INTRODUCTION
Relationships between levels of parasitism and male mating success have received much attention over the past few decades (e.g. Hamilton and Zuk, Reference Hamilton and Zuk1982; Read, Reference Read, Barnard and Behnke1990; Clayton, Reference Clayton1991; Dunn, Reference Dunn2005). Three main hypotheses are usually put forward to explain the observed decrease in infected male mating success. First, parasite infection may affect male ability to compete with other males for access to reproduction and fertilization (Howard and Minchella, Reference Howard and Minchella1990; Forbes, Reference Forbes1991). Infection can alter male potential to find and secure a territory (Borgia, Reference Borgia1986) or a mate (e.g. in arthropods, Carmichael et al. Reference Carmichael, Moore and Bjostad1993; Zohar and Holmes, Reference Zohar and Holmes1998; Bollache et al. Reference Bollache, Gambade and Cézilly2001). Infected males may also be less able to directly interfere with competitors to gain access to females (Zohar and Holmes, Reference Zohar and Holmes1998; Bollache et al. Reference Bollache, Gambade and Cézilly2001). Second, females may refuse to mate with infected males (Milinski and Bakker, Reference Milinski and Bakker1990). They should prefer to consort with uninfected males to avoid contamination by parasites (Able, Reference Able1996). They should also favour males that resist parasite infection as this could provide them with pathogen-resistant offspring (Hamilton and Zuk, Reference Hamilton and Zuk1982). Third, the mating success reduction of infected male hosts may result from parasite adaptations (Hurd, Reference Hurd2001; Moore, Reference Moore2002; Lefèvre et al. Reference Lefèvre, Roche, Poulin, Hurd, Renaud and Thomas2008). Parasites with complex life cycles sometimes present strategies to increase their chance of transmission from an intermediate host to a definitive host (Poulin, Reference Poulin1994; Lafferty, Reference Lafferty1999; Lagrue et al. Reference Lagrue, Kaldonski, Perrot-Minnot, Motreuil and Bollache2007). In case of trophic transmission, parasites can manipulate host behaviour and physiology to make it more susceptible to predation by a definitive host (Lafferty, Reference Lafferty1999; Lagrue et al. Reference Lagrue, Kaldonski, Perrot-Minnot, Motreuil and Bollache2007). Manipulation can hence induce modifications in some aspects of host behaviour, such as general activity or spatial and temporal distribution, reducing their probability of encountering mates (Rasmussen, Reference Rasmussen1959; Thomas et al. Reference Thomas, Renaud, Derothe, Lambert, Meeüs and Cézilly1995; Zohar and Holmes, Reference Zohar and Holmes1998; Tain et al. Reference Tain, Perrot-Minnot and Cézilly2006). Manipulative parasites can also modify host physiology, leading to fecundity alteration, suspension or even castration with significant effects on mating behaviour (Baudoin, Reference Baudoin1975; Thompson and Kavaliers, Reference Thompson and Kavaliers1994; Bollache et al. Reference Bollache, Rigaud and Cézilly2002; Ferreira et al. Reference Ferreira, Jensen, Martins, Sousa, Marques and Pardal2005). Most studies have focused on the influence of infected female fecundity reduction on male mating preferences (Poulton and Thompson, Reference Poulton and Thompson1987; Bollache et al. Reference Bollache, Rigaud and Cézilly2002; Dunn et al. Reference Dunn, Andrews, Ingrey, Riley and Wedell2006). On the other hand, the effects of manipulative parasite on spermatogenesis and male mating success have been poorly documented (Bierbower and Sparkes, Reference Bierbower and Sparkes2007).
Cyathocephalus truncatus (Cestoda: Spathebothriidea) is a tapeworm widespread in Europe. It almost exclusively infects amphipod crustaceans, such as Gammarus pulex, as intermediate hosts, and fishes as definitive hosts (Okaka, Reference Okaka1984). Franceschi et al. (Reference Franceschi, Rigaud, Moret, Hervant and Bollache2007) showed that C. truncatus was able to manipulate the behaviour of its G. pulex intermediate host. Infected individuals have been described to be significantly less photophobic than uninfected ones. This alteration in infected G. pulex behaviour makes them more conspicuous to visual predators, and explains the previously observed increase of C. truncatus-infected gammarid predation rate (Knudsen et al. Reference Knudsen, Gabler, Kuris and Amundsen2001). In addition, Franceschi et al. (Reference Franceschi, Rigaud, Moret, Hervant and Bollache2007) observed various C. truncatus pathogenic effects, especially on intermediate host survival, swimming activity and oxygen consumption.
Mating behaviour in G. pulex is characterized by a pre-copulatory mate-guarding phase (also called amplexus or pre-copula) during which a male carries a female beneath his ventral surface for several days (up to 20 days, e.g. Galipaud et al. Reference Galipaud, Dechaume-Moncharmont, Oughadou and Bollache2011). This mate-guarding period usually begins when the female initiates vitellogenesis and thus becomes receptive to pairing. The pre-copula ends with female moulting. The female then becomes receptive for reproduction with the guarding male for about a day (Sutcliffe, Reference Sutcliffe1992; Bollache et al. Reference Bollache, Gambade and Cézilly2000). Pre-copulatory mate-guarding behaviour is thought to have evolved as a male competitive strategy in response to this brief period of female sexual receptivity (Parker, Reference Parker1974; Grafen and Ridley, Reference Grafen and Ridley1983). In amphipods, parasite infection often correlates with a decrease in male ability to successfully pair with a female in nature (Ward, Reference Ward1986; Thomas et al. Reference Thomas, Renaud, Derothe, Lambert, Meeüs and Cézilly1995; Zohar and Holmes, Reference Zohar and Holmes1998; Bollache et al. Reference Bollache, Gambade and Cézilly2001). According to previous studies, several processes related to sexual selection may explain this pattern. Both female mate choice and male-male competition for females have been suggested as important components of infected males, lower pairing success (Zohar and Holmes, Reference Zohar and Holmes1998; Bollache et al. Reference Bollache, Gambade and Cézilly2001). The aim of this study was to combine field observations and laboratory experiments to assess the influence of C. truncatus on male G. pulex (i) sperm reserves and (ii) pairing success and competitive ability.
MATERIALS AND METHODS
Field collection
All gammarids were collected from March to May 2009 in a small tributary of River Suzon, Burgundy, eastern France (N: 47°24,215′; E: 4°52,974′) using a hand net and the kick sampling method described by Hynes (Reference Hynes1954). The relative large worm size and its white colour, visible through the gammarid cuticle, make infected hosts easy to recognize. All infected individuals sampled in the field were only infected by one larva.
Following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997), we estimated the prevalence of C. truncatus in the field by measuring the proportion of infected individuals in a first sample. For laboratory experiments, we sampled gammarids for a second time in the field (hereafter referred to as ‘the second field sample’), looking specifically for infected males and uninfected individuals. Uninfected males were either found unpaired or paired with an uninfected female. Infected males, however, were only found unpaired in the field. In this second field sample, G. pulex males were thus found in the following 3 different field states: (i) uninfected paired, (ii) uninfected unpaired or (iii) infected. We used males from this second field sample (paired males where separated from their previous female) either for the inclination experiment and sperm measurement (n=105) or for the competition experiment (n=66), as described below.
Laboratory studies
In the laboratory, gammarids were maintained under a constant photo-period (12:12 h) in well-aerated tanks containing UV-treated water at 15°C and leaf litter. For experiments, gammarids were individually housed in small plastic cups (h=7 cm; Ø=9 cm). At the end of each experiment, all individuals were killed, using 70% alcohol, and measured (size of the fourth coxal plate, e.g. Bollache et al. Reference Bollache, Rigaud and Cézilly2002) using a stereoscopic microscope (Nikon SMZ 1500) and the Lucia G 4.81 software. With the same apparatus, we also measured the total body length of cestodes. No gammarids were used more than once for experiments. Individuals that moulted or died during experiments were excluded from the dataset.
Male inclination to pair
We first investigated the effect of male field states (infected unpaired, uninfected unpaired and uninfected paired) on male inclination to pair with a new uninfected female. Males were first individually allowed to acclimatize for 1 h in a plastic cup. A female was then added to each cup. All females used in this experiment had already been caught in pre-copula to control for their receptivity to pairing. Their position in their intermoult (i.e. the time between 2 successive moults) was approximately assessed (either close to moult, in the middle of intermoult or at the beginning of intermoult) based on the developmental stage of embryos in their brood pouch (Geffard et al. Reference Geffard, Xuereb, Chaumot, Geffard, Biagianti, Noël, Abbaci, Garric, Charmantier and Charmantier-Daures2010). Cups were first checked after 1 h and then after a period of 24 h to determine whether individuals had entered into pre-copula. All individuals were then measured. Males were also dissected for sperm number assessment as described below. The effect of males field states on their pairing success was tested using a logistic regression model with sperm number, female position in their intermoult, and male and female size as covariates.
Sperm reserve
We also assessed sperm reserve of males from the inclination experiment using the protocol described by Lemaître et al. (Reference Lemaître, Rigaud, Cornet and Bollache2009). Briefly, 1 testis per male was removed and isolated in a watch glass, in 1 ml of Crustacean Ringer solution. The gonad was cut into small fragments with fine forceps under a binocular microscope. This allowed sperm to mix with the Ringer. The solution was then exposed to ultrawaves for 10 sec to separate sperm from membranes without damaging the gametes (Ultra-waves tank, Branson 2200 Branson cleaning Equipment Company, Shelton, Co, USA). The solution was homogenized with a micropipette (i.e. by pushing and pulling liquid for 30 sec) and 4×10 μl drops per male were placed on a slide and dried for 10 min. Slides were then gently rinsed with demineralized water to eliminate Ringer's crystals before allowing them to dry again for 30 min. Sperms of each slide were counted under optic microscopy (Nikon Eclipse E600, magnification × 100). Total sperm reserve of each individual was therefore estimated by combining sperm number of all 4 drops (40 μl). Using an ANCOVA, we tested for the effect of male field state on sperm reserve with male size as covariate. Sperm reserve data were Box-Cox transformed to meet normality. Homogeneity of variance was verified with a Bartlett test.
Male-male competition
We also studied the ability of infected G. pulex males to pair with a female in the presence of an uninfected competitor male. Two males of similar size (t-test; t=0·83, P=0·406), 1 infected and 1 uninfected (previously paired in the field), were introduced in a plastic cup and allowed to acclimatize for 1 h. A previously paired female (i.e. receptive for pairing) was then added to each cup. Females used for this experiment were always smaller than the 2 males in their cups. Trials (n=33 replicates) were examined every hour during 1 day (i.e. 12 h). Once 1 of the 2 males had formed pre-copula, the 3 gammarids were removed from the cups and measured. After 24 h, every gammarid was removed from the apparatus. We used a binomial test to compare uninfected and infected male pairing success in competition. However, this did not distinguish between the two confounding effects of male-male interaction and male inclination to pair on male pairing probability. In order to disentangle these two effects, we also compared the pairing success of infected males in the inclination experiment (i.e. with no competition) to the pairing success of infected males in the competition experiment with a Fisher exact test. For more consistency, we also calculated the odds ratio as a measure of effect size of the difference and its 95% confidence interval (Nakagawa and Cuthill, Reference Nakagawa and Cuthill2007).
RESULTS
Field studies
Overall, 536 pre-copula pairs and 1113 unpaired gammarids (643 males and 470 females) were collected in the first sample. Parasite prevalence was extremely low in the field (0·23% of C. truncatus-infected individuals in the first field sample, n=5). Because of this low proportion of infected individuals found in this first field sample, we were unable to reliably test for a parasite prevalence difference in males (0·25%, n=3) and females (0·20%, n=2). For the same reason, we were also unable to test for a difference between infected and uninfected male pairing success in this first field sample. None of the infected males collected in the first field sample were paired. On the other hand, 45·6% of uninfected males were found in amplexus. In the second field sample (i.e. gammarids dedicated to laboratory experiments), males showed size differences according to their field states (Kruskal-Wallis, χ 22=9·72, P<0·01). Infected unpaired males (n=33) were significantly larger than uninfected unpaired males (n=39, post-hoc test: P<0·01) but did not differ in size with uninfected paired males (n=33, post-hoc test: P=0·69). Uninfected paired and unpaired males did not differ in size either (post-hoc test: P=0·06).
Laboratory studies
Male inclination to pair
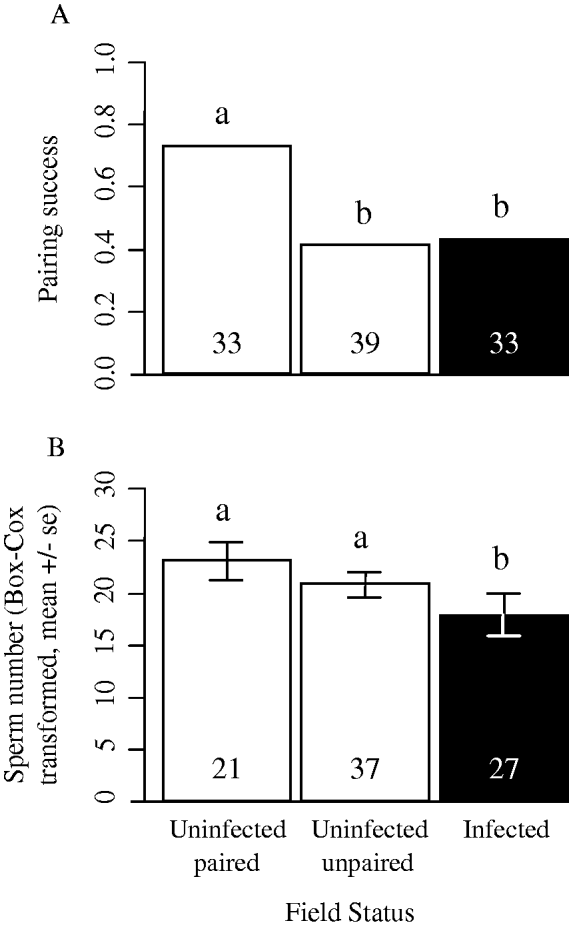
Male inclination to pair with a female was significantly related to male field state, but not to female's time left to moult, number of sperm or male and female body size (Table 1). Males infected with C. truncatus were significantly less likely to enter into pre-copula than uninfected paired males (post-hoc test, Z=−2·44, P <0·05, Fig. 1A). Similarly, uninfected unpaired males formed significantly fewer pre-copula than uninfected paired males (post-hoc test, Z=−2·64, P<0·01, Fig. 1A). However, there was no difference in pairing probability between uninfected unpaired males and C. truncatus-infected males (post-hoc test, Z=0·14, P=0·89, Fig. 1A). Thus, among 105 individuals, uninfected males found paired in the field were more likely to pair again with a new female (70·59%) compared to uninfected males found unpaired in the field or infected males (41·02% and 42·42% respectively, Fig. 1A).

Fig.1. Pairing success (A) (proportion of males entering in pre-copula) and sperm number (B) of infected males and paired or unpaired uninfected males. Numbers inside bars represent sample sizes for each male field state. Categories sharing the same letter above their bars did not differ significantly.
Table 1. Logistic regression of pairing success in male Gammarus pulex in the laboratory as a function of male field state, male and female body size, time left to the female moult and sperm number

Sperm reserve
Total sperm reserve (i.e. the estimated number of sperm in 1 testis) was significantly affected by male field state (F2,85=3·33, P=0·04, Fig. 1B). Infected males had lower sperm reserve than uninfected paired males (post-hoc test; t=2·296, P<0·05, Fig. 1B) or uninfected unpaired males (post-hoc test; t=2·177, P<0·05, Fig. 1B). However, uninfected paired and unpaired males did not differ regarding their sperm reserve (post-hoc test; t=0·289, P=0·774, Fig. 1B). Larger males carried more sperm in their testes than smaller males (F1,85=6·45, P=0·01). The interaction between male size and male field state had no effect on sperm number (F2,85=0·17, P=0·84). Among infected males, we found a positive correlation between male size and cestode size (Pearson correlation, r=0·63, n=26, P<0·001). However, none of the following variables significantly influenced infected male sperm number: male size (F1,26=0·79, P=0·38), cestode size (F1,26=0·38, P=0·54), the interaction between male and cestode size (F1,26=2·21, P=0·15).
Male-male competition
Overall, 33 assays were performed involving 2 males and a receptive female, but only 70% (23/33) resulted in a pairing. In competitive situations, infected male pairing success was strongly decreased. Only in 2 out of 23 trials (8·7%) did C. truncatus-infected males succeed in entering into pre-copula when competing with an uninfected male (binomial test: P<0·001). In non-competitive trials (i.e. in the inclination experiment), infected male pairing success was even significantly better than in competitive situations (odds ratio: OR=12·78, 0·95 confidence interval ranging from 2·06 to 43·3, Fisher exact test: P<0·01, see Table 2 for sample sizes).
Table 2. Number of parasite-infected and uninfected males that succeeded in pairing with a female in the inclination and the competition experiments

DISCUSSION
G. pulex males exposed to C. truncatus infection incur a severe decline in their pairing success. Both their inclination to pair with a receptive female and their competitive ability decreased. Manipulative parasites have been reported to alter male mating success in several field-based studies (Oetinger, Reference Oetinger1987; Zohar and Holmes, Reference Zohar and Holmes1998; Bollache et al. Reference Bollache, Gambade and Cézilly2001; Sparkes et al. Reference Sparkes, Weil, Renwick and Talkington2006; Bierbower and Sparkes, Reference Bierbower and Sparkes2007). In this study, no male infected by C. truncatus was ever found paired with a female in the field, in either of our samples. This would tend to support the pattern observed in laboratory experiments. However, the low parasitic prevalence we measured does not allow us to draw a definitive conclusion about infected male pairing success in nature. Among uninfected males, those found unpaired in the field also showed a weak tendency to pair with a new female. This is consistent with previous findings on reproductive behaviour of G. pulex males. They appear to show more willingness in initiating a new pre-copula after they already spent some time paired with another female (Lemaître et al. Reference Lemaître, Rigaud, Cornet and Bollache2009). This suggests either that (i) a common phenomenon causes a weak paring pattern in both infected and uninfected unpaired males or, (ii) that different phenomena lead to the same difficulties in initiating pre-copula in both male states. Following the first hypothesis, it is possible that males which do not succeed in pairing with a female are also more susceptible to parasite infection. In our field samples, infected males may thus simply be weakened males, already unable to successfully pair with a female. However, the size difference we observed between uninfected unpaired males and infected males makes this hypothesis unrealistic in G. pulex. In the rest of the discussion, we consider the second hypothesis, acknowledging relative roles of male and female strategies and parasite manipulation to explain G. pulex male-mating pattern.
One hallmark of C. truncatus infection in males is a reduction in sperm. Such reductions have not been reported for crustaceans infected with acanthocephalan parasites: i.e. amphipod (Moore, Reference Moore1984; Zohar and Holmes, Reference Zohar and Holmes1998) or isopod (Bierbower and Sparkes, Reference Bierbower and Sparkes2007). Two main phenomena could explain this effect. First, the substantial tapeworm size (up to 30% of host mass, Okaka, Reference Okaka1984; Franceschi et al. Reference Franceschi, Rigaud, Moret, Hervant and Bollache2007) and its position in the host body cavity may induce pathogenic effects or mechanical harm on G. pulex, potentially resulting in reduced sperm production in infected males. This may occur either directly, by physically curtailing gametogenesis, or indirectly by acting on host nutrient availability (see Hurd, Reference Hurd2001 for a review). For instance, C. truncatus-infected gammarids have been shown to suffer a decrease in swimming activity, which may affect their foraging efficiency (Franceschi et al. Reference Franceschi, Rigaud, Moret, Hervant and Bollache2007). Second, by limiting or diverting energy normally allocated to reproduction, the parasites may reduce host fecundity. Parasites often directly compete with their host for nutrients, which can reduce energy available for host gamete production. Under these conditions, a negative correlation between parasite biomass and host fecundity is expected (Hurd, Reference Hurd2001). In this study, no correlation was found between gammarid sperm number and tapeworm size, raising doubts about any effect of nutrient competition on host sperm reserve.
The reduced pairing of infected males could be linked to sperm reserve. But male pairing success is also expected to be affected by other parasite-induced pathogenic effects or by female mating behaviour. In this section, we consider these 3 hypotheses to explain the infected male-pairing pattern.
First, with low sperm reserves, males may change their mating behaviour, as has been suggested for other arthropod species (Kendall and Wolcott, Reference Kendall and Wolcott1999; Ortigosa and Rowe, Reference Ortigosa and Rowe2003; van Son and Thiel, Reference van Son and Thiel2006). Uninfected unpaired males did not differ in sperm number with uninfected paired males, although they paired less often with a new female. Thus, for uninfected unpaired males, pairing propensity does not seem to be linked to sperm reserve. It would thus be surprising that the low sperm reserve in C. truncatus-infected males influences their inclination to pair. Lemaître et al. (Reference Lemaître, Rigaud, Cornet and Bollache2009) also found no effect of sperm reserve on male pairing decision in G. pulex.
Second, pathogenic effects induced by parasites, such as reduced swimming activity or oxygen consumption (Franceschi et al. Reference Franceschi, Rigaud, Moret, Hervant and Bollache2007) may alter male-pairing success. These pathogenic effects could make struggles prior to pre-copula more difficult for infected males (Sparkes et al. Reference Sparkes, Weil, Renwick and Talkington2006). Franceschi et al. (Reference Franceschi, Rigaud, Moret, Hervant and Bollache2007) also suggested that the low survival observed in C. truncatus-infected individuals may be due to the large amount of energy that is lost to the parasite infection. Pre-copulatory mate guarding is a long lasting and energy-expensive behaviour in G. pulex (Plaistow et al. Reference Plaistow, Bollache and Cézilly2003), and it is therefore possible that infected males, who may be already energy depleted, are less able to afford the energetic cost of holding a female for several days. Under these circumstances, they would not be able to pair as often as healthy males, and this could explain their low inclination to pair in our experiments. Perhaps owing to this weakened body condition, tapeworm-infected males suffered even lower pairing success when directly competing with healthy males. Our results revealed that infected males paired even less in competitive situations when compared to non-competitive situations. Evidence for such an effect of parasites on male competitive ability are scarce in the literature (Zohar and Holmes, Reference Zohar and Holmes1998; Bollache et al. Reference Bollache, Gambade and Cézilly2001). It is difficult to distinguish between the relative roles of interference competition versus scramble competition when explaining the decreased competitive ability observed in infected males. It is possible that C. truncatus-infected males might have had their females usurped by rival healthy males (i.e. ‘take-over’, Grafen and Ridley, Reference Grafen and Ridley1983). However, take-overs are rarely, if ever, observed in G. pulex (Franceschi et al. Reference Franceschi, Lemaître, Cézilly and Bollache2010). It is thus more likely that their poorer propensity to pair resulted in a disadvantage in rapidly securing the female.
Third, female sexual behaviour would likely play a role in male pairing success. In several amphipod species displaying pre-copulatory mate guarding, females resist male guarding attempts as a form of mate choice (Elwood et al. Reference Elwood, Gibson and Neil1987; Jormalainen, Reference Jormalainen1998; Cothran, Reference Cothran2008a, b). Male size, for instance, has been proposed to play a role in female mate choice (Wellborn and Bartholf, Reference Wellborn and Bartholf2005; Cothran, Reference Cothran2008a). Our data showed that infected males were larger than uninfected unpaired males. However, they suffered an equally low mating success. If pairing is under female control, female mate choice based on male size alone does not explain the pairing pattern we observed. On the other hand, females may base their choice on other male traits such as sperm reserve. In species in which females do not store sperm, as in the case of amphipods (Hunte et al. Reference Hunte, Myers and Doyle1985; Jormalainen, Reference Jormalainen1998), sperm limitation during mating can result in a fecundity cost for them (Hou and Sheng, Reference Hou and Sheng1999; Sadek, Reference Sadek2001; Sparkes et al. Reference Sparkes, Keogh and Orsburn2002, Dunn et al. Reference Dunn, Andrews, Ingrey, Riley and Wedell2006). Sparkes et al. (Reference Sparkes, Keogh and Orsburn2002) demonstrated that in a stream-dwelling isopod, females avoid mating with newly mated, possibly sperm-limited males. By resisting pairing with infected males (i.e. sperm limited), G. pulex females may thus prevent possible fecundity costs. Female mate choice for uninfected males could also result from other deleterious effects linked with male infection. Infected males may be of lower genetic quality (Hamilton and Zuk, Reference Hamilton and Zuk1982). Females may also risk parasite infection when mating with infected males (Keymer and Read, Reference Keymer, Read, Toft, Aeschlimann and Bolis1991), although C. truncatus horizontal transmission has never been reported between intermediate hosts. However, manipulative parasites induce behavioural and physiological changes in their intermediate host to facilitate transmission to a definitive host (Poulin, Reference Poulin1994). Pairing with infected individuals could thus come with a higher predation risk in intermediate host species (Sparkes et al. Reference Sparkes, Keogh and Orsburn2002). G. pulex have a central position in the food web as a prey of numerous fish species (MacNeil et al. Reference MacNeil, Dick and Elwood1999). It may then be particularly risky for females of this species to be held by a C. truncatus-infected male.
In conclusion, various effects related to sexual selection can explain the observed pairing success of G. pulex males. We observed a sperm reduction in infected males, but not in uninfected unpaired males, although they both showed a reduced pairing success. Thus, sperm number does not seem to influence male-pairing success. Rather, it seems that other infection-induced pathogenic effects related to a male's body condition may have deleterious effects on both their inclination to pair and their competitive ability. Future studies should carefully assess the influence of female mate choice, as several parasite-related deleterious effects (lower mate quality, predation risk) should alter their motivation to mate with infected males. Here, we also emphasize the importance of considering the pairing success of healthy males found unpaired in the field when studying the role of parasites on reproductive behaviour in species displaying pre-copulatory mate guarding. This provides useful cues about possible pre-existing mating bias in uninfected males, hence pondering the effect of parasite infection on host reproductive success.
ACKNOWLEDGMENTS
This study was founded by the Conseil Régional de Bourgogne. M.G. was supported by a doctoral grant from the Ministère de la Recherche et de l'Enseignement Supérieur. We thank Mark Gillingham and Brian Preston for correction of the English, and Thierry Rigaud, François-Xavier Dechaume-Moncharmont, Clément Lagrue, Anne Besson and two anonymous referees for insightful comments about the manuscript.