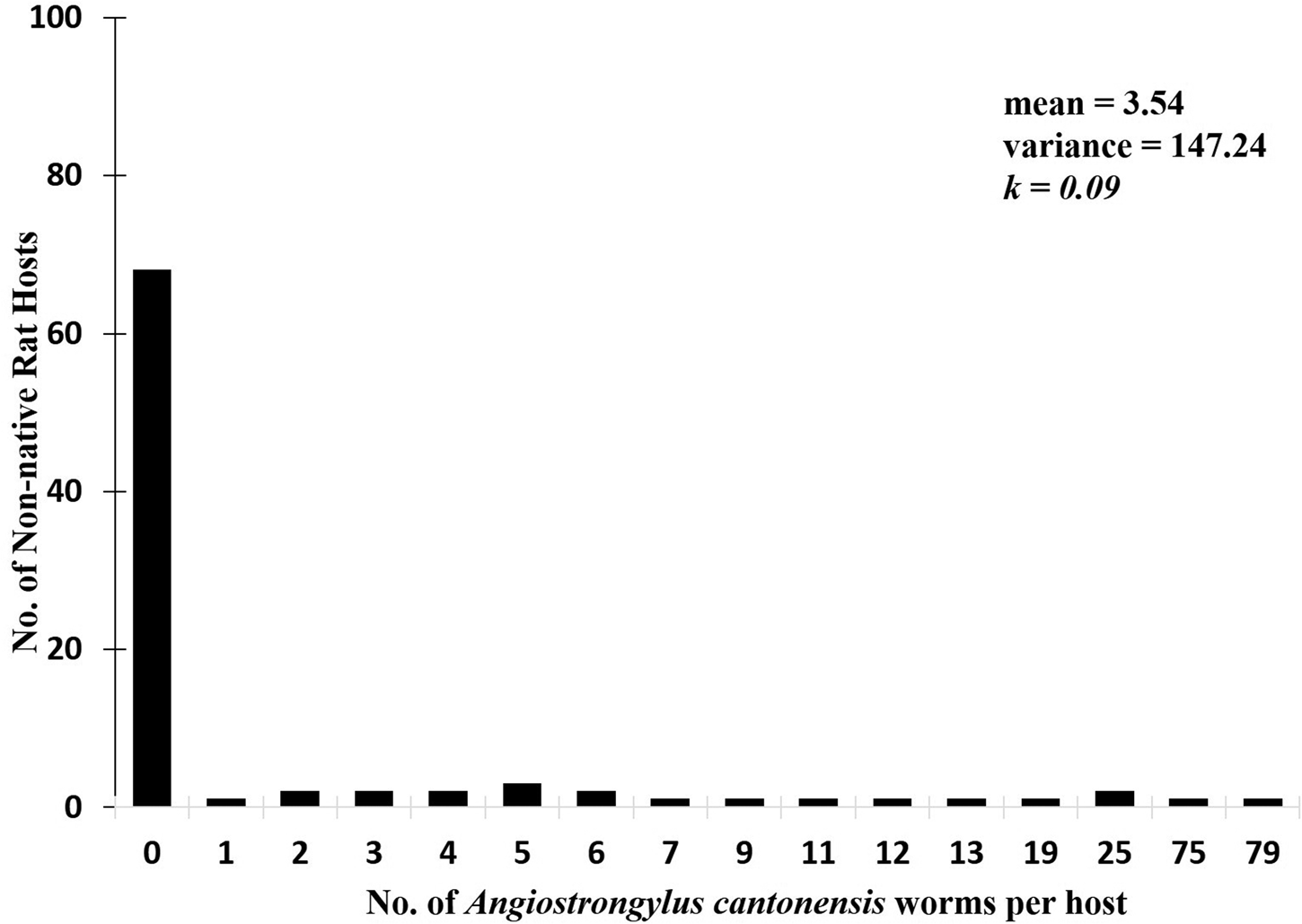
Fig. 3. Frequency distribution of A. cantonensis collected from non-native rats (Rattus spp.) in Philippine MMFR and adjacent areas showing a highly aggregated distribution of the parasite in its host population.
Discussion
Rats play an important role in the life cycle of A. cantonensis. The occurrence of non-native rats in various ecosystems in MMFR and its adjacent areas has several implications on food security and biodiversity threatening endemic species, economic losses and public health. In this study, R. tanezumi was ubiquitously found in all types of habitats. This may suggest that it can act as bridge species carrying pathogens across different habitats. While R. norvegicus and R. exulans were found only in residential and agricultural areas, respectively. Rattus norvegicus was the dominant host species in the residential areas associated with poor sanitation. This rat species is opportunistic and highly adaptive to a wide range of condition enabling them to successfully inhabit and survive in human habitations. Rattus exulans, on the other hand, is omnivorous and known to be a major agricultural pest. Its diet includes rice, coconut, maize, palms, sweet potatoes, gastropod and insects, which accounts for their abundance in agricultural areas. Habitat preferences of rats are also influenced by availability of food.
This study revealed an overall prevalence of A. cantonensis in non-native rats (Rattus spp.) at 24.44%. The highest prevalence rate was significantly higher in agricultural and agro-forest areas than in residential areas. The map in Fig. 1 demonstrates the spatial distribution of Angiostrongylus-infected rats across all habitats from agro-forest, agriculture, and residential areas. However, most of the infected rats were found in agricultural areas which could be attributed to the presence and distribution of the intermediate hosts of A. cantonensis. Angiostrongylus cantonensis require gastropod as intermediate hosts which abound in agriculture and agro-forest areas. Fontanilla and Wade (Reference Fontanilla and Wade2008) reported the presence through molecular identification of A. cantonensis in a snail terrestrial gastropod, Achatina fulica, in MMFR. This gastropod is also a common sight in backyard gardens in residential areas. The presence of this snail vector and rats in residential areas may pose health risks to humans. Also, some of the local people in the area, particularly farmers, were reportedly eating raw vegetables and snails posing transmission of the parasites to humans. This parasite is zoonotic causing angiostrongyliasis which can result to oeosinophilic meningitis. So far, there have been only a few and sporadic reported cases in the Philippines (Alto, Reference Alto2001; Hochberg et al., Reference Hochberg, Park, Blackburn, Sejvar, Gaynor, Chung, Leniek, Herwaldt and Efler2007), but the number could be higher. The problem lies in the lack of diagnosis for angiostrongyliasis in the country. Recent studies in the Philippines by Tujan et al. (Reference Tujan, Fontanilla and Paller2016) and Castillo and Paller (Reference Castillo and Paller2018) also reported significant high prevalences of A. cantonensis infection in R. tanezumi and R. norvegicus rat species in the northern rice granary of the Philippines.
Furthermore, the occurrence of A. cantonensis was associated with the various characteristics of the rat hosts. This study revealed that both male and female were vulnerable to infections. However, some studies reported that males were more active and travel significantly than females making them more likely to get infected (Tew and Macdonald, Reference Tew and Macdonald1994; Heaney et al., Reference Heaney, Balete, Rickart, Utzurrum and Gonzales1999; Soliman et al., Reference Soliman, Marzouk, Main and Montasser2001). Factors such as sex hormones, immune-competence, mobility, body size, home range and dispersal rates differ between sexes. For instance, in this study, it was observed that in agriculture and agro-forest habitats, male rats tend to travel to alternative habitats; whereas breeding females continued to forage in rice fields to meet the nutritional requirements needed to raise litters. These behaviours render both sexes vulnerable to infections.
In addition, infection of animals can also be attributed to the influence of abiotic factors such as environmental conditions. However, the current study observed no significant difference in prevalence of non-native rats captured during wet and dry seasons. O'connor et al. (Reference O'connor, Kahn and Walkden-Brown2007) reported that moisture and high humidity in wet seasons promote a better rate of growth and survival of parasite eggs and larvae in the environment. However, in a tropical country like the Philippines wherein weather conditions do not quite vary, it could be inferred that infective stages of parasites are likely to be present in the environment throughout the year. These environmental conditions could pose public health threats for parasites transmission in the area.
Moreover, results revealed a positive association between parasite intensity and rat's host body length and weight, that is, as the host body weight and length increase, the parasite infection also increases. This result can be attributed to the behaviour of a large-bodied host in relation to foraging and roaming activities which make them more available to acquiring infective stages of parasites in the environment. Moreover, older hosts have longer exposure to parasitic infections than younger hosts. A study by Winternitz et al. (Reference Winternitz, Yabsley and Altizer2012) reported that hosts with large body size and weight could forage more effectively which leads to increased exposure to parasite infection. The results also agreed with the findings of Castillo and Paller (Reference Castillo and Paller2018) who mentioned that large-bodied rat hosts have more niche and nutrients available for parasites. Also, heavier hosts are more likely to engage in breeding functions and could potentially trade off reproduction against immune defense causing them more susceptible for chronic infections (Perrin et al., Reference Perrin, Christe and Richner1996; Schwanz, Reference Schwanz2008).
Furthermore, the distribution of parasites in the rat hosts observed in this study appeared to be highly aggregated. It revealed that only a few members of the rat population exhibited high infection rates, and that majority had low to moderate infections. The overly dispersed characteristics of parasite populations may contribute to their survival, reproductive success, as well as survival of the rat host population. This pattern of distribution is commonly exhibited by parasites (Anderson and Gordon, Reference Anderson and Gordon1982; Calabrese et al., Reference Calabrese, Brunner and Ostfeld2011) and marked as spatial heterogeneities of infection (Morrill and Forbes, Reference Morrill and Forbes2012). This tends to stabilize host–parasite dynamics showing only a few infected among the susceptible hosts available for infection. Parasite aggregation is important to study in host–parasite ecology since it has a direct effect on host fitness and the stability of the host population dynamics.
Finally, the recovery of A. cantonensis from the non-native rat species has implications to public health. MMFR is a semi-pristine area, however, due to increased human activities, its foothill and adjacent areas are being utilized for agro-forestry and partly converted into residential and ecotourism areas. The movement and possible encroachment of native and non-native rats to human habitations could lead to possible spillover of zoonotic agents which could pose threats to public health.
Conclusion
This study provides important data on the extent of A. cantonensis infection of non-native rats in MMFR in the Philippines and its implication on zoonotic transmission. This may pose a potential health risk to other wild animals and humans residing in adjacent areas. Rodent control and eradication measures must be put into place to prevent rodent-borne disease outbreaks particularly angiostrongyliasis. Moreover, the study emphasized that the local people can be infected by the accidental ingestion of infective stage larvae found in contaminated water, soil and farm produce. Thus, interventions must be done in the local communities regarding hygiene and sanitation measures as well as good farming practices to lessen the risk of transmission. Understanding the transmission dynamics of A. cantonensis involving non-native rats, its intermediate and other reservoirs hosts, vis-à-vis human behaviour, climate and environmental changes are significant in the control and prevention of zoonotic health threats.
Acknowledgements
The authors would like to thank Makiling Center for Mountain Ecosystem (MCME) and the local government units (LGUs) of Los Baños, Laguna, Philippines for providing permits and assistance during sample collection. Also, the Animal Biology Division of the Institute of Biological Sciences, University of the Philippines Los Baños (UPLB) for the facilities and equipment.
Financial support
This work was supported by the Philippine Commission of Higher Education (CHED) for the research fund granted to Mr L.A. Estaño.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Prior to the conduct of study, the Institutional Animal Care and Use Committee (IACUC) of the University of the Philippines Los Baños approved the experimental protocol with assigned protocol number IBS-201601.








