INTRODUCTION
Gametocytes are the sexual stage of the malaria parasite which develop in red blood cells and are essential for transmission to the mosquito vector. It has long been recognized that patients treated for malaria should be cleared of gametocytes in order to prevent them transmitting the infection to others (Darling, Reference Darling1910). Gametocytes are also well recognized as a vulnerable stage in parasite development and therefore have become a key target in the parasite life cycle against which control strategies can be developed, for the ambitious goal of malaria elimination. There are currently no transmission-blocking vaccines or licensed antimalarial drugs with the potential to kill or inhibit gametocytes which are safe for community-wide use.
Historically antimalarial discovery and development programmes focused on asexual intra-erythrocytic stage parasites as these stages are responsible for the clinical symptoms of malaria. Compounds with activity against gametocytes were not seen as a priority and methods used to identify compounds that inhibit asexual stage parasite growth could not be used to evaluate the effect of compounds on gametocytes which are terminally differentiated. In contrast to asexual stage parasites gametocyte survival cannot be monitored using cell multiplication markers. In addition working with gametocyte stage parasites is technically challenging and until very recently methods for the production of large numbers of gametocytes suitable for use in in vitro screening programmes were not available. Fortunately, the renewed emphasis on the eradication of malaria that has occurred in recent years has highlighted the need for anti-gametocyte drugs and tools with which to identify them.
GAMETOCYTOGENESIS
Gametocytes are the only stage of the malaria parasite life cycle able to mediate transmission from the human host to the mosquito vector and in the case of Plasmodium falciparum, generally appear 10–14 days after the first appearance of asexual parasites in the host bloodstream. The process undertaken by the asexual erythrocytic stage parasites which leads to the development of these sexual stages within the host bloodstream is called gametocytogenesis. The onset of gametocytogenesis represents a transition period during which the parasite differentiates morphologically and biochemically from a life of asexual reproduction within the human host to one of sexual development within the mosquito vector. Asexual blood stage parasites and immature gametocytes (stage I–III) are thought to have similar metabolic profiles. As an example, both stages of development rely on haemoglobin digestion as a source of amino acids (Smalley, Reference Smalley1977; Hanssen et al. Reference Hanssen, Knoechel, Dearnley, Dixon, Le Gros, Larabell and Tilley2011) and are hence both likely to be vulnerable to drugs that affect haemoglobin metabolism. Mature (stage IV–V) gametocytes are less active metabolically than asexual parasites and are relatively insensitive to all currently used antimalarial drugs, with the exception of primaquine.
Only a small proportion of the asexual parasite population enters the sexual pathway and this number varies greatly between isolates and during the course of a natural infection (Ponnudurai et al. Reference Ponnudurai, Meuwissen, Leeuwenberg, Verhave and Lensen1982; Graves et al. 1984; Day et al. Reference Day, Karamalis, Thompson, Barnes, Peterson, Brown, Brown and Kemp1993; Dunyo et al. Reference Dunyo, Milligan, Edwards, Sutherland, Targett and Pinder2006; McKenzie et al. Reference McKenzie, Jeffery and Collins2007) (reviewed in Alano and Carter, Reference Alano and Carter1990). We still do not fully understand what triggers the switch from the asexual pathway to the production of the sexual stages but we do know that this is very flexible and sensitive to environmental stimuli (Carter and Miller, Reference Carter and Miller1979; Dyer and Day, Reference Dyer and Day2000; Peatey et al. Reference Peatey, Skinner-Adams, Dixon, McCarthy, Gardiner and Trenholme2009b).
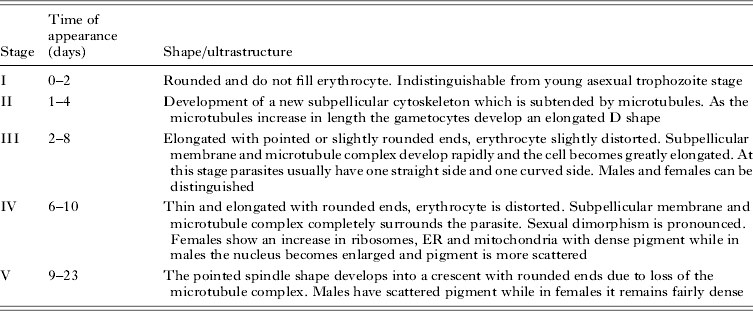
While most Plasmodium sexual stage parasites reach maturity within 27–30 h, P. falciparum gametocytes require 10–12 days to reach maturity and once mature can reportedly be carried by infected individuals for up to 55 days post clearance of asexual stages depending on treatment (Bousema et al. Reference Bousema, Okell, Shekalaghe, Griffin, Omar, Sawa, Sutherland, Sauerwein, Ghani and Drakeley2010). This maturation process can be divided into five distinct stages (I–V) based on light and electron microscopy (Table 1) (Hawking et al. Reference Hawking, Wilson and Gammage1971; Sinden, Reference Sinden1982). Stage I and early stage II gametocytes are indistinguishable from asexual stage parasites and only become morphologically distinguishable at late stage II–III (72–96 h post invasion) with mature stage V parasites exhibiting the characteristic crescent shape. Immature stage I–IV gametocytes sequester in host tissue including bone marrow and possibly the spleen (Smalley et al. Reference Smalley, Abdalla and Brown1981; Buffet et al. Reference Buffet, Safeukui, Deplaine, Brousse, Prendki, Thellier, Turner and Mercereau-Puijalon2011; Farfour et al. Reference Farfour, Charlotte, Settegrana, Miyara and Buffet2012). Mature stage V gametocytes are released into the peripheral circulation and can undergo further development only when taken up by the mosquito vector.
Table 1. Morphology of gametocytogenesis based on light (Hawking et al. Reference Hawking, Wilson and Gammage1971) and electron microscopy (Sinden, Reference Sinden1982)

It is known that numerous genetic and metabolic changes occur during gametocyte maturation and while we do not fully understand the biology of many of these changes it is clear that they impact on drug efficacy. While some drugs are active against immature gametocytes they are inactive against mature gametocytes. Targeting mature stage gametocytes is problematic as they are refractory to most antimalarial drugs. Transmission-blocking drugs can exert effects by both directly killing gametocytes within humans or by effecting viability or development within the vector.
An unintended effect
Field studies show that even if a drug is effective against immature gametocytes this does not always result in a reduction of mature infective gametocyte stages, demonstrating that many factors affect a patient's gametocytaemia. These include the time between acquisition of infection and treatment, the choice of drug, the dose administered and asexual parasitemia. These factors all influence the number of gametocytes circulating in a patient's bloodstream and therefore their propensity to transmit malaria. Importantly there is evidence that some drugs, such as amodiaquine and sulfadoxine–pyrimethamine, can stimulate the production of gametocytes in the field and this impacts the emergence of antimalarial resistance (Barnes et al. Reference Barnes, Little, Mabuza, Mngomezulu, Govere, Durrheim, Roper, Watkins and White2008; Sowunmi et al. Reference Sowunmi, Balogun, Gbotosho and Happi2008). Furthermore, sub-optimal concentrations of many antimalarials can increase the production of gametocytes in vitro (Buckling et al. Reference Buckling, Ranford-Cartwright, Miles and Read1999; Peatey et al. Reference Peatey, Skinner-Adams, Dixon, McCarthy, Gardiner and Trenholme2009b; Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011). A renewed focus on reducing gametocytaemia as well as asexual stage parasites during treatment is therefore imperative to the efforts to reduce malaria transmission.
CURRENTLY USED DRUGS WITH ACTIVITY AGAINST MATURE GAMETOCYTES
Primaquine
Primaquine is currently the only licensed antimalarial that is effective against mature stage V P. falciparum gametocytes in vivo. It is an 8-aminoquinoline that is active against gametocytes of all species. Primaquine is also effective against the exo-erythrocytic stages of Plasmodium vivax and Plasmodium ovale (Alving et al. Reference Alving, Arnold and Robinson1952; Miller et al. Reference Miller, Marcus and Cameron1965). However it has only weak activity against asexual stage parasites and so must be used in combination with other schizonticides (Rieckmann et al. Reference Rieckmann, McNamara, Frischer, Stockert, Carson and Powell1968).
Primaquine treatment reduces gametocytaemia and results in reduced gametocyte carriage times when given singularly or with a partner drug (Burgess and Bray, Reference Burgess and Bray1961; Rieckmann et al. Reference Rieckmann, McNamara, Frischer, Stockert, Carson and Powell1968; Kamtekar et al. Reference Kamtekar, Gogtay, Dalvi, Karnad, Chogle, Aigal and Kshirsagar2004; Pukrittayakamee et al. Reference Pukrittayakamee, Chotivanich, Chantra, Clemens, Looareesuwan and White2004; Lederman et al. Reference Lederman, Maguire, Sumawinata, Chand, Elyazar, Estiana, Sismadi, Bangs and Baird2006; Shekalaghe et al. Reference Shekalaghe, Drakeley, Gosling, Ndaro, van Meegeren, Enevold, Alifrangis, Mosha, Sauerwein and Bousema2007; Bousema et al. Reference Bousema, Okell, Shekalaghe, Griffin, Omar, Sawa, Sutherland, Sauerwein, Ghani and Drakeley2010; Smithuis et al. Reference Smithuis, Kyaw, Phe, Win, Aung, Oo, Naing, Nyo, Myint, Imwong, Ashley, Lee and White2010; Arango et al. Reference Arango, Upegui and Carmona-Fonseca2012; Kolaczinski et al. Reference Kolaczinski, Leslie, Ali, Durrani, Lee, Barends, Beshir, Ord, Hallett and Rowland2012). The drug has been shown to block transmission in animal models (Coleman et al. Reference Coleman, Clavin and Milhous1992; Portela et al. Reference Portela, Moreira, Valente, Constantino, Iley, Pinto, Rosa, Cravo and do Rosario1999; Vale et al. Reference Vale, Nogueira, do Rosario, Gomes and Moreira2009; Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012) and human trials (Chen et al. Reference Chen, Li, Guo, He, Fu, Fu and Song1994; reviewed in: Jeffery et al. Reference Jeffery, Young and Eyles1956; Rieckmann et al. Reference Rieckmann, McNamara, Frischer, Stockert, Carson and Powell1968; Graves et al. Reference Graves, Gelband and Garner2012).
The mode of action of primaquine against P. falciparum gametocytes remains poorly defined but is thought to be mediated primarily by metabolites as its activity against mature stage gametocytes in vivo does not directly translate to the in vitro situation (Peatey et al. Reference Peatey, Andrews, Eickel, MacDonald, Butterworth, Trenholme, Gardiner, McCarthy and Skinner-Adams2009a; Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011) . In addition, only a small percentage of primaquine is excreted unchanged (Greaves et al. Reference Greaves, Price-Evans, Gilles and Baty1979; Strother et al. Reference Strother, Fraser, Allahyari and Tilton1981; Mihaly et al. Reference Mihaly, Ward, Edwards, Orme and Breckenridge1984; Baird and Hoffman, Reference Baird and Hoffman2004). Little is known about the metabolism of primaquine or the identity of the derived metabolite(s) responsible for its activity against gametocytes. Primaquine contains many biologically reactive groups suggesting that its metabolism is likely to be complex (Vasquez-Vivar and Augusto, Reference Vasquez-Vivar and Augusto1992). Available data indicate that primaquine is metabolized into highly reactive unstable products (Strother et al. Reference Strother, Allahyari, Buchholz, Fraser and Tilton1984) and only two metabolites, carboxyprimaquine and 6-methoxy-8-aminoquinoline have been isolated in humans in vivo (Baty et al. Reference Baty, Price Evans and Robinson1975; Mihaly et al. Reference Mihaly, Ward, Edwards, Orme and Breckenridge1984), but other as yet unidentified metabolites are also known to exist (Mihaly et al. Reference Mihaly, Ward, Edwards, Orme and Breckenridge1984). As the isolation of the active primaquine metabolites in humans has been unsuccessful, alternative systems have been used and a range of primaquine metabolites have been isolated from a variety of organisms including bacteria, yeast, rat livers, mice, dogs and monkeys (Strother et al. Reference Strother, Fraser, Allahyari and Tilton1981; Hufford et al. Reference Hufford, Clark, Quinones, Baker and McChesney1983, Reference Hufford, Baker, McChesney and Clark1986; Clark et al. Reference Clark, Baker and McChesney1984a, Reference Clark, Hufford, Gupta, Puri and McChesneyb; Strother et al. Reference Strother, Allahyari, Buchholz, Fraser and Tilton1984; Baker et al. Reference Baker, Yarber, Nanayakkara, McChesney, Homo and Landau1990; Ni et al. Reference Ni, Xu and Wang1992; Portela et al. Reference Portela, Moreira, Valente, Constantino, Iley, Pinto, Rosa, Cravo and do Rosario1999). Recently Avula et al. (Reference Avula, Tekwani, Chaurasiya, Nanayakkara, Wang, Khan, Adelli, Sahu, Elsohly, McChesney, Khan and Walker2013) developed a method of chromatography enabling the metabolites of primaquine produced after incubation with human hepatocytes to be analysed in a more sensitive way. Fourteen primaquine metabolites were detected and most interestingly carboxyprimaquine, previously thought to be inactive and benign, was shown to be further metabolized into a quinone-imine metabolite that is likely to be antimalarial and toxic (Avula et al. Reference Avula, Tekwani, Chaurasiya, Nanayakkara, Wang, Khan, Adelli, Sahu, Elsohly, McChesney, Khan and Walker2013).
Only a proportion of identified primaquine metabolites have been assessed for activity against parasites (Bates et al. Reference Bates, Meshnick, Sigler, Leland and Hollingdale1990). When the effects of primaquine metabolites on Plasmodium berghei exo-erythrocytic schizonts were assessed in vitro it was clear that many metabolites are more active than primaquine, particularly those which are hydroxylated (Bates et al. Reference Bates, Meshnick, Sigler, Leland and Hollingdale1990). However extrapolating these results into an in vivo setting against P. falciparum gametocytes is difficult; therefore direct evidence linking a specific metabolite to anti-gametocyte activity is still incomplete.
The parasite mitochondria have been implicated as the site of primaquine action with evidence coming from in vitro morphological studies with P. falciparum gametocytes and the exo-erythrocytic forms of the avian malaria parasite Plasmodium fallax, as well as an in vivo study examining murine exo-erythrocytic schizonts after treatment (Beaudoin and Aikawa, Reference Beaudoin and Aikawa1968; Aikawa and Beaudoin, Reference Aikawa and Beaudoin1970; Boulard et al. Reference Boulard, Landau, Miltgen, Ellis and Peters1983; Lanners, Reference Lanners1991). These studies demonstrate that primaquine treatment causes morphological and physiological changes to the mitochondria resulting in thickened mitochondrial membranes that are often swollen and contain multiple layers (Beaudoin and Aikawa, Reference Beaudoin and Aikawa1968; Aikawa and Beaudoin, Reference Aikawa and Beaudoin1970; Boulard et al. Reference Boulard, Landau, Miltgen, Ellis and Peters1983; Lanners, Reference Lanners1991). An action within mitochondria may explain why primaquine is more potent against mid to mature stage gametocytes than against asexual stage parasites. Initially it was thought that as gametocytes develop the number of mitochondria increase with mature stage parasites possessing 4–6 mitochondria compared with only 1–2 in asexual stage parasites (Krungkrai et al. Reference Krungkrai, Prapunwattana and Krungkrai2000). However, recent evidence suggests that one mitochondrion is present which undergoes significant segmentation forming a highly lobed structure (Okamoto et al. Reference Okamoto, Spurck, Goodman and McFadden2009). In any case, the gametocyte mitochondria undergo significant changes during maturation (Sinden, Reference Sinden1982; Kato et al. Reference Kato, Tanabe, Miki, Ichimori and Waki1990) including changes in the transcriptome of these organelles (Young et al. Reference Young, Fivelman, Blair, de la Vega, Le Roch, Zhou, Carucci, Baker and Winzeler2005). It has been hypothesized that primaquine effects mitochondrial function by altering electron transport (Vaidya et al. Reference Vaidya, Lashgari, Pologe and Morrisey1993). Hydroxylated primaquine metabolites may also inflict extensive oxidative damage to the parasite as they are known to increase the oxidative stress on human red blood cells (Baird et al. Reference Baird, McCormick and Canfield1986; Fletcher et al. Reference Fletcher, Barton and Kelly1988; Ganesan et al. Reference Ganesan, Tekwani, Sahu, Tripathi and Walker2009), a factor that contributes to the toxicity of primaquine.
The fact that primaquine impacts on gametocytes and transmission and has a role to play in malaria elimination programmes is well recognized. Indeed, the World Health Organization currently recommends that a single dose of primaquine be administered after clearance of P. falciparum asexual stages in order to reduce subsequent malaria transmission (World Health Organization, 2011). However the effectiveness of this regimen in reducing transmission has recently been questioned (Graves et al. Reference Graves, Gelband and Garner2012).
Unfortunately there are known side-effects to treatment with primaquine that decreases its usefulness. It is known to cause haemolytic anemia and methaemoglobin formation (Anders et al. Reference Anders, Chung and Theoharides1988; Bolchoz et al. Reference Bolchoz, Budinsky, McMillan and Jollow2001) particularly in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD) (Alving et al. Reference Alving, Carson, Flanagan and Ickes1956) for whom primaquine is contraindicated. G6PD deficiency is highly prevalent in malaria endemic areas and severely limits the use of primaquine (Howes et al. Reference Howes, Piel, Patil, Nyangiri, Gething, Dewi, Hogg, Battle, Padilla, Baird and Hay2012). So while primaquine is an effective gametocytocidal drug, its potential negative effects on health mean that continued research to find a less toxic alternative with similar efficacy against gametocytes and exo-erythrocytic parasites is imperative.
Bulaquine
Bulaquine is an 8-aminoquinoline analogue of primaquine; it was formerly known as CDRI80/53 and is currently only licensed for use in India where it is used for radical cure of P. vivax. Data show that bulaquine has gametocytocidal activity in vivo against both P. falciparum and Plasmodium cynomolgi (Puri and Dutta, Reference Puri and Dutta2005; Gogtay et al. Reference Gogtay, Kamtekar, Dalvi, Mehta, Chogle, Aigal and Kshirsagar2006), it also appears to be less toxic than primaquine in G6PD-deficient individuals and as such is deserving of further evaluation.
Artemisinin derivatives
A crude extract of the plant Artemisia annua has been for used for centuries in traditional Chinese medicine to treat many illnesses including malaria but the full extent of its antimalarial properties were only determined in the late 1970s (Jiang et al. Reference Jiang, Li, Guo, Kong and Arnold1982). Artemisinin, the active constituent, is relatively cheap to manufacture but it has physical properties such as poor bioavailability that limit its effectiveness. To circumvent this problem a number of derivatives including water-soluble artesunate or oil, soluble artemether and arteether (reviewed in Cui and Su, Reference Cui and Su2009) have been developed. Artemisinin and other endoperoxide derivatives have been shown to reduce gametocyte carriage in a number of field studies (Hatz et al. Reference Hatz, Abdulla, Mull, Schellenberg, Gathmann, Kibatala, Beck, Tanner and Royce1998; von Seidlein et al. Reference von Seidlein, Bojang, Jones, Jaffar, Pinder, Obaro, Doherty, Haywood, Snounou, Gemperli, Gathmann, Royce, McAdam and Greenwood1998; Priotto et al. Reference Priotto, Kabakyenga, Pinoges, Ruiz, Eriksson, Coussement, Ngambe, Taylor, Perea, Guthmann, Olliaro and Legros2003; Ndayiragije et al. Reference Ndayiragije, Niyungeko, Karenzo, Niyungeko, Barutwanayo, Ciza, Bosman, Moyou-Somo, Nahimana, Nyarushatsi, Barihuta, Mizero, Ndaruhutse, Delacollette, Ringwald and Kamana2004; Bousema et al. Reference Bousema, Okell, Shekalaghe, Griffin, Omar, Sawa, Sutherland, Sauerwein, Ghani and Drakeley2010). The introduction of artemisinin for the treatment of malaria in Thailand resulted in a decline in gametocyte carriage and cases of clinical malaria (Price et al. Reference Price, Nosten, Luxemburger, ter Kuile, Paiphun, Chongsuphajaisiddhi and White1996). Reduced transmissibility of P. falciparum after treatment with an artemisinin derivative has also been confirmed in a group of children treated with artesunate (Targett et al. Reference Targett, Drakeley, Jawara, von Seidlein, Coleman, Deen, Pinder, Doherty, Sutherland, Walraven and Milligan2001). In this study a low level of gametocyte carriage correlated directly with a reduction in the number of infected mosquitoes (Targett et al. Reference Targett, Drakeley, Jawara, von Seidlein, Coleman, Deen, Pinder, Doherty, Sutherland, Walraven and Milligan2001). Treatment of stage V P. falciparum gametocytes with artesunate in vitro prior to membrane feeding also reduces subsequent oocyst development in Anopheles dirus (Chotivanich et al. Reference Chotivanich, Sattabongkot, Udomsangpetch, Looareesuwan, Day, Coleman and White2006).
The effects of artemisinin derivatives on P. falciparum gametocytaemia during infection could stem from the rapid clearance of asexual stage parasites, thereby reducing the potential for gametocyte formation, activity against immature sequestered gametocytes and possibly inhibition of mature gametocytes. Artemisinin has effects on stage I–III gametocytes in vitro with significant inhibition seen when treated with 0·1 and 1 μ m drug preparations (Kumar and Zheng, Reference Kumar and Zheng1990).
The activity of artemisinin derivatives against P. falciparum gametocytes has also been demonstrated in vitro. Many artemisinin derivatives are rapidly converted to dihydroartemisinin (DHA) in vivo. DHA has been shown to inhibit stage I–III gametocytes by 50⩾75% at concentrations of 12–120 nmin vitro (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011; Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011). It has also been shown to be active against mature gametocytes (25–50% inhibition at concentrations of 24 and 120 nm). However, other reports of the activity of DHA against mature gametocytes were reported to be lower (40% inhibition at 10 μ m) (Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012). This activity would also fit well with reports suggesting that 2–10 μ m artesunate is required to inhibit mature gametocytes by 50% (Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012; Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012). Artemisinin appears to be inactive against stage IV to V gametocytes at concentrations as high as 1 μ min vitro (Kumar and Zheng, Reference Kumar and Zheng1990). However, the clinical relevance of these in vitro data are hard to predict given the short half-life of these drugs and the metabolism of artemisisin and artesunate into DHA. Interestingly, while concentrations of the artemisinin drugs may wane quickly after administration, plasma concentrations of DHA approximating 9 μ m have been achieved (reviewed in Morris et al. Reference Morris, Duparc, Borghini-Fuhrer, Jung, Shin and Fleckenstein2011). These concentrations are within reason to have some effect on gametocytes given the in vitro data, however gametocytes still form and mature in patients after treatment with artesunate who were negative for gametocytaemia at the start of treatment (Targett et al. Reference Targett, Drakeley, Jawara, von Seidlein, Coleman, Deen, Pinder, Doherty, Sutherland, Walraven and Milligan2001).
The mode of action of artemisinin and its derivatives is still not well understood but is dependent on their endoperoxide dioxygen bridge (Cumming et al. Reference Cumming, Ploypradith and Posner1997). It is believed that the endoperoxides accumulate in parasite compartments such as the cytosol and digestive vacuole and presence of haeme generates hydroperoxide, a powerful oxidizing agent which releases carbon-centred free radicals (Haynes and Vonwiller, Reference Haynes and Vonwiller1994; Posner et al. Reference Posner, Oh, Wang, Gerena, Milhous, Meshnick and Asawamahasadka1994) and other metabolites (Butler et al. Reference Butler, Gilbert, Hulme, Irvine, Renton and Whitwood1998). These metabolites bind with membrane-associated proteins (reviewed in Meshnick et al. Reference Meshnick, Taylor and Kamchonwongpaisan1996), causing damage to parasite organelles such as the mitochondria, food vacuole and the nuclear envelope (reviewed in Meshnick, Reference Meshnick2002) although how this occurs is unclear. Most likely the free radicals attack particular proteins that effect structural function (reviewed in Price and Douglas, Reference Price and Douglas2009). Gametocytes continue to digest haemoglobin and produce haeme until they reach stage IV (Hanssen et al. Reference Hanssen, Knoechel, Dearnley, Dixon, Le Gros, Larabell and Tilley2011), at which stage digestion ceases. This could reflect the more potent effects of artemisinin and derivatives on immature vs mature stage gametocytes.
DRUGS THAT ARE ACTIVE AGAINST IMMATURE GAMETOCYTES
Unlike mature gametocytes which are relatively inactive metabolically, immature gametocytes are similar to asexual stage parasites in that they are still metabolically active and are actively digesting haemoglobin. It is therefore not surprising that many drugs which are effective against asexual stage parasites also kill immature gametocytes.
Chloroquine
Chloroquine inhibits immature stage I–III gametocytes (IC50 42 nm), likely affecting their ability to digest haemoglobin (Smalley, Reference Smalley1977; Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011). However, it has no effect on mature stage (IV and V) gametocytes in vitro (Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012) or in vivo (Sowunmi and Fateye, Reference Sowunmi and Fateye2003a; Sowunmi et al. Reference Sowunmi, Fateye, Happi, Gbotosho and Oduola2003b). In addition, chloroquine can increase gametocytogenesis when given at sub-curative concentrations in vitro (Bucking et al. Reference Buckling, Ranford-Cartwright, Miles and Read1999; Peatey et al. Reference Peatey, Skinner-Adams, Dixon, McCarthy, Gardiner and Trenholme2009b) and in animal models (Buckling et al. Reference Buckling, Taylor, Carlton and Read1997). Chloroquine treatment has also been associated with an increase in infectivity of patients to mosquitoes (Wilkinson et al. Reference Wilkinson, Noeypatimanondh and Gould1976).
Mefloquine
Mefloquine is a synthetic analogue of quinine that is active against stage I–II immature gametocytes in vitro at an IC50 of 95 nm, similar to the asexual stage IC50in vitro at between 36–80 nm (Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011). Mefloquine has activity against mature stages of gametocyte development in vitro, however at far higher concentrations than the asexual IC50in vitro. Studies published recently report a 50–60% inhibition of stage V gametocytes at 5–10 μ m (Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012; Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012) and approximately 20% inhibition at 10 μ m against mixed stages III–V (Tanaka and Williamson, 2011). There is little evidence that mefloquine is active against gametocytes in vivo (Price et al. Reference Price, Nosten, Luxemburger, ter Kuile, Paiphun, Chongsuphajaisiddhi and White1996; Suputtamongkol et al. Reference Suputtamongkol, Chindarat, Silpasakorn, Chaikachonpatd, Lim, Chanthapakajee, Kaewkaukul and Thamlikitkul2003; Sowunmi et al. Reference Sowunmi, Nkogho, Okuboyejo, Gbotosho, Happi and Adewoye2009).
Amodiaquine and piperaquine
Amodiaquine in the form of its active metabolite desethylamodiaquine is active against immature stage I–II gametocytes in vitro at clinically relevant concentrations (30 nm) (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011), however in vivo efficacy against mature gametocytes has not been demonstrated, with some studies finding the drug actually causes an increase in gametocytaemia (Sowunmi et al. Reference Sowunmi, Balogun, Gbotosho, Happi, Adedeji and Fehintola2007, Reference Sowunmi, Balogun, Gbotosho and Happi2008).
Piperaquine also shows moderate efficacy in vitro against immature gametocyte stages at concentrations as low as 17 nm (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011). Data on the effect of piperaquine against mature gametocytes in the field is limited; however studies where piperaquine was used in combination with DHA did not indicate a significant effect on the clearance of gametocytaemia in patients (Grande et al. Reference Grande, Bernasconi, Erhart, Gamboa, Casapia, Delgado, Torres, Fanello, Llanos-Cuentas and D'Alessandro2007; Mens et al. Reference Mens, Sawa, van Amsterdam, Versteeg, Omar, Schallig and Kager2008). The mechanism of action of amodiaquine and piperaquine is poorly defined. However as a result of structural similarities to chloroquine and additional factors such as evidence of cross resistance (Childs et al. Reference Childs, Boudreau, Milhous, Wimonwattratee, Pooyindee, Pang and Davidson1989; Basco and Le Bras, Reference Basco and Le Bras1993; Ochong et al. Reference Ochong, van den Broek, Keus and Nzila2003) and similar effects on parasite morphology (Sachanonta et al. Reference Sachanonta, Chotivanich, Chaisri, Turner, Ferguson, Day and Pongponratn2011), both drugs are thought to effect haemoglobin digestion within the parasite (Ginsburg et al. Reference Ginsburg, Famin, Zhang and Krugliak1998). Such a mechanism of action would also fit well with their activity against immature gametocytes that still digest haemoglobin.
Pyronaridine
Pyronaridine has been used as an antimalarial in China for more than 30 years (Croft et al. Reference Croft, Duparc, Arbe-Barnes, Craft, Shin, Fleckenstein, Borghini-Fuhrer and Rim2012) and is currently being assessed for its efficacy as a partner drug for artemisinin, with a phase III clinical trial completed in 2009 (Tshefu et al. Reference Tshefu, Gaye, Kayentao, Thompson, Bhatt, Sesay, Bustos, Tjitra, Bedu-Addo, Borghini-Fuhrer, Duparc, Shin and Fleckenstein2010). The drug is effective against chloroquine-sensitive and -resistant field isolates in vitro (Pradines et al. Reference Pradines, Mabika Mamfoumbi, Parzy, Owono Medang, Lebeau, Mourou Mbina, Doury and Kombila1999; Kurth et al. Reference Kurth, Pongratz, Belard, Mordmuller, Kremsner and Ramharter2009; Okombo et al. Reference Okombo, Kiara, Mwai, Pole, Ohuma, Ochola and Nzila2011) and clears parasites in patients with uncomplicated P. falciparum infections at a rate equivalent to artermether-lumerfantrine (Tshefu et al. Reference Tshefu, Gaye, Kayentao, Thompson, Bhatt, Sesay, Bustos, Tjitra, Bedu-Addo, Borghini-Fuhrer, Duparc, Shin and Fleckenstein2010).
Pyronaridine has been assessed both in vitro and in vivo for activity against P. falciparum gametocytes. The drug is moderately effective against stage I–II gametocytes in vitro demonstrating between 25–50% inhibition at the asexual IC50 concentration of 17 nm (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011) and has an IC50 of between 6–20 nm against stage II–III gametocytes (Chavalitshewinkoon-Petmitr et al. Reference Chavalitshewinkoon-Petmitr, Pongvilairat, Auparakkitanon and Wilairat2000). However, reports on the effect of pyronaridine against stage V gametocytes in vitro are conflicting. Some studies report a significant effect against stage V gametocytes in vitro (IC50 280 nm) (Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012), while others do not demonstrate any effect at clinically relevant concentrations (IC50 3·2 μ m, approximately 700 times the asexual IC50) (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011; Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012). These studies used a variety of assay methods including microscopy (Chavalitshewinkoon-Petmitr et al. Reference Chavalitshewinkoon-Petmitr, Pongvilairat, Auparakkitanon and Wilairat2000), a gametocyte-specific GFP-luciferase reporter (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011) and an ATP-based bioluminescent assay (Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012; Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012) which may account for this variability.
A limited number of field studies have assessed the effect of pyronaridine on stage V gametocytes but no effect on gametocyte carriage was seen when patients were treated with this drug in Cameroon (Ringwald et al. Reference Ringwald, Meche and Basco1999). Furthermore, no difference in gametocyte clearance times were seen when patients were treated with either artermether-lumerfantrine or pyronaridine-artesunate (Tshefu et al. Reference Tshefu, Gaye, Kayentao, Thompson, Bhatt, Sesay, Bustos, Tjitra, Bedu-Addo, Borghini-Fuhrer, Duparc, Shin and Fleckenstein2010). Current evidence suggests pyronaridine is likely to have limited effect on gametocytes in vivo and therefore its use with a gametocytocidal drug such as primaquine may be warranted.
Atovaquone
Atovaquone is effective in vitro against stage (I–II) gametocytes, although the extent of this effect varies with different methods of assessment (Fleck et al. Reference Fleck, Pudney and Sinden1996; Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011; Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011). The drug has not shown efficacy against mature stage gametocytes in vitro (Fleck et al. Reference Fleck, Pudney and Sinden1996; Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011). However, atovaquone-proguanil (Malarone) can clear gametocytes at a faster rate than chloroquine (Enosse et al. Reference Enosse, Butcher, Margos, Mendoza, Sinden and Hogh2000). Interestingly, treatment of P. falciparum mature gametocytes with serum taken from patients taking atovaquone does affect transmission to mosquitoes, although this may reflect inhibition of stages within the mosquito (Butcher and Sinden, Reference Butcher and Sinden2003).
Atovaquone is a hydroxynathquinone that is thought to inhibit the mitochondrial respiratory chain and indirectly inhibit de novo pyrimidine synthesis, a process that is essential for the replication of DNA within the parasite (Fry and Pudney, Reference Fry and Pudney1992; Ittarat et al. Reference Ittarat, Asawamahasakda and Meshnick1994). As gametocytes are terminally differentiated, DNA replication is thought not to occur or is significantly reduced until exflagellation of the male gametocyte (Canning and Sinden, Reference Canning and Sinden1975; Sinden et al. Reference Sinden, Canning, Bray and Smalley1978; Raabe et al. Reference Raabe, Billker, Vial and Wengelnik2009). RNA replication is thought to continue until day 6 of maturation (reviewed in Baker, Reference Baker2010) representing a possible target for atovaquone within the immature stages of gametocyte development.
DRUGS IN CLINICAL DEVELOPMENT WITH ACTIVITY AGAINST GAMETOCYTES
Tafenoquine
Tafenoquine is an 8-aminoquinoline analogue of primaquine that is currently undergoing Phase III clinical trials. It appears to be active against multiple stages of parasite development including liver stages of P. vivax. Tafenoquine, initially named WR 238605, was developed as part of a study aimed at uncovering a less toxic and longer-acting alternative to primaquine. It has many advantages over primaquine including a higher therapeutic index (Edstein et al. Reference Edstein, Kocisko, Walsh, Eamsila, Charles and Rieckmann2003), increased activity against P. falciparum asexual stages (Pradines et al. Reference Pradines, Mamfoumbi, Tall, Sokhna, Koeck, Fusai, Mosnier, Czarnecki, Spiegel, Trape, Kombila and Rogier2006) and a longer plasma half-life (12 to 16 days; 50 times that of primaquine) (Mihaly et al. Reference Mihaly, Ward, Edwards, Nicholl, Orme and Breckenridge1985; Brueckner et al. Reference Brueckner, Lasseter, Lin and Schuster1998b; Edstein et al. Reference Edstein, Kocisko, Brewer, Walsh, Eamsila and Charles2001). Preclinical in vivo studies demonstrate that tafenoquine is an effective prophylactic for P. falciparum (Brueckner et al. Reference Brueckner, Coster, Wesche, Shmuklarsky and Schuster1998a; Lell et al. Reference Lell, Faucher, Missinou, Borrmann, Dangelmaier, Horton and Kremsner2000; Hale et al. Reference Hale, Owusu-Agyei, Fryauff, Koram, Adjuik, Oduro, Prescott, Baird, Nkrumah, Ritchie, Franke, Binka, Horton and Hoffman2003; Walsh et al. Reference Walsh, Eamsila, Sasiprapha, Sangkharomya, Khaewsathien, Supakalin, Tang, Jarasrumgsichol, Cherdchu, Edstein, Rieckmann and Brewer2004a) and P. vivax (Walsh et al. Reference Walsh, Eamsila, Sasiprapha, Sangkharomya, Khaewsathien, Supakalin, Tang, Jarasrumgsichol, Cherdchu, Edstein, Rieckmann and Brewer2004a; Elmes et al. Reference Elmes, Nasveld, Kitchener, Kocisko and Edstein2008; Nasveld et al. Reference Nasveld, Edstein, Reid, Brennan, Harris, Kitchener, Leggat, Pickford, Kerr, Ohrt and Prescott2010). Tafenoquine is also effective in clearing exo-erythrocytic stages of P. vivax (Walsh et al. Reference Walsh, Looareesuwan, Wilairatana, Heppner, Tang, Brewer, Chokejindachai, Viriyavejakul, Kyle, Milhous, Schuster, Horton, Braitman and Brueckner1999, Reference Walsh, Wilairatana, Tang, Heppner, Brewer, Krudsood, Silachamroon, Phumratanaprapin, Siriyanonda and Looareesuwan2004b). However, given limited activity against P. falciparum asexual parasites (IC50 4 μ min vitro) (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011) tafenoquine should be used in combination with a fast-acting blood schizonticide (Fisk et al. Reference Fisk, Millet, Collins and Nguyen-Dinh1989; Obaldia et al. Reference Obaldia, Rossan, Cooper, Kyle, Nuzum, Rieckmann and Shanks1997; Puri and Dutta, Reference Puri and Dutta2003).
Although tafenoquine is believed to be active against gametocytes there is little evidence to support this assumption, particularly for mature stage V gametocytes. In vitro studies demonstrate tafenoquine has no effect against stage IV and V gametocytes and has only limited effect on stages I–III (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011). However there is the possibility that tafenoquine, as with primaquine, maybe active through a metabolite. In vitro studies demonstrate that tafenoquine is metabolized (Idowu et al. Reference Idowu, Peggins, Brewer and Kelley1995), however, the mechanisms are poorly defined and metabolites have not been detected in humans (Charles et al. Reference Charles, Miller, Nasveld, Reid, Harris and Edstein2007). In the in vitro rat liver microsome model tafenoquine is metabolized to form aminophenolic metabolites that may be capable of redox cycling (Idowu et al. Reference Idowu, Peggins, Brewer and Kelley1995), therefore the mode of action of tafenoquine against Plasmodium may be similar to that of primaquine, i.e. through oxidative stress and the disruption of mitochondrial function (Baird et al. Reference Baird, McCormick and Canfield1986; Fletcher et al. Reference Fletcher, Barton and Kelly1988; Vaidya et al. Reference Vaidya, Lashgari, Pologe and Morrisey1993; Ganesan et al. Reference Ganesan, Tekwani, Sahu, Tripathi and Walker2009). This mechanism has also been indicated as the possible mode of action of tafenoquine against other protozoa. Tafenoquine inhibits cytochrome c reductase of Leishamania species, depolarizing mitochondrial membrane potential and increasing reactive oxygen species (Carvalho et al. Reference Carvalho, Luque-Ortega, Manzano, Castanys, Rivas and Gamarro2010).
In vivo studies in mice do not demonstrate tafenoquine to be effective against mature gametocytes (Coleman, Reference Coleman1990; Coleman et al. Reference Coleman, Clavin and Milhous1992; Peters et al. Reference Peters, Robinson and Milhous1993), however when administered at the start of P. berghei infection tafenoquine appears to prevent gametocytes forming (Coleman, Reference Coleman1990; Coleman et al. Reference Coleman, Clavin and Milhous1992). Administration of tafenoquine on day 2 and 4 post infection had no effect on mature stage gametocyte viability or exflagellation. In these studies tafenoquine effected P. berghei oocyst and sporozoite development, however only when the drug was ingested by the mosquito.
Further studies to determine the effects of tafenoquine against P. falciparum gametocytes in vitro and in vivo are required to conclusively establish the transmission-blocking potential of this drug.
Methylene blue
Methylene blue was the first synthetic compound to be used against malaria, but was not considered suitable for large-scale use as it causes discolouration of the eyes and skin (Wainwright and Amaral, Reference Wainwright and Amaral2005). The discovery that methylene blue is highly active against all gametocyte stages both in vitro at clinically relevant concentrations (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011), and in vivo (Coulibaly et al. Reference Coulibaly, Zoungrana, Mockenhaupt, Schirmer, Klose, Mansmann, Meissner and Muller2009) make it a promising transmission-blocking agent and has renewed interest in its potential as an antimalarial drug. It is for this reason that methylene blue has recently undergone clinical evaluation as both a monotherapy and as part of a combination (with chloroquine, artesunate or amodiaquine) (Meissner et al. Reference Meissner, Mandi, Coulibaly, Witte, Tapsoba, Mansmann, Rengelshausen, Schiek, Jahn, Walter-Sack, Mikus, Burhenne, Riedel, Schirmer, Kouyate and Muller2006; Zoungrana et al. Reference Zoungrana, Coulibaly, Sie, Walter-Sack, Mockenhaupt, Kouyate, Schirmer, Klose, Mansmann, Meissner and Muller2008; Bountogo et al. Reference Bountogo, Zoungrana, Coulibaly, Klose, Mansmann, Mockenhaupt, Burhenne, Mikus, Walter-Sack, Schirmer, Sie, Meissner and Muller2009). Results from these trials suggest methylene blue has significant potential as a component of an effective antimalarial combination therapy. Furthermore, methylene blue has been shown to significantly reduce transmission to mosquitoes in in vitro membrane feeding experiments at clinically relevant concentrations (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011).
Methylene blue interacts with the antioxidant enzyme glutathione reductase (GR) but its mode of action or interaction(s) with GR or other potential targets is unclear. One theory is that it interferes with GR metabolism either directly or by causing NADPH (which is required for GR production) to be sequestered, altering GR levels in the parasite and also causing the production of reactive oxygen species (Kelner and Alexander, Reference Kelner and Alexander1985; Farber et al. Reference Farber, Arscott, Williams, Becker and Schirmer1998; Sarma et al. Reference Sarma, Savvides, Becker, Schirmer, Schirmer and Karplus2003; Arora and Srivastava, Reference Arora and Srivastava2005; Buchholz et al. Reference Buchholz, Schirmer, Eubel, Akoachere, Dandekar, Becker and Gromer2008; Haynes et al. Reference Haynes, Cheu, Tang, Chen, Guo, Guo, Coghi and Monti2011). However, transgenic P. berghei blood-stage parasites lacking GR remain sensitive to methylene blue suggesting that GR may not be the primary target of this drug in these parasites (Pastrana-Mena et al. Reference Pastrana-Mena, Dinglasan, Franke-Fayard, Vega-Rodriguez, Fuentes-Caraballo, Baerga-Ortiz, Coppens, Jacobs-Lorena, Janse and Serrano2010). The oocysts of these transgenic parasites were however highly susceptible to methylene blue, suggesting it may have different targets in different stages of parasite development (Pastrana-Mena et al. Reference Pastrana-Mena, Dinglasan, Franke-Fayard, Vega-Rodriguez, Fuentes-Caraballo, Baerga-Ortiz, Coppens, Jacobs-Lorena, Janse and Serrano2010).
It has also been suggested that methylene blue may inhibit haemozoin formation in a similar method to chloroquine (Atamna et al. Reference Atamna, Krugliak, Shalmiev, Deharo, Pescarmona and Ginsburg1996; Deharo et al. Reference Deharo, Garcia, Oporto, Gimenez, Sauvain, Jullian and Ginsburg2002; Blank et al. Reference Blank, Davioud-Charvet and Elhabiri2012). Interestingly, the level of resistance induced experimentally in a P. berghei mouse model is moderate (Thurston, Reference Thurston1953), providing additional evidence that methylene blue may have multiple targets in parasites (Thurston, Reference Thurston1953; Schirmer et al. Reference Schirmer, Coulibaly, Stich, Scheiwein, Merkle, Eubel, Becker, Becher, Muller, Zich, Schiek and Kouyate2003; Blank et al. Reference Blank, Davioud-Charvet and Elhabiri2012).
The potent activity of methylene blue across multiple stages of parasite development and its reported low production cost (Schirmer et al. Reference Schirmer, Coulibaly, Stich, Scheiwein, Merkle, Eubel, Becker, Becher, Muller, Zich, Schiek and Kouyate2003) makes it an attractive potential addition to treatment formulations for malaria, particularly as a transmission-blocking agent. However, a note of caution is required as adverse effects, usually red cell haemolysis, have been reported after methylene blue is administered to G6PD-deficient patients during treatment for non-malaria ailments (Rosen et al. Reference Rosen, Johnson, McGehee and Beutler1971; Gauthier, Reference Gauthier2000; Foltz et al. Reference Foltz, Dalal, Wadsworth, Broady, Chi, Eisenhauer, Kobayashi and Kollmannsburger2006). Nevertheless clinical trials assessing methylene blue as an antimalarial in malaria endemic areas with high prevalence of G6PD deficiency recently demonstrated that this drug is well tolerated with only a few adverse effects seen in children (Mandi et al. Reference Mandi, Witte, Meissner, Coulibaly, Mansmann, Rengelshausen, Schiek, Jahn, Sanon, Wust, Walter-Sack, Mikus, Burhenne, Riedel, Schirmer, Kouyate and Muller2005; Meissner et al. Reference Meissner, Mandi, Witte, Coulibaly, Mansmann, Rengelshausen, Schiek, Jahn, Sanon, Tapsoba, Walter-Sack, Mikus, Burhenne, Riedel, Schirmer, Kouyate and Muller2005, Reference Meissner, Mandi, Coulibaly, Witte, Tapsoba, Mansmann, Rengelshausen, Schiek, Jahn, Walter-Sack, Mikus, Burhenne, Riedel, Schirmer, Kouyate and Muller2006; Muller et al. Reference Muller, Mockenhaupt, Marks, Meissner, Coulibaly, Kuhnert, Buchner, Schirmer, Walter-Sack, Sie and Mansmann2012).
Trioxaquines
Trioxaquines are dual molecule antimalarials that contain two therapeutically active moieties (Dechy-Cabaret et al. Reference Dechy-Cabaret, Benoit-Vical, Robert and Meunier2000). They combine a chemically active group of artemisinin, a trioxane motif, with a 4-aminoquinoline moiety known to enable molecules to easily enter the parasite (Dechy-Cabaret et al. Reference Dechy-Cabaret, Benoit-Vical, Robert and Meunier2000). These compounds were designed to act on the haemoglobin digestion pathway via two different mechanisms with the goal of reducing the development of resistance (Dechy-Cabaret et al. Reference Dechy-Cabaret, Benoit-Vical, Robert and Meunier2000, Reference Dechy-Cabaret, Benoit-Vical, Loup, Robert, Gornitzka, Bonhoure, Vial, Magnaval, Seguela and Meunier2004). Trioxaquines are highly active against asexual stages of P. falciparum in vitro, including chloroquine-resistant strains (Dechy-Cabaret et al. Reference Dechy-Cabaret, Benoit-Vical, Robert and Meunier2000). Trioxaquines have also successfully cleared parasites in several murine models (Dechy-Cabaret et al. Reference Dechy-Cabaret, Benoit-Vical, Loup, Robert, Gornitzka, Bonhoure, Vial, Magnaval, Seguela and Meunier2004; Benoit-Vical et al. Reference Benoit-Vical, Lelievre, Berry, Deymier, Dechy-Cabaret, Cazelles, Loup, Robert, Magnaval and Meunier2007; Cosledan et al. Reference Cosledan, Fraisse, Pellet, Guillou, Mordmuller, Kremsner, Moreno, Mazier, Maffrand and Meunier2008) and lead inhibitors are now undergoing preclinical development (Cosledan et al. Reference Cosledan, Fraisse, Pellet, Guillou, Mordmuller, Kremsner, Moreno, Mazier, Maffrand and Meunier2008).
While widespread studies have not been performed, trioxaquine compounds have also demonstrated activity against all stages of gametocyte development in vitro (Benoit-Vical et al. Reference Benoit-Vical, Lelievre, Berry, Deymier, Dechy-Cabaret, Cazelles, Loup, Robert, Magnaval and Meunier2007). It is not known how these agents affect mature stage gametocytes, however their activity against immature gametocytes is believed to be linked to their ability to target haemoglobin digestion. Further studies examining the potential of these drugs as anti-transmission agents are urgently required.
Endoperoxides: OZ439 and OZ277
Ozonide OZ439 is a synthetic peroxide antimalarial candidate that is in phase IIa clinical trials. Delves et al (Reference Delves, Plouffe, Scheurer, Meister, Wittlin, Winzeler, Sinden and Leroy2012) have shown that endoperoxides such as OZ439 are strong inhibitors of exflagellation. However activity against mature gametocytes is not proven in vitro or in vivo. OZ277 (arterolane), a synthetic endoperoxide designed on the basis of the artemisinin pharmacophore is currently in Phase III trials. OZ277 has been shown to have in vitro activity against stage V gametocytes (IC50 6·4 μ m) (Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012) and inhibit exflagellation at 10 μ m (Delves et al. Reference Delves, Plouffe, Scheurer, Meister, Wittlin, Winzeler, Sinden and Leroy2012) but its effectiveness against sexual stages in vivo is yet to be determined.
EXPERIMENTAL DRUGS/DRUGS IN PRE-CLINICAL DEVELOPMENT WITH ACTIVITY AGAINST GAMETOCYTES
While research to discover drugs with activity against gametocytes has been ongoing for some time, it has been slow and complicated by the difficulties associated with studying these terminally differentiated parasites. The recent malaria eradication agenda has, however, spurred significant interest in this area of research. As primaquine remains the only clinically available drug with significant activity against mature stage gametocytes, it has been recognized that if eradication is to be achieved new, safer drugs with activity against mature gametocytes are required. Multiple international groups have recently begun searching for compounds with activity against mature gametocytes. Additional research examining the mode of action of active drugs is also ongoing. These studies have identified a number of agents that while still experimental may be useful as anti-gametocyte agents or tools to learn more about gametocyte biology and vulnerabilities.
Quinoline compounds: primaquine derivatives
In an effort to retain gametocyte activity while reducing the toxic side-effects of primaquine, researchers have investigated the gametocytocidal activity of primaquine analogues. While many of these derivatives retain their activity against gametocytes and have improved pharmacokinetic profiles, they often remain haemolytic in G6PD deficient individuals. The 8-aminoquinoline analogue NPC-1161C or more particularly its (−)-enantiomer, NPC-1161B, is perhaps one of the most promising primaquine derivatives currently under investigation. NPC-1161B demonstrates significantly reduced haematotoxicity and retains activity against gametocytes (in vitro IC50 3·8 μ m (Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012)). NPC-1161C also completely inhibits P. falciparum exflagellation in vitro (10 μ m) (Delves et al. Reference Delves, Plouffe, Scheurer, Meister, Wittlin, Winzeler, Sinden and Leroy2012) and is more active (IC50 50–500 nm) than primaquine (IC50 0·5–2·5 μ m) against asexual intra-erythrocytic parasites under similar conditions (Delves et al. Reference Delves, Plouffe, Scheurer, Meister, Wittlin, Winzeler, Sinden and Leroy2012). These observations suggest that NPC-1161B may not require metabolic activation to be active against all stages of parasite development. Additional analogues with modifications to the terminal primary amino group of primaquine including bulaquine, imidazoquines and the trioxaquines have also shown promise as anti-gametocyte agents (Benoit-Vical et al. Reference Benoit-Vical, Lelievre, Berry, Deymier, Dechy-Cabaret, Cazelles, Loup, Robert, Magnaval and Meunier2007; Kiszewski, Reference Kiszewski2011; Dechy-Cabaret and Benoit-Vical, Reference Dechy-Cabaret and Benoit-Vical2012) and are discussed in previous sections of this review.
Decoquinate
Decoquinate, a well-known veterinary product used to control Coccidial infections in ruminants, has recently been shown to kill P. falciparum stage I–II gametocytes (IC50 36 nm) (da Cruz et al. Reference da Cruz, Martin, Buchholz, Lafuente-Monasterio, Rodrigues, Sonnichsen, Moreira, Gamo, Marti, Mota, Hannus and Prudencio2012). Decoquinate also inhibits the growth of asexual intra-erythrocytic and liver stage parasites. While additional studies are required to determine the activity of decoquinate against mature stage gametocytes, these initial studies are encouraging. The mode of action of decoquinate against immature gametocytes has yet to be specifically demonstrated. However, data show that it kills asexual parasites by selectively and specifically inhibiting the parasite mitochondrial bc1 complex (da Cruz et al. Reference da Cruz, Martin, Buchholz, Lafuente-Monasterio, Rodrigues, Sonnichsen, Moreira, Gamo, Marti, Mota, Hannus and Prudencio2012). An action against bc1 might be of some concern if cross-resistance with atovaquone exists, however, data suggest that decoquinate has little cross-resistance with atovaquone. The potent and broadspectrum activity displayed by this new antimalarial lead certainly warrants further investigation.
9-Anilinoacridines
9-anilinoacridine compounds have demonstrated activity against both asexual and sexual stage malaria parasites (Figgitt et al. Reference Figgitt, Denny, Chavalitshewinkoon, Wilairat and Ralph1992; Chavalitshewinkoon-Petmitr et al. Reference Chavalitshewinkoon-Petmitr, Pongvilairat, Ralph, Denny and Wilairat2001). These agents, originally developed for use as anti-cancer agents, inhibit DNA topoisomerase II (Schneider et al. Reference Schneider, Hsiang and Liu1990). They have a similar core structure to the antimalarial pyronaridine which has activity against gametocytes (discussed above) and malaria parasite topoisomerase II (Chavalitshewinkoon-Petmitr et al. Reference Chavalitshewinkoon-Petmitr, Pongvilairat, Auparakkitanon and Wilairat2000). Pyronaridine is also known to target haematin (Auparakkitanon et al. Reference Auparakkitanon, Chapoomram, Kuaha, Chirachariyavej and Wilairat2006). While 9–anilinoacridines can inhibit gametocytes (stage II–III) at μ m concentrations (IC50 8–97 μ m) they are more active against asexual erythrocytic parasites (IC50 0·01–21 μ m) (Gamage et al. Reference Gamage, Tepsiri, Wilairat, Wojcik, Figgitt, Ralph and Denny1994; Chavalitshewinkoon-Petmitr et al. Reference Chavalitshewinkoon-Petmitr, Pongvilairat, Ralph, Denny and Wilairat2001). There may be issues with the ability of some of these drugs to enter cells and also to be rapidly metabolized, however, this reduced activity against gametocytes is likely to be associated with their action against either haematin or DNA topoisomerase II. In comparison to asexual stages parasites, gametocytes are relatively metabolically inactive and synthesize little DNA (Sinden and Smalley, Reference Sinden and Smalley1979). It is also important to note that some of the more active 9-anilinoacridine compounds have yet to be tested against gametocytes.
HIV-protease inhibitors
HIV protease inhibitors have been shown to inhibit malaria parasite growth at clinically relevant concentrations (Skinner-Adams et al. Reference Skinner-Adams, McCarthy, Gardiner, Hilton and Andrews2004; Andrews et al. Reference Andrews, Fairlie, Madala, Ray, Wyatt, Hilton, Melville, Beattie, Gardiner, Reid, Stoermer, Skinner-Adams, Berry and McCarthy2006; Redmond et al. Reference Redmond, Skinner-Adams, Andrews, Gardiner, Ray, Kelly and McCarthy2007). These drugs inhibit the growth of malaria parasites in mice (Andrews et al. Reference Andrews, Fairlie, Madala, Ray, Wyatt, Hilton, Melville, Beattie, Gardiner, Reid, Stoermer, Skinner-Adams, Berry and McCarthy2006) and sera taken from HIV patients receiving HIV protease inhibitors inhibits the growth of P. falciparum in vitro (Redmond et al. Reference Redmond, Skinner-Adams, Andrews, Gardiner, Ray, Kelly and McCarthy2007). Studies investigating the stage-specific activity of HIV protease inhibitors against malaria parasites have also demonstrated that they have activity against pre-erythrocytic stages (Hobbs et al. Reference Hobbs, Voza, Coppi, Kirmse, Marsh, Borkowsky and Sinnis2009) and can directly kill gametocytes of all stages at concentrations between 5–29 μ m depending on the inhibitor (Peatey et al. Reference Peatey, Andrews, Eickel, MacDonald, Butterworth, Trenholme, Gardiner, McCarthy and Skinner-Adams2009a, Reference Peatey, Skinner-Adams, Dixon, McCarthy, Gardiner and Trenholmeb; Hobbs et al. Reference Hobbs, Tanaka, Muratova, Van Vliet, Borkowsky, Williamson and Duffy2013). Furthermore, a field study conducted in Uganda indicated a significant reduction of gametocytaemia in children receiving protease inhibitor-based treatment for HIV compared with children receiving non-protease inhibitor-based drugs (Ikilezi et al. Reference Ikilezi, Achan, Kakuru, Ruel, Charlebois, Clark, Rosenthal, Havlir, Kamya and Dorsey2013). Such a broad spectrum of activity against a range of life-cycle stages, including gametocytes is very unusual, and while these drugs are not suitable to be used as first-line antimalarials in their own right (essentially due to cost, instability and modest activity (asexual IC50 range 0·4–3·8 μ m) (Skinner-Adams et al. Reference Skinner-Adams, McCarthy, Gardiner, Hilton and Andrews2004)), these data indicate that they may represent a promising lead towards a new group of drugs that could reduce clinical disease, relapse and disease transmission.
Natural products
Natural products have traditionally been very important to the control and treatment of malaria so it is not surprising that several of the new and promising treatments with activity against gametocytes are natural products. Neem, a complex product derived from Azadirachta india, which has been used for many years as an insecticide and treatment for a variety of diseases is active against all gametocyte stages (Udeinya et al. Reference Udeinya, Brown, Shu, Udeinya and Quakeyie2006). The gametocytocidal activity of Neem has been attributed to several specific components including azadirachtin, gedunin and nimbolide. Azadiractin is believed to be the primary active component and appears to act by inhibiting the parasites’ cytoskeletal system (Billker et al. Reference Billker, Shaw, Jones, Ley, Mordue and Sinden2002).
The proteosome inhibitor epoxomicin (Hanada et al. Reference Hanada, Sugawara, Kaneta, Toda, Nishiyama, Tomita, Yamamoto, Konishi and Oki1992) has also been identified as a potent anti-gametocyte agent (Czesny et al. Reference Czesny, Goshu, Cook and Williamson2009). This compound originally derived from Actinomycetes bacteria has activity against all stages of Plasmodium gametocytes (24 h IC50 54 nm) and asexual intra-erythrocytic parasites (24 h IC50 41 nm) (Czesny et al. Reference Czesny, Goshu, Cook and Williamson2009; Tanaka and Williamson, Reference Tanaka and Williamson2011). It also has limited mammalian cell toxicity and is cytotoxic to parasites, suggesting a high therapeutic index (Czesny et al. Reference Czesny, Goshu, Cook and Williamson2009). While epoxomicin appears to affect the morphology of gametocytes it does not inhibit exflagellation in vitro (Czesny et al. Reference Czesny, Goshu, Cook and Williamson2009). Additional proteosome inhibitors include non-specific inhibitors such as thiostepton and compounds that are already in clinical use and are now also under investigation as potential anti-gametocyte agents (Aminake et al. Reference Aminake, Schoof, Sologub, Leubner, Kirschner, Arndt and Pradel2011).
Harmonine, a defence compound from the harlequin ladybird Harmonia axyridis which has activity against a broad spectrum of microbes, also has moderate activity against P. falciparum (Rohrich et al. Reference Rohrich, Ngwa, Wiesner, Schmidtberg, Degenkolb, Kollewe, Fischer, Pradel and Vilcinskas2012). In a recent study harmonine was shown to be active against asexual intra-erythrocytic parasites (IC50 4·8–7·6 μ m) and stage II gametocytes (18% reduction in gametocyte numbers when treated with 4·8 μ m) (Rohrich et al. Reference Rohrich, Ngwa, Wiesner, Schmidtberg, Degenkolb, Kollewe, Fischer, Pradel and Vilcinskas2012). It also inhibited the exflagellation of microgametocytes (IC50 5·8 μ m) (Rohrich et al. Reference Rohrich, Ngwa, Wiesner, Schmidtberg, Degenkolb, Kollewe, Fischer, Pradel and Vilcinskas2012). While harmonine may be useful as a lead towards novel agents active against gametocytes, its moderate activity and low therapeutic index (cytotoxicity 20–60 μ m) (Rohrich et al. Reference Rohrich, Ngwa, Wiesner, Schmidtberg, Degenkolb, Kollewe, Fischer, Pradel and Vilcinskas2012) suggest it will not be useful in its own right.
Riboflavin or vitamin B2 has been found to be active against P. falciparum gametocytes in vitro (Akompong et al. Reference Akompong, Eksi, Williamson and Haldar2000a). It is effective against both immature and mature gametocytes. However, inhibition is highest when gametocytes are young. Riboflavin is also active against asexual erythrocytic stage parasites (Akompong et al. Reference Akompong, Ghori and Haldar2000b) with studies suggesting that activity may be mediated by an effect on haemoglobin digestion (Akompong et al. Reference Akompong, Ghori and Haldar2000b). Unfortunately, riboflavin has a short half-life (2–6 h; Jusko and Levy, Reference Jusko and Levy1967), and since concentrations as high as 10–100 μ m are required to inhibit all parasite stages it is likely to have limited use as an antimalarial agent. Interestingly, however, riboflavin has been shown to potentiate the activity of mefloquine, pyrimethamine and quinine against asexual erythrocytic stages, suggesting that it may be valuable as a component of a combination therapy (Akompong et al. Reference Akompong, Eksi, Williamson and Haldar2000a).
IDENTIFYING NEW DRUGS AND DRUG TARGETS
Thanks to the sustained efforts of researchers over the last decades there are currently a number of promising anti-gametocyte compounds at various stages along the development pipeline, but given the rigorous selection criteria they must pass along the way, it is certain that only a small proportion will make it through to clinical use as a licensed drug. For this reason the search for good lead compounds must continue and below we examine the prospects for discovery of new anti-gametocyte compounds.
Traditionally there are two approaches to identifying new drugs for a given disease, target-based approaches which are directed at identifying novel targets such as essential enzymes within the parasite to which new drugs can be designed (for example: McGowan et al. Reference McGowan, Porter, Lowther, Stack, Golding, Skinner-Adams, Trenholme, Teuscher, Donnelly, Grembecka, Mucha, Kafarski, Degori, Buckle, Gardiner, Whisstock and Dalton2009; Phillips and Rathod, Reference Phillips and Rathod2010; Skinner-Adams et al. Reference Skinner-Adams, Stack, Trenholme, Brown, Grembecka, Lowther, Mucha, Drag, Kafarski, McGowan, Whisstock, Gardiner and Dalton2010) and cell-based approaches based on more general phenotypic screens to identify compounds which can kill the infectious organism but whose mode of action remains undefined (Cervantes et al. Reference Cervantes, Stout, Prudhomme, Engel, Bruton, Cervantes, Carter, Tae-Chang, Hay, Aalbersberg, Kubanek and Le Roch2012; Duffy and Avery, Reference Duffy and Avery2012; Guiguemde et al. Reference Guiguemde, Shelat, Garcia-Bustos, Diagana, Gamo and Guy2012). Each has its advantages and disadvantages. While studies on the asexual stages of P. falciparum were greatly facilitated by the development of an in vitro culture system (Trager and Jensen, Reference Trager and Jensen1976), our limited understanding of P. falciparum gametocytes has made studies with these stages much more difficult. Difficulties have been predominately associated with being able to differentiate immature stage gametocytes from asexual forms and obtaining adequate numbers of pure gametocytes. As a consequence of these issues we have little information with respect to the differences in protein expression and metabolism between mature stage gametocytes and asexually replicating parasites and hence cell-based approaches are the most feasible option to identify new drugs that target gametocytes. To identify new agents that can kill gametocytes methods for cultivation of large numbers of pure gametocytes are required. A number of methodologies for small-scale gametocyte culture have been published (Campbell et al. Reference Campbell, Chin, Collins and Moss1980; Ifediba and Vanderberg, Reference Ifediba and Vanderberg1981; Ponnudurai et al. Reference Ponnudurai, Meuwissen, Leeuwenberg, Verhave and Lensen1982), but these all have the disadvantage that they do not yield a pure gametocyte culture, but a mix of both sexual and asexual forms. Recently new methodologies have been developed to overcome some of these shortcomings (Fivelman et al. Reference Fivelman, McRobert, Sharp, Taylor, Saeed, Swales, Sutherland and Baker2007). However, most gametocyte preparations are inherently limited by the fact that even in parasite lines with relatively high conversion rates gametocytes usually represent less than 1% of the parasitized cells with a given culture and are essentially terminally differentiated unless ingested by an Anopheles mosquito. While it has been reported that gametocytogenesis can be increased by the addition of drugs (Peatey et al. Reference Peatey, Skinner-Adams, Dixon, McCarthy, Gardiner and Trenholme2009b), or conditioned media (Fivelman et al. Reference Fivelman, McRobert, Sharp, Taylor, Saeed, Swales, Sutherland and Baker2007) the increase in gametocyte production is generally only 2–3-fold (Dyer and Day, Reference Dyer and Day2000). Dixon et al. reported the development of an assay utilizing a green fluorescent protein chimera of the early sexual blood stage protein Pfs16 as a marker for commitment to gametocytogenesis (Dixon et al. Reference Dixon, Peatey, Gardiner and Trenholme2009). This reporter system allows accurate identification of gametocytes well before they are morphologically distinguishable from asexual parasites and made it possible for the first time to isolate relatively large numbers of pure gametocytes suitable for use in drug screening and high throughput screening assays (HTS) (Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012). Nonetheless this method has the disadvantage of using a transgenic parasite line which alters the drug resistance profile of the parasite due to the introduction of the transgene (Peatey et al. Reference Peatey, Skinner-Adams, Dixon, McCarthy, Gardiner and Trenholme2009b). Gametocyte culture methods are continuously improving however, with a recent publication by Lucantoni and Avery (Reference Lucantoni and Avery2012) reporting that their culture method can achieve gametocytaemia levels of 1–4%. Although limited information on this method was reported, achievement of such a high level of gametocyte production is a significant step towards the development of a HTS.
The most commonly used methods for gametocyte production start with either synchronous or asynchronous asexual stage parasite cultures which are then ‘induced’ to produce higher levels of gametocytes either by the use of conditioned media, reduction of culture haematocrit, or a combination of the two (Williams, Reference Williams1999; Fivelman et al. Reference Fivelman, McRobert, Sharp, Taylor, Saeed, Swales, Sutherland and Baker2007; Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011; Tanaka and Williamson, Reference Tanaka and Williamson2011; Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012). Asexual parasites are removed by the use of either sorbitol (Saul et al. Reference Saul, Graves and Edser1990) or N-acetyl glucosamine (NAG) (Fivelman et al. Reference Fivelman, McRobert, Sharp, Taylor, Saeed, Swales, Sutherland and Baker2007). Thus it is possible to obtain a uniform population of gametocytes of one specific age. These gametocytes can then be purified from the uninfected red cells by the use of magnetic separation (MACS) (Fivelman et al. Reference Fivelman, McRobert, Sharp, Taylor, Saeed, Swales, Sutherland and Baker2007; Ribaut et al. Reference Ribaut, Berry, Chevalley, Reybier, Morlais, Parzy, Nepveu, Benoit-Vical and Valentin2008). Preparations with 90% gametocyte purity can readily be achieved using this method (Fivelman et al. Reference Fivelman, McRobert, Sharp, Taylor, Saeed, Swales, Sutherland and Baker2007). Gametocytes can then be used in drug assays immediately as in the majority of the published literature (Peatey et al. Reference Peatey, Skinner-Adams, Dixon, McCarthy, Gardiner and Trenholme2009bReference Peatey, Leroy, Gardiner and Trenholme2012; Chevalley et al. Reference Chevalley, Coste, Lopez, Pipy and Valentin2010; Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011; Tanaka and Williamson, Reference Tanaka and Williamson2011; Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012) or frozen in liquid nitrogen for later use. While freezing results in a loss of viability of the gametocytes of up to 30% (Peatey et al. Reference Peatey, Spicer, Hodder, Trenholme and Gardiner2011), due to variability in gametocytes numbers collected in a single culture, stockpiling frozen gametocytes may be a convenient way to perform a true high throughput screen. Large-scale production of gametocytes requires the selection of an appropriate parasite isolate as isolates differ in their propensity for producing gametocytes (Graves et al. Reference Graves, Carter and McNeill1984).
A number of manuscripts have been published detailing different methods for undertaking drug assays on gametocytes, however assays are generally in a 96-well format so relatively few compounds are screened (N<50). Methods for assessing inhibition include: ATP bioluminescence (Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012; Peatey et al. Reference Peatey, Leroy, Gardiner and Trenholme2012), expression of a gametocyte-specific fluorescent reporter measured by flow cytometry (Peatey et al. Reference Peatey, Andrews, Eickel, MacDonald, Butterworth, Trenholme, Gardiner, McCarthy and Skinner-Adams2009a; Buchholz et al. Reference Buchholz, Burke, Williamson, Wiegand, Wirth and Marti2011), measurement of transgenic luciferase activity (Adjalley et al. Reference Adjalley, Johnston, Li, Eastman, Ekland, Eappen, Richman, Sim, Lee, Hoffman and Fidock2011) and measurement of metabolic activity using a metabolic indicator such as alamar blue (Tanaka and Williamson, Reference Tanaka and Williamson2011) or hydroethidine (Chevalley et al. Reference Chevalley, Coste, Lopez, Pipy and Valentin2010). To date two publications have reported gametocyte assays in a 384 format (Lelievre et al. Reference Lelievre, Almela, Lozano, Miguel, Franco, Leroy and Herreros2012; Lucantoni and Avery, Reference Lucantoni and Avery2012), although the report by Lucantoni and Avery (Reference Lucantoni and Avery2012) mentions a 384-well formatted assay, but no experimental results are given beyond a description of the signal to noise ratio and the Z′ score of the assay. A 1534-well assay has recently been reported using alamar blue as a viability indicator. This assay requires 20 000 gametocytes per well and more importantly does not require the removal of RBCs (Tanaka et al. Reference Tanaka, Dehdashti, Nguyen, McKew, Zheng and Williamson2013). Miniaturization of the assay did not result in loss of sensitivity therefore, along with reductions in labour requirements and reagents, this assay represents a most promising step toward a HTS of a significant number of compounds (>300 000) being performed.
While all of the described assays are useful in determining the gametocytocidal activity of known antimalarial compounds or a relatively small number of unknown compounds, none reported have yet been miniaturized enough to undertake the screening of large compound libraries where the number of compounds can exceed 300 K individual chemical entities. Most methods use relatively large numbers of gametocytes, in the order of 104 to 106 per well, thus making them difficult to use, at least in their current format, for a HTS. A method that uses gametocytes in the range of 102–103 per well is likely to be required for a HTS to be undertaken. Nonetheless, even given all currently reported methods have their limitations, these assays allow us for the first time to investigate the transmission blocking effects of current and experimental gametocytocidal agents, particularly as a number of HTS against asexual stage parasites have been undertaken (Kurosawa et al. Reference Kurosawa, Dorn, Kitsuji-Shirane, Shimada, Satoh, Matile, Hofheinz, Masciadri, Kansy and Ridley2000; Baldwin et al. Reference Baldwin, Michnoff, Malmquist, White, Roth, Rathod and Phillips2005; Plouffe et al. Reference Plouffe, Brinker, McNamara, Henson, Kato, Kuhen, Nagle, Adrian, Matzen, Anderson, Nam, Gray, Chatterjee, Janes, Yan, Trager, Caldwell, Schultz, Zhou and Winzeler2008; Gamo et al. Reference Gamo, Sanz, Vidal, de Cozar, Alvarez, Lavandera, Vanderwall, Green, Kumar, Hasan, Brown, Peishoff, Cardon and Garcia-Bustos2010; Guiguemde et al. Reference Guiguemde, Shelat, Bouck, Duffy, Crowther, Davis, Smithson, Connelly, Clark, Zhu, Jimenez-Diaz, Martinez, Wilson, Tripathi, Gut, Sharlow, Bathurst, El Mazouni, Fowble, Forquer, McGinley, Castro, Angulo-Barturen, Ferrer, Rosenthal, Derisi, Sullivan, Lazo, Roos, Riscoe, Phillips, Rathod, Van Voorhis, Avery and Guy2010; Rottmann et al. Reference Rottmann, McNamara, Yeung, Lee, Zou, Russell, Seitz, Plouffe, Dharia, Tan, Cohen, Spencer, Gonzalez-Paez, Lakshminarayana, Goh, Suwanarusk, Jegla, Schmitt, Beck, Brun, Nosten, Renia, Dartois, Keller, Fidock, Winzeler and Diagana2010; Duffy and Avery, Reference Duffy and Avery2012) and large numbers of compounds with activity against asexual stage parasites identified. The most potent and diverse have been assembled into focused groups which include the GlaxoSmithKline TCAMS library (13 500 compounds), Novartis-GNF malaria box (5600 compounds) and the Medicines for Malaria Venture malaria box (400 compounds). The relatively small size of these focused libraries makes them a good starting point for screening in order to identify compounds with gametocytocidal activity and within the capacity of current assay methods. The ability to screen large (>300 000 compounds) chemical and natural product libraries for gametocytocidal agents is now tantalizingly close, however it is important to remember that this is only the first step in a long development process albeit a step of great significance.
CONCLUSION
While very few currently available agents have activity against gametocytes, the renewed interest in studying the activity of drugs against these stages and the improved techniques developed to perform these studies are paving the way towards the identification and optimization of new gametocytocidal compounds. These drugs are urgently needed if the current eradication agenda is to be successful.
FINANCIAL SUPPORT
ASB is supported by an NHMRC PhD Scholarship and a QIMR PhD Top-Up Scholarship.