Introduction
Knowledge of taxonomy and life cycles of trematodes has advanced over time, both through classical experimental studies (revised by Yamaguti, Reference Yamaguti1975; Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003; Bolek et al., Reference Bolek, Stigge, Gustafson, Janovy and Esch2016; Blasco-Costa and Poulin, Reference Blasco-Costa and Poulin2017) and molecular tools (Blasco-Costa et al., Reference Blasco-Costa, Cutmore, Miller and Nolan2016; Assis et al., Reference Assis, Lopez-Hernández, Pulido-Murillo, Melo and Pinto2019, Gonchar et al., Reference Gonchar, Jouet, Skírnisson, Krupenko and Galaktionov2019; Locke et al., Reference Locke, Drago, Núñez, Souza and Takemoto2020). However, studies focused on life cycle of trematodes parasitizing cold-blooded tetrapods, especially amphibians, are comparatively scarce worldwide. This scientific gap is more pronounced in South America, where despite the great diversity of these parasites reported in amphibians, either as definitive or second intermediate hosts (Hamann and González, Reference Hamann and González2009; Campião et al., Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Silva2014; Fernandes and Kohn, Reference Fernandes and Kohn2014; Hamann et al., Reference Hamann, Fernández and González2019, Reference Hamann, González and Fernández2020a; Queiroz et al., Reference Queiroz, López-Hernández, Locke, Pinto and Anjos2019, Reference Queiroz, Pontes, Neto, Campião and Anjo2020), most species remain with their life cycle and phylogenetic position unknown. In fact, only one species of trematode that uses anurans as definitive hosts (Halipegus dubius Klein, 1905) had its life cycle elucidated by experimental studies in South America (Paraense, Reference Paraense1992; Ostrowski de Núñez and Gil de Pertierra, Reference Ostrowski de Núñez, Gil de Pertierra, Ranzani-Paiva, Takemoto and Lizama2004). Moreover, only recently have trematodes from anurans been studied by molecular means in this continent (Gomes et al., Reference Gomes, Melo, Giese, Furtado, Gonçalves and Santos2013; Queiroz et al., Reference Queiroz, López-Hernández, Locke, Pinto and Anjos2019; Hamann et al., Reference Hamann, León-Règagnon, Fernández and González2020b).
Among the poorly known trematodes found in anurans are the members of the superfamily Paramphistomoidea, parasites with a worldwide distribution (Sey, Reference Sey1991; Jones, Reference Jones, Jones, Bray and Gibson2005a). About four dozen species of amphistomes from two families (Cladorchiidae Fischoeder, 1901 and Diplodiscidae Cohn, 1904) have been reported in amphibians from different parts of the world (Skryabin, Reference Skryabin1949; Yamaguti, Reference Yamaguti1971; Sey, Reference Sey1991; Jones, Reference Jones, Jones, Bray and Gibson2005a). Among these, only members of the diplodiscid genus Catadiscus Cohn, 1904 (12 species) occur in South America (Sey, Reference Sey1983, Reference Sey1991; Campião et al., Reference Campião, Morais, Dias, Aguiar, Toledo, Tavares and Silva2014; Fernandes and Kohn, Reference Fernandes and Kohn2014; Hamann et al., Reference Hamann, Kehr and González2014). From an evolutionary point of view, the Neotropical region was considered the centre of early radiation and diversification of the paramphistomoids in amphibians, with subsequent colonization of these hosts in other zoogeographical realms (Sey, Reference Sey1991). Despite the high diversity of species, the knowledge about the life cycle of these amphibian parasites is restricted to species of the genus Diplodiscus Diesing, 1836, Megalodiscus Chandler, 1923 and Opisthodiscus Cohn, 1904 from Europe, North America and Asia (Sey, Reference Sey1991). These studies have shown that either a tadpole or adult becomes infected when ingesting encysted metacercariae in food or its own cast-off skin; an alternative route of infection by swallowing the cercariae was also proposed (Yamaguti, Reference Yamaguti1975; Grabda-Kazubska, Reference Grabda-Kazubska1980; Bolek and Janovy Jr., Reference Bolek and Janovy2008; Besprozvannykh et al., Reference Besprozvannykh, Rozhkovan, Ermolenko and Izrailskaya2018). The paucity of knowledge on the life cycle of anuran amphistomes can be due to difficulties in the maintenance of this host under laboratory conditions for experimental infection studies. The scarcity or even absence of molecular information for most species, which would make it easier to link larval stages to their adult counterparts, is a limiting factor as well. In this last aspect, data from no more than six species of trematodes from anurans were included in recent high-level phylogenetic studies involving trematodes or exclusively members of Paramphistomoidea (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003; Pérez-Ponce de Léon and Hernández-Mena, Reference Pérez-Ponce de Léon and Hernández-Mena2019; Alves et al., Reference Alves, Assis, López-Hernández, Pulido-Murillo, Melo, Locke and Pinto2020).
In the current study, adult parasites recovered from anurans and amphistome cercariae found in planorbid snails in Brazil were subjected to morphological, experimental and molecular studies. The life cycle of Catadiscus marinholutzi Freitas and Lent, Reference Freitas and Lent1939 was here studied for the first time using this integrative taxonomic approach. Moreover, the first phylogenetic placement of a species of Catadiscus within the Paramphistomoidea is attempted and associated implications for the systematics of the group discussed.
Materials and methods
Study area and host sampling
The anurans and snails evaluated in this study were collected in a temporary marsh pond located in a riparian forest of Véstia stream (20°23′43.4″S, 51°23′39.8″W), municipality of Selvíria, State of Mato Grosso do Sul, the Central-West region of Brazil. The pond contains water about 10 months a year, has a maximum depth of 1.6 m, a perimeter of 314 m and a surface area of 5000 m2. Adult anurans were collected by the scan searching method (Halliday, Reference Halliday and Sutherland2006), with 1 h of capture effort during 23 samplings between December 2016 and February 2019. The animals were placed individually in plastic bags and transported to the laboratory, where they were measured (snout-vent length) and euthanized [sodium thiopental solution (Thiopentax®, Itapira, SP, Brazil), via intraperitoneal]. Tadpoles were collected using a rectangular dip-net (75 cm × 50 cm) and transported to the laboratory. The anurans were subjected to macroscopic inspection, and after this process, viscera were removed, transferred to Petri dishes containing physiological solution (0.9% NaCl) and examined under a stereomicroscope for detecting the presence of parasites. The taxonomic identification of anurans was based on morphological criteria, according to Provete et al. (Reference Provete, Garey, Silva and Rossa-feres2011).
A long-term malacological survey was carried out monthly between May 2017 and February 2019 (29 field expeditions with a duration of 1 h). The snails were also collected using a rectangular dip-net, placed in a plastic sack and transported to the laboratory for the parasitological analyses. In the laboratory, the collected snails were placed individually in polystyrene microplates and subjected to artificial photostimulation for 2 h followed by exam using a stereomicroscope. A new exam was performed after an overnight period (Fernandez et al., Reference Fernandez, Thiengo, Amaral, Amaral, Thiengo and Pieri2008). Subsamples of the snails collected were used for taxonomic identification, which was based on morphological traits, according to Paraense and Deslandes (Reference Paraense and Deslandes1956, Reference Paraense and Deslandes1957) and PAHO (1968).
Experimental infection tests
For the current study, a morphotype of amphistome cercariae found in Drepanotrema lucidum (Pfeiffer, 1839) was selected to experimental infection study. Aiming to obtain parasite-free tadpoles, we sampled anuran eggs from the pond at the beginning of the rainy season of 2018. Eggs of two anuran species were collected: Physalaemus cuvieri Fitzinger, 1826 (Leptodactylidae) and Trachycephalus typhonius (Linnaeus, 1758) (Hylidae). The laboratory-reared tadpoles were kept in rectangular plastic trays (60 cm × 35 cm × 15 cm) with 3 L of filtered water and aquarium pumps. They were fed daily with fish feed. In the first experiment, we evaluated the formation of metacercariae on tadpole skin. For this, tadpoles of T. typhonius (Gosner stage 26–28) (n = 3) and P. cuvieri (Gosner stage 27–38) (n = 3) were placed individually in wells of a 6-well polystyrene plate containing 10 mL chlorine-free water and at least ten cercariae. The plate was examined under a stereomicroscope for the presence of encysted metacercariae on tadpole or at the bottom of the well after 2, 4, 6, 12 and 24 h exposure to the cercariae. We tried to experimentally infect tadpoles of T. typhonius (n = 6) to obtain adult parasites. The laboratory-reared anurans were placed with one infected snail in a 1 L aquarium for 80 h. After the exposure, the tadpoles were necropsied in 2 days interval for the recovery of trematodes.
Morphological study
Samples of adult amphistomes from different species of anuran hosts naturally infected were slightly compressed between glass slide and coverslip and fixed in formalin, or killed in hot water (70°C) and fixed in 2.5% glutaraldehyde solution diluted in a buffer solution of pH 7.4. Samples of worms were stained with haematoxylin, serially dehydrated in ethanol solutions, clarified in methyl salicylate and mounted on permanent slides with Canada balsam (adapted from Amato et al., Reference Amato, Boeger and Amato1991). The cercariae that emerged from the snail hosts were initially studied alive in non-permanent preparations stained with vital dyes (0.05% neutral red or Nile blue sulphate). For morphometric analyses, they were killed in hot water (70°C), fixed in 2.5% glutaraldehyde solution diluted in a buffer solution of pH 7.4. Two naturally infected snails were dissected to recover rediae.
The developmental stages were studied under a Leica DM 2500 optical microscope with a differential phase contrast system and photographed with a camera coupled to the microscope. Measurements were taken with the aid of Leica software. Morphometric data are presented in micrometres as the mean followed by the standard deviation and range in parentheses. For scanning electron microscopy (SEM), some heat-killed (70°C) and glutaraldehyde-fixed trematodes were passed through increasingly concentrated acetone, which was later removed by critical point drying in a Leica Microsystems model CPD300. Samples were then sputter-coated with gold for 3 min (film thickness: 5 nm) on a Quorum model Q150TE and photographed with a Zeiss microscope model EVO-LS15 using SmartSEM. The taxonomic identification of adult trematodes was based on morphological traits according to different authors (Travassos et al., Reference Travassos, Freitas and Kohn1969; Sey, Reference Sey1983, Reference Sey1991; Jones, Reference Jones, Jones, Bray and Gibson2005a, Reference Jones, Jones, Bray and Gibson2005b). The quantitative descriptors of parasitism followed Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Voucher specimens were deposited in the collection of trematodes of UFMG (UFMG-TRE 119).
Molecular analyses
Samples of adult parasites recovered from naturally infected Leptodactylus podicipinus (Cope, 1862) (Leptodactylidae) and cercariae emerged from Drepanotrema depressissimum (Moricand, 1839) (Planorbidae) were fixed in ethanol for molecular analyses. The DNA was extracted using the QIAamp DNA micro kit (Qiagen Ltd., Crawley, UK) and quantified using a microvolume spectrophotometer (NanoDrop Lite; Thermo Fisher Scientific). A partial region of the 28S rDNA gene (~1200 bp) was amplified using the primers (dig12 and 1500R) and polymerase chain reaction (PCR) conditions described by Tkach et al. (Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003). PCR reactions were performed in a final volume of 25 μL, which included 12.5 μL Platinum Hot Start PCR Master Mix, 1.25 pmol of each primer and about 50 ng of template DNA. Amplification products were visualized in 1% agarose gel electrophoresis stained with UniSafe Dye 20.000× (Uniscience) and purified with polyethylene glycol 20% (PEG 8000, Promega, USA). The sequencing was done in two directions by capillary electrophoresis using the BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Inc., Foster City, CA) in an ABI3730 sequencer, with the same primers used in PCR.
Contiguous sequences were assembled in ChromasPro (Technelysium Pty Ltd., Australia) and aligned with 44 sequences available for paramphistomoids in the GenBank (Table S1) using default parameters of MAFFT (Katoh and Standley, Reference Katoh and Standley2013) implemented in the Guidance2 web server (http://guidance.tau.ac.il/; Sela et al., Reference Sela, Ashkenazy, Katoh and Pupko2015). Unreliable positions in the alignment were identified and removed using the Gblock web server (http://phylogeny.lirmm.fr/; Dereeper et al., Reference Dereeper, Guignon, Blanc, Audic, Buffet, Chevenet, Dufayard, Guindon, Lefort, Lescot, Claverie and Gascuel2008) with less stringent settings. Uncorrected P-distances were calculated using MEGA 7.0 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Phylogenetic reconstructions were performed with the Bayesian inference (BI) and maximum likelihood (ML) criteria, based on the GTR + F + I + G4 model, predicted as the best estimator by the small sample size corrected Akaike information criterion in ModelFinder (Kalyaanamoorthy et al., Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) within IQ-TREE (Nguyen et al., Reference Nguyen, Schmidt, Von Haeseler and Minh2015). Microscaphid and mesometrid digeneans were used as outgroups (see Table S1) based on previous studies (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003; Pérez-Ponce de Léon and Hernández-Mena, Reference Pérez-Ponce de Léon and Hernández-Mena2019).
The ML trees were generated via IQ-TREE and nodal supports were estimated with 10 000 UFBoot iterations (Minh et al., Reference Minh, Nguyen and Von Haeseler2013). BI analyses were conducted in MrBayes ver. 3.2 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) running two independent MC3 runs of four chains (one cold, three heated) for 10 million generations, sampling tree topologies every 1000th generation, with the first 25% of samples discarded as burn-in; all above-mentioned analyses were run on the computational resource CIPRES (Miller et al., Reference Miller, Pfeiffer and Schwartz2010). Tracer v.1.6 (Rambaut et al., Reference Rambaut, Suchard, Xie and Drummond2014) was used to check the convergence and mixing of different parameters and to confirm that the effective sample size (ESS) of each parameter was adequate to provide reasonable estimates of the variance in model parameters (i.e. ESS values >200). FigTree ver. 1.4.2 (Rambaut, Reference Rambaut2012) was used for tree visualization and Adobe® Photoshop® CS5 (Adobe Inc., USA) for additional editing. Newly generated sequences were deposited in the GenBank database (accession number MW618662 and MW618663).
Results
Herpetological and malacological surveys
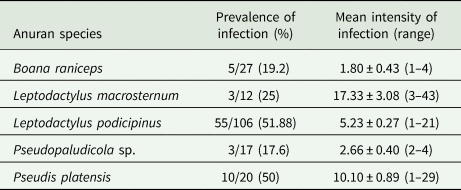
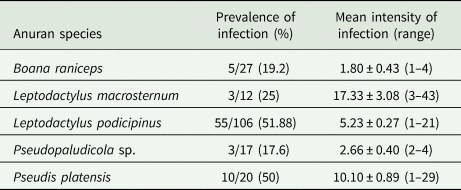
In the herpetological study, 296 specimens of adult anurans of 17 species were collected and evaluated for trematode infections. From these, 76 specimens (24.9%) belonging to five species [Boana raniceps (Cope, 1862), Pseudis platensis Gallardo, 1961‒ both Hylidae; Leptodactylus macrosternum Miranda-Ribeiro, 1926, L. podicipinus, Pseudopaludicola sp.,‒ all Leptodactylidae] were found harbouring amphistomes in the large intestine. Basic descriptive ecological parameters of the infections are shown in Table 1. The following species of adult anurans were not found infected with amphistome trematodes: Dendropsophus elianeae (Napoli and Caramaschi, 2000) (n = 7), Dendropsophus minutus (Peters, 1872) (n = 22), Dendropsophus nanus (Boulenger, 1889) (n = 44), Scinax fuscovarius (Lutz, 1925) (n = 6), Scinax nasicus (Cope, 1862) (n = 12), T. typhonius (n = 2) ‒ all Hylidae; Leptodactylus mystaceus (Spix, 1824) (n = 2), L. mystacinus (Burmeister, 1861) (n = 1), P. cuvieri (n = 2), Physalaemus nattereri (Steindachner, 1863) (n = 8) ‒ all Leptodactylidae; Dermatonotus muelleri (Boettger, 1885) (n = 6) ‒ Microhylidae; Pithecopus azureus (Cope, 1862) (n = 6) ‒ Phyllomedusidae. Additionally, 261 specimens of tadpoles belonging to 11 species [B. raniceps (n = 49); D. minutus (n = 4), D. nanus (n = 67), D. muelleri (n = 50), L. podicipinus (n = 20), P. platensis (n = 8); P. cuvieri (n = 6), P. nattereri (n = 14), S. fuscovarius (n = 18), S. nasicus (n = 6) and T. typhonius (n = 19)] were examined; however, none of them was found harbouring encysted metacercariae on skin or trematodes in the intestine.
Table 1. Ecological parameters for Catadiscus marinholutzi recovered in five anuran species sampled in Selviria, Central-Western Brazil, from December 2016 to February 2019
In the malacological study, a same morphotype of amphistome cercaria was found in 3/420 (0.7%) of the specimens of D. depressissimum, and 6/2236 (0.3%) of D. lucidum (Pfeiffer, 1839) (Planorbidae) collected in the current study. Specimens of Biomphalaria occidentalis (Planorbidae) (n = 1013), Pomacea sp. (Ampullariidae) (n = 66) and Physa marmorata Guilding, 1828 (Physidae) (n = 360) were also collected and examined for infection with larval trematodes, but no amphistome cercariae were found in these species.
Experimental infection study
During the experimental infection study carried out with the amphistome cercariae found in snails, larval encystment on tadpole skin was not observed. However, live cercariae and encysted metacercariae were found at the bottom of the container in all intervals. After 24 h of exposure, only metacercariae were observed. The keeping of tadpoles of T. typhonius in the same aquarium containing one naturally infected snail resulted in the infection of one of the six exposed specimens (precise pathway of infection not observed). In total, 14 immature worms were recovered in the intestine of this tadpole 8 days after the exposure.
Morphological study and taxonomic identification
Morphological examination of adult parasites found in anurans enabled us to identify these amphistomes as Catadiscus marinholutzi. Members of Catadiscus are typified by having the lumen of acetabulum constricted in two regions (Jones, Reference Jones, Jones, Bray and Gibson2005b) and may be distinguished from each other by several morphological traits (e.g. caeca length, arrangement and extent of vitelline follicles, pharynx/acetabulum and acetabulum/body ratios) (Sey, Reference Sey1991). Catadiscus marinholutzi can be readily distinguished from most of its congeners by the presence of vitelline follicles confluent anteriorly. An exception is Catadiscus propinquus Freitas and Dobbin Jr., Reference Freitas and Dobbin1956, of which C. marinholutzi differs by the presence of larger vitelline follicles and pharynx/acetabulum ratio (Sey, Reference Sey1991). In all infected host species, we observed the presence of ovigerous worms at different stages of maturation, i.e. juvenile as well as fully mature (gravid) specimens with eggs in the uterus and, as previously reported for C. propinquus by Hamann (Reference Hamann2004). Comparative morphometric data, based on fully mature adult specimens obtained from L. podicipinus, are shown in Table 2. Measurements obtained from selected mature worms from other four anuran host are presented in Table S2, which are also compatible with C. marinholutzi. Overall, the morphology of immature worms obtained experimentally in tadpoles matches that of the adult forms. The link between cercariae and adults is also supported using molecular data (100% similarity in the 28S rDNA). The morphometric data obtained for larval stages are shown in Table 3 and compared with morphologically similar species reported in South America.
Table 2. Morphometric data of Catadiscus marinholutzi found in anurans from Brazil and of the most similar species, Catadiscus propinquus
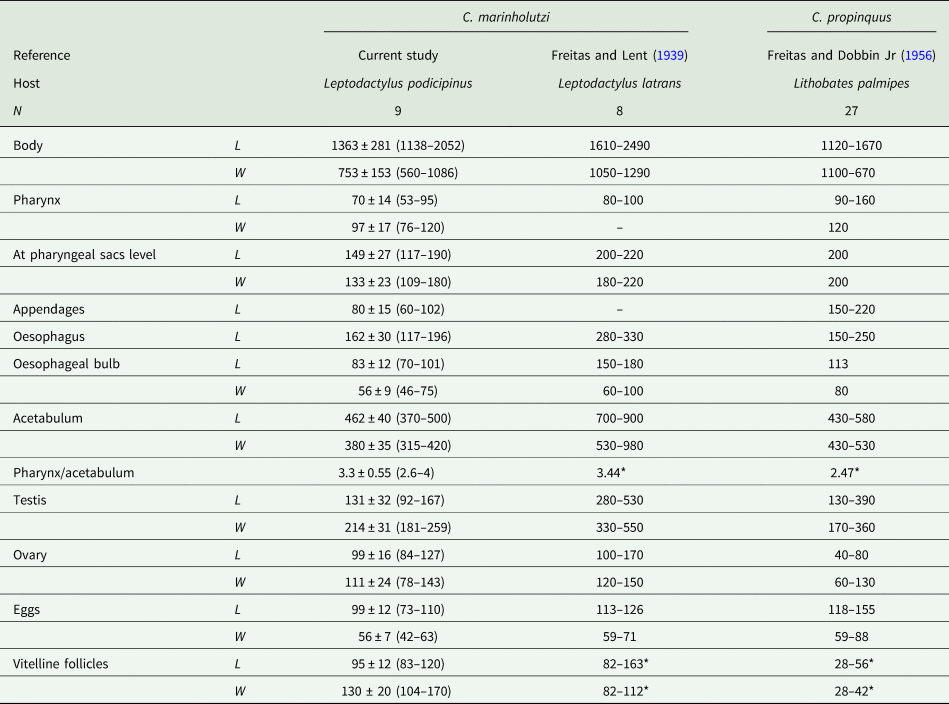
L, length, W, width.
Asterisks indicate data calculated from figures presented in the cited references.
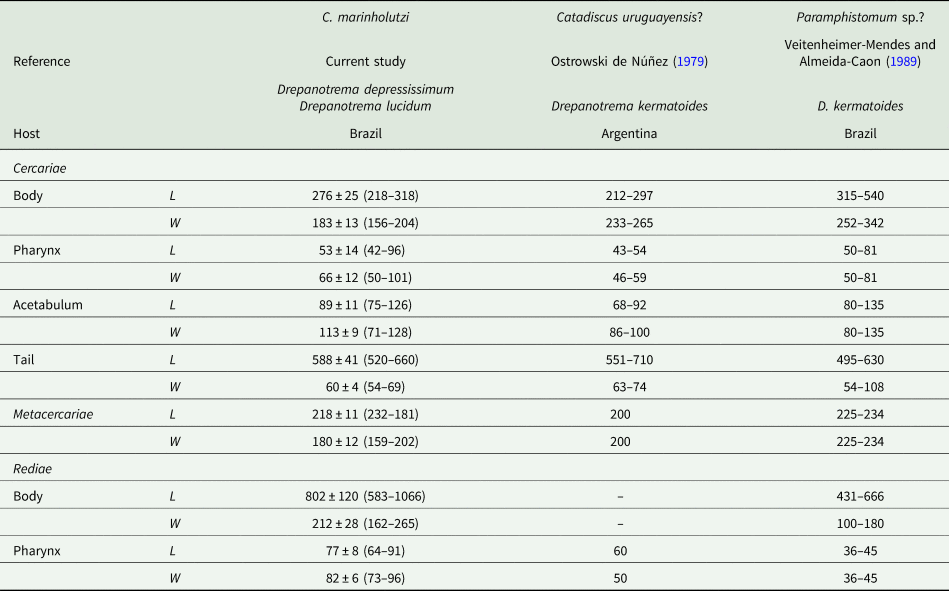
Table 3. Morphometric data of cercariae, metacercariae and rediae of Catadiscus marinholutzi found in Drepanotrema spp. from Brazil
L, length; W, width.
Data reported for amphistome cercariae found in D. kermatoides in South America by other authors are shown for comparison.
Taxonomic summary
Catadiscus marinholutzi Freitas and Lent, Reference Freitas and Lent1939 (Figs 1–4; Tables 2 and 3).
Type-host and locality: Leptodactylus latrans (Steffen, 1815), Aquidauana, State of Mato Grosso do Sul, Brazil.
Definitive hosts: Leptodactylus macrosternum (New Host Record – NHR), Leptodactylus podicipinus, Pseudopaludicola sp. (NHR) (Anura: Leptodactylidae), Boana raniceps (NHR), Pseudis platensis (NHR) (Anura: Hylidae).
First intermediate hosts: Drepanotrema lucidum and Drepanotrema depressissimum (Mollusca: Planorbidae).
Locality: Selvíria (New Locality Record), State of Mato Grosso do Sul, Brazil.
Representative DNA sequences (partial 28S rDNA): MW618663 from D. lucidum (snail host) and MW618662 from L. podicipinus (anuran host).
Morphological description
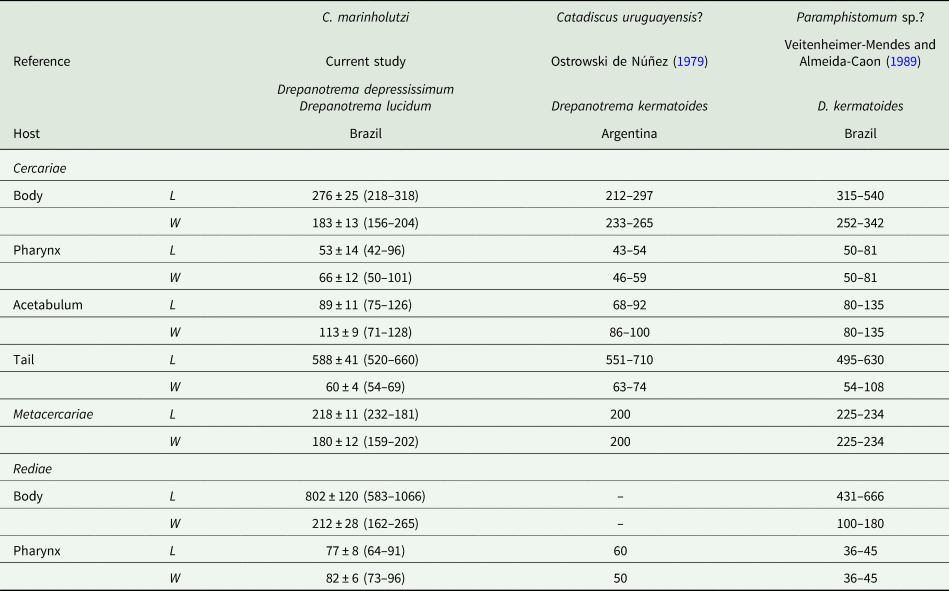
Adults (Figs 1 and 2, Table 1): Body piriform with smooth tegument (Figs 1A, B and 2A). Mouth terminal with undulated edges surrounding oral aperture (Fig. 2A, B). Pharynx muscular, with two evident pharyngeal sacs (Fig. 1C). Oesophagus long, with a muscular bulb. Two small caeca reaching up to equatorial region. Genital pore small, pre-equatorial, opening at caeca bifurcation level, with a prominent sucker-like structure (genital papillae) surrounding the genital pore (Figs 1A, D and 2A, C). Cirrus sac globose . Acetabulum ventral and subterminal (Figs 1A, B and 2A); large, with a transversal constriction (Figs 1A, B and 2A, D). Testis single, oval, smooth, intercaecal, median or submedian (Fig. 1A and B). Ovary rounded or oval, sometimes elongated, testicular or post-testicular (Fig. 1A and B). Vitellaria with large follicles, larger than eggs, lying between the acetabulum and caecal bifurcation, confluent anteriorly (Fig. 1A and E). Uterus sinuous, dorsal, overlapping the acetabulum zone, extending to bifurcal region. Eggs oval, operculated, smaller than vitelline follicles.
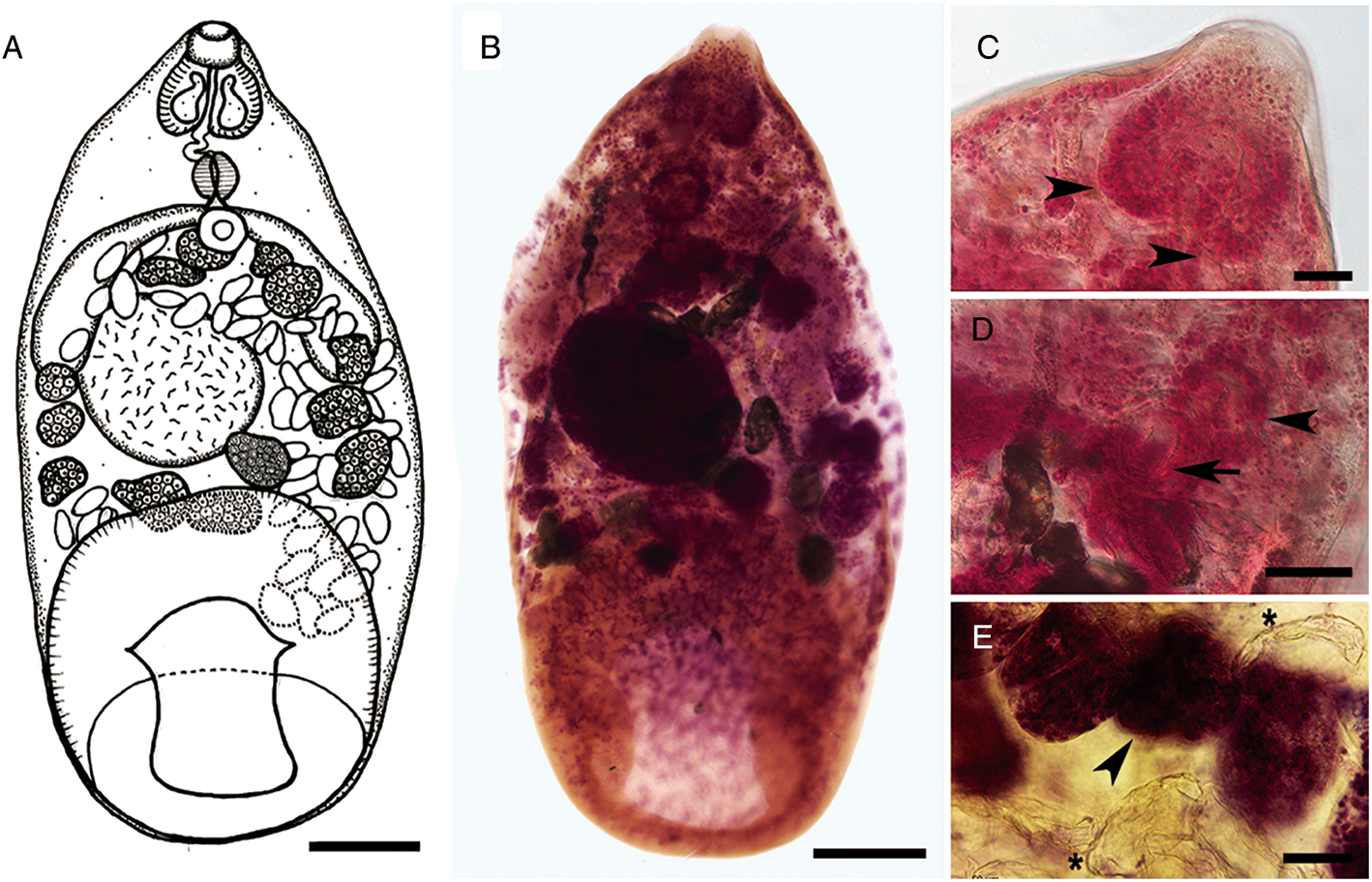
Fig. 1. Catadiscus marinholutzi adult recovered from Leptodactylus podicipinus from Brazil: (A) Line drawing of a mature worm. (B) Body in ventral view of a haematoxylin-stained worm. (C) Details of pharynx with primary sacs (arrowhead). (D) Details of oesophageal bulb (arrowhead) and genital pore (arrow). (E) Details of the relative size of vitelline follicles (arrowhead) and eggs (asterisks). Scale bars: A, B = 200 μm; C, E = 50 μm, D = 100 μm.
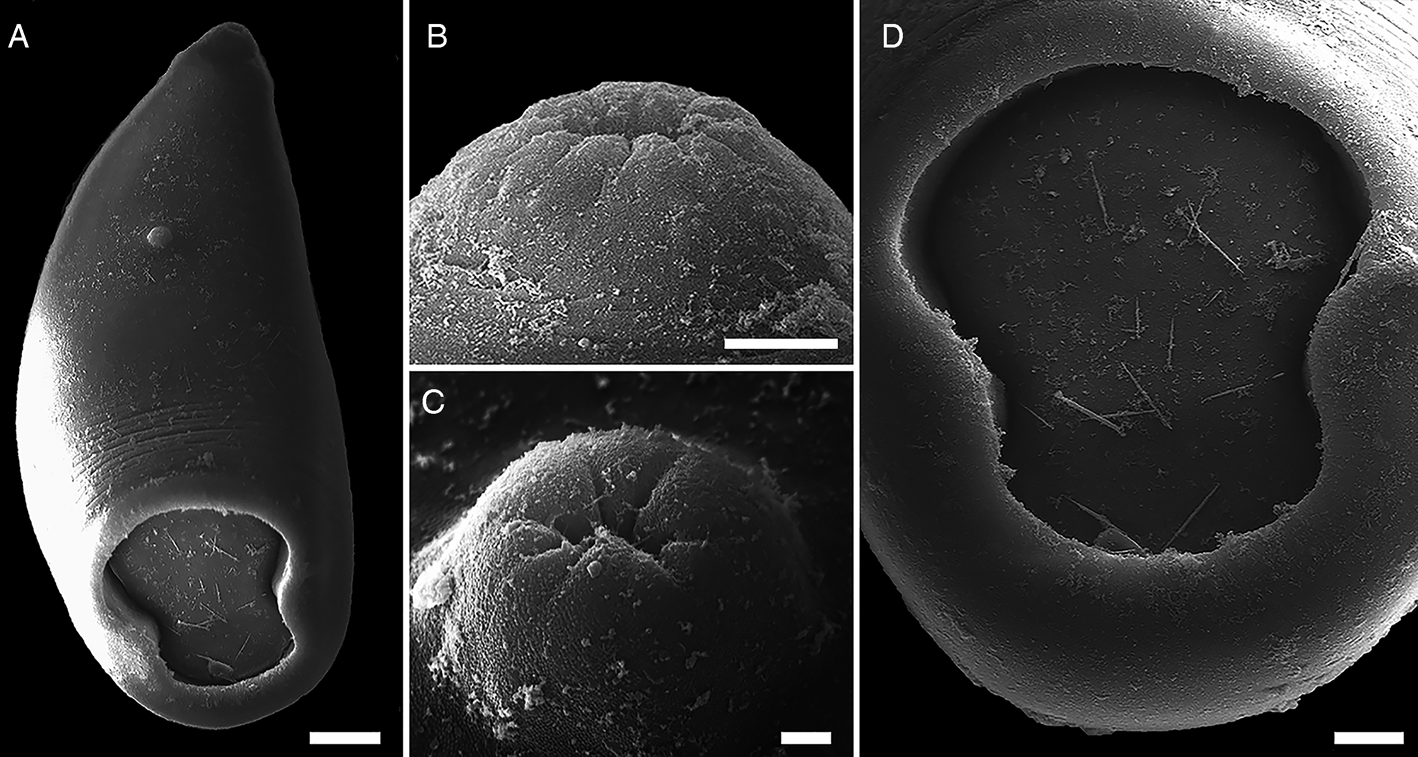
Fig. 2. SEMs of Catadiscus marinholutzi from Leptodactylus podicipinus. (A) Body in ventral view showing the genital pore. (B) Oral opening surrounded by undulated edges. (C) Details of the genital pore bearing a genital papillae. (D) Details of the median transversal constriction of acetabulum. Scale bars: A = 50 μm, B = 10 μm, C = 2 μm, D = 20 μm.
Cercariae (Figs 3 and 4A‒C, Table 3): Body ovoid with black pigmentation, except in mouth and pharynx region (Fig. 4A). Two pigmented, square-shaped eyespots in the anterior body, between pharyngeal sacs and oesophageal bulb (Figs 3 and 4B). Abundant cystogenous cells in two shapes, with granular contents in lateral-body and with rod-shaped contents in the mid-body (Figs 3 and 4B). Mouth terminal with undulated border, followed by a muscular pharynx with two pharyngeal sacs longer and wider than pharynx. Oesophagus long, ending with a muscular bulb. Two relatively short caeca, posteriorly enlarged, running laterally up to the mid-body, distant from the acetabulum. Acetabulum large, subterminal, constricted in the middle region (Figs 3 and 4A, C). Excretory system constituted by an oval excretory vesicle, pre-acetabular, two main collecting ducts, with numerous small granules inside, united above excretory vesicle, running sinuously up to posteriormost pharyngeal region, surpassing the eyespots. Medial anastomosis of main collecting ducts absent. Additional data of the excretory system were not observed because of the intense pigmentation of the body. Tail long, more than twice the body length, cylindrical, progressively tapered, presenting a long caudal excretory duct slightly enlarged towards tip.
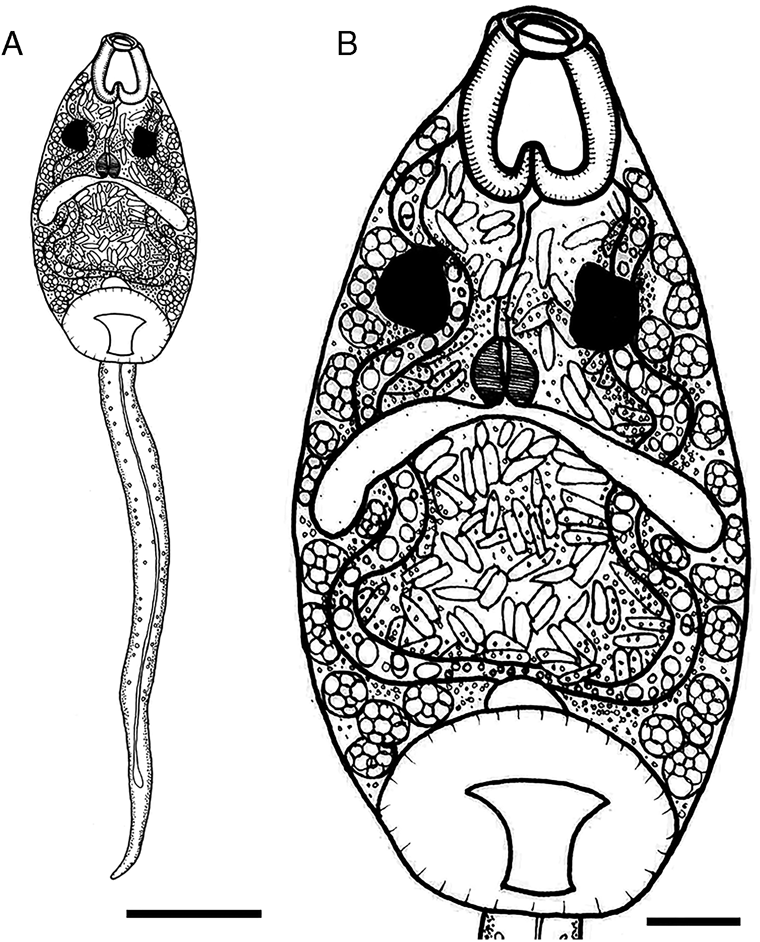
Fig. 3. Line drawing of cercariae of Catadiscus marinholutzi found in Drepanotrema lucidum. (A) Whole view of cercaria. (B) Details of cercarial body. Scale bars: A = 100 μm, B = 25 μm.
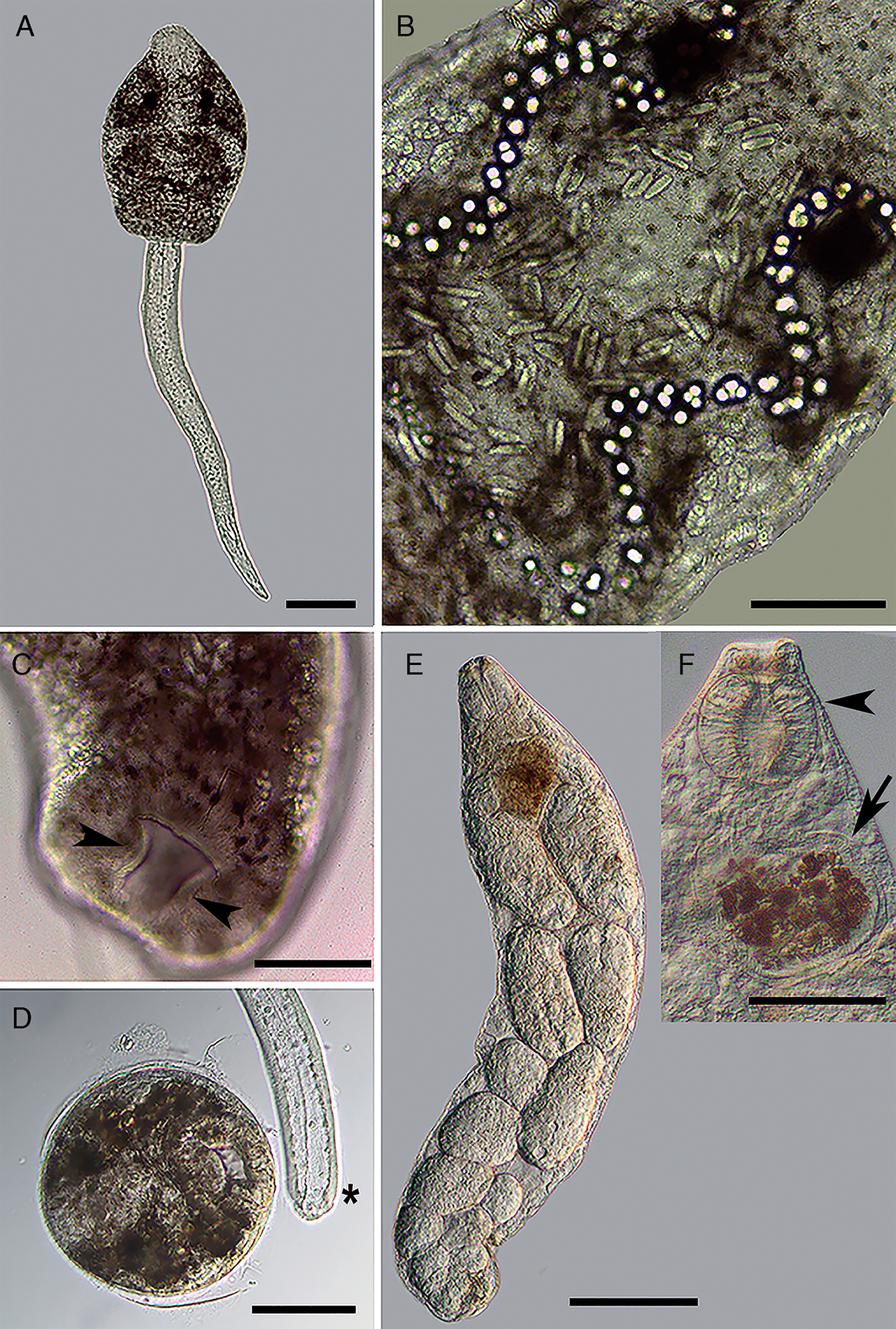
Fig. 4. Larval stages of Catadiscus marinholutzi found in Drepanotrema lucidum. (A) Whole-mount of the amphistome cercaria, ventral view. (B) Details of cercarial body. (C) Details of the constriction in the acetabulum of cercaria (arrowhead). (D) Recently encysted metacercaria and detail of the detachment of the tail from the body (asterisk). (E) Whole-mount of redia. (F) Details of anterior region of redia, evidencing the pharynx (arrowhead) and caecum (arrow). Scale bars: A, D, F = 100 μm; B, C = 50 μm. E = 200 μm.
Metacercariae (Fig. 4D): Cysts spherical, cystic wall thin.
Rediae (Fig. 4E and F): Body transparent, elongated. Pharynx subterminal. Caecum short, sac-like, with brown granules inside. Several germinal balls (8–10) at different stages of development scattered in the body.
Remarks
The morphology and measurements of adults of C. marinholutzi reported in this study are compatible with those described by Freitas and Lent (Reference Freitas and Lent1939). Although full range of variation for most morphometric data is higher in our specimens than those reported in the original description, the mean body length and width of our individuals are slightly smaller than the interval reported by Freitas and Lent (Reference Freitas and Lent1939). These differences may come from different fixation and mounting processes, comparison of worms at different stages of the ontogenic development, or it indicates a high morphometric variability within the species. Such variability, which may be host-induced (e.g. host size, age and geographical distribution), cannot be overlooked in species delineation of Catadiscus based on morphological features alone.
Few cercariae of C. marinholutzi reported here in Drepanotrema spp. emerged from the snails on the morning and encysted on the bottom of the container. They belong to the Diplocotylea group of amphistome cercariae, characterized mainly by the presence of pharyngeal sacs and absence of medial anastomosis in the main collecting ducts (Sewell, Reference Sewell1922; Dawes, Reference Dawes1968; Schell, Reference Schell1985). The presence of a transverse constriction in the acetabulum, a trait known for the adult stages of the genus Catadiscus, is reported here in the cercariae. Considering amphistome cercariae reported in Drepanonetrema spp. from South America, those described as the probable larval stage of Catadiscus uruguayensis Freitas and Lent, Reference Freitas and Lent1939 by Ostrowski de Núñez (Reference Ostrowski de Núñez1979) presents measurements similar to the morphometric data gathered from C. marinholutzi. However, some morphological differences are noticeable, especially concerning the position of the caeca (long, extending up to the acetabulum), extension of main ducts of the excretory system and position of the excretory vesicle (dorsal to acetabulum), absence of an oesophageal bulb and especially the non-constricted acetabulum. The amphistome cercariae reported from Drepanotrema kermatoides (Orbigny, 1835) in Brazil by Veitenheimer-Mendes and Almeida-Caon (Reference Veitenheimer-Mendes and Almeida-Caon1989) is significantly longer and wider than the cercariae of C. marinholutzi and possess medial anastomosis in the main excretory ducts, eyespots with lenses and an acetabulum without transverse constrictions. Despite unequivocal experimental evidence is still required to determine the route of infection, the specimen of T. typhonius became infected probably by the ingestion of encysted metacercariae present in the food. Anyway, the results obtained reveal that the ingestion of its own shed skin is not a mandatory event, given the recuperation of parasites occurs before metamorphosis.
Phylogenetic analyses
The two identical, newly generated 28S sequences from cercariae and adults of C. marinholutzi were 1219 bp long. The alignment of 46 sequences comprised of 1431 positions, of which 453 were excluded, resulting in a final alignment of 978 positions with 278 parsimony-informative sites. The phylogenetic trees resulting from the BI and ML analyses were identical in topology, except for the relationship among representatives of Diplodiscus (see ML tree depicted in Fig. S1). The ingroup formed a virtual polytomy composed of five clades equally related to each other: (i) amphistome parasites of warm-blooded terrestrial hosts (collapsed clade), including representatives of the Zygocotylidae, Gastrodiscidae, Paramphistomidae, Gastrothylacidae and Olveriidae; (ii) cladorchiids (Cladorchiidae) that have colonized aquatic and terrestrial cold-blooded hosts ‒ except for Solenorchis travassosi Hilmy, 1949, known from the marine mammal Dugong dugon (Müller, 1776) ‒ from unrelated hosts and geographical regions; (iii) fish cladorchiids from the Neotropical region; (iv) members of Diplodiscus, type genus of the Diplodiscidae from the Palaearctic region and (v) isolates of C. marinholutzi (also Diplodiscidae) from its intermediate and final hosts (Fig. 5).
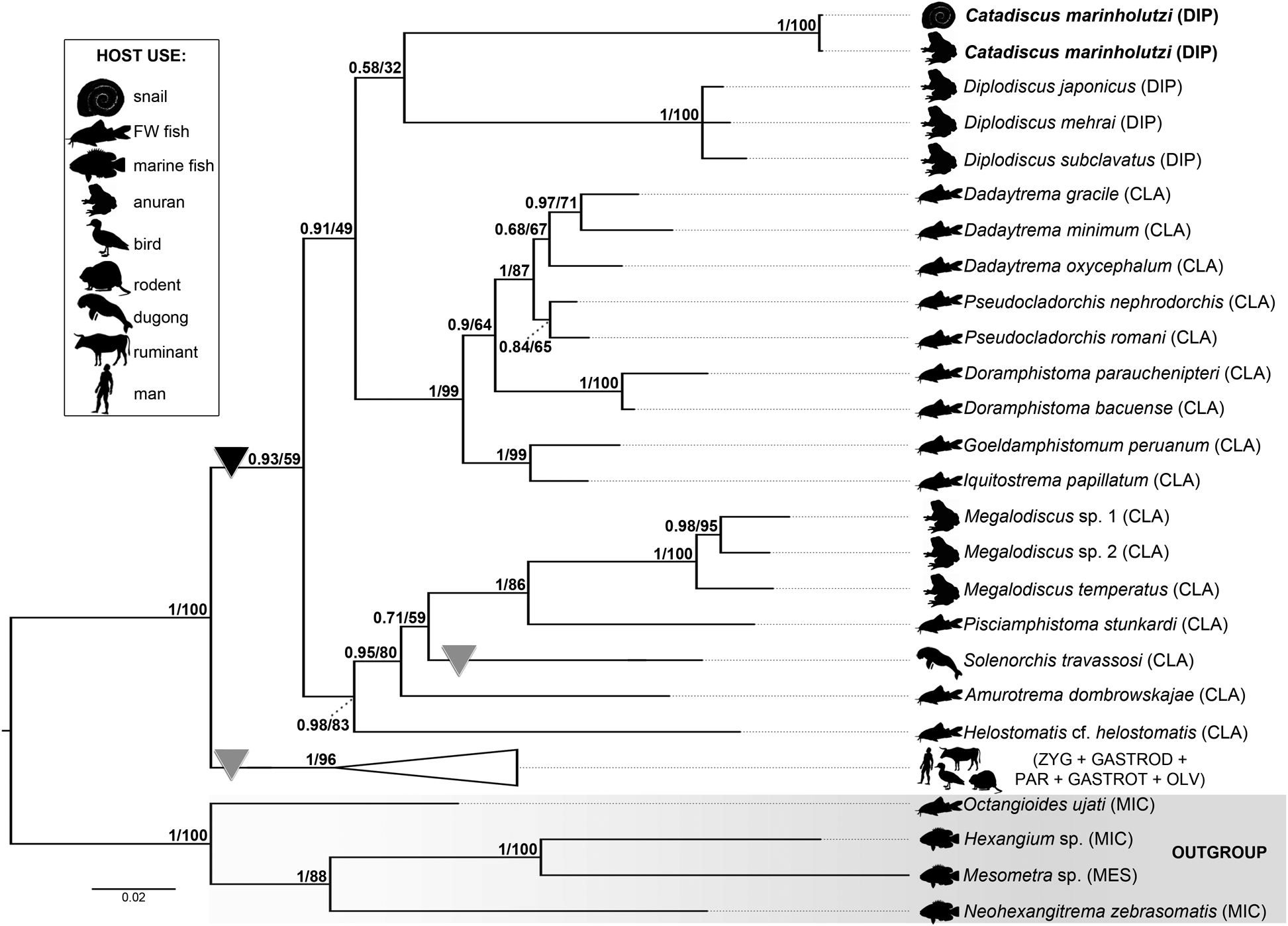
Fig. 5. BI phylogram based on partial 28S sequences of Catadiscus marinholutzi and selected members of the Paramphistomoidea. BI posterior probabilities and ML UFbootstrap support values are shown above nodes. Newly generated sequences are highlighted in bold. Parasitism in cold- and warm-blooded hosts are indicated by a black and a grey triangle, respectively. Branch length scale bar indicates the number of substitutions per site. CLA, Cladorchiidae; DIP, Diplodiscidae; GASTROD, Gastrodiscidae; GASTROT, Gastrothylacidae; MES, Mesometridae; MIC, Microscaphidiidae, OLV, Olveriidae; PAR, Paramphistomidae; ZYG, Zygocotylidae.
Even though C. marinholutzi appeared as an independent lineage within the Paramphistomoidea, its phylogenetic relationships remain unclear. In addition, there is no evidence for the monophyly of the Diplodiscidae considering the branch support of the group formed by C. marinholutzi and Diplodiscus spp. (32% UFbootstrap and 0.58 posterior probability support values) and the relatively high genetic divergence verified between them (ranging from 10.06 to 10.84%; 65–70 nt differences). The species of Megalodiscus, found primarily in amphibians and once included within Diplodiscidae, grouped in a moderately supported clade with other cladorchiids from unrelated hosts and zoogeographical realms, while the remaining representatives of Cladorchiidae from Neotropical fishes formed a well-supported clade; genetic divergence between C. marinholutzi and Megalodiscus spp. ranged from 12.48 to 13.47% (80–87 nt differences).
Pairwise comparisons within well-supported monophyletic assemblages revealed lower intergeneric divergences in the Gastrodiscidae (1.08%; 7 nt differences), Zygocotylidae (1.70%; 11 nt differences), and Neotropical cladorchiids (1.39–6.19%; 9–40 nt differences) than that exhibited between the diplodiscids Catadiscus and Diplodiscus (see above).
Discussion
The results obtained here for C. marinholutzi from anuran and snail hosts bring new insights into the biology and taxonomy of the genus Catadiscus, formed by poorly studied trematodes found primarily infecting amphibians from Neotropics. Using an integrative taxonomic approach for studying C. marinholutzi, we successfully linked cercariae found in snails and adults recovered from amphibians. Moreover, this study represents the first attempt to include a species of Catadiscus into a phylogenetic context. Our results suggest that a taxonomic reorganization may be necessary to accommodate species of this genus.
The species identified in the current study as C. marinholutzi was originally described by Freitas and Lent (Reference Freitas and Lent1939) from anurans identified as Leptodactylus ocellatus (L.) and Leptodactylus caliginosus Girard, 1853 [both currently considered synonyms to Leptodactylus latrans (Steffen, 1815)] collected in Salobra and Aquidauana (district of Camisão), localities from State of Mato Grosso Sul, Brazil, not so far from our sampling area (about 600 km). Later, additional reports of the infection of leptodactylids (L. latrans and L. podicipinus) with this amphistome were registered in different localities in Brazil (Travassos, Reference Travassos1940; Goldberg et al., Reference Goldberg, Bursey, Caldwell and Shepard2009; Graça et al., Reference Graça, Oda, Lima, Guerra, Gambale and Takemoto2017). The species was also reported in Rhinella fernandezae (Gallardo, 1957) from Argentina (Hamann et al., Reference Hamann, Kehr and González2013), greatly expanding its distribution. Out of South America, the species was found in colubrid snakes [Coniophanes bipunctatus (Günther, 1858), Coniophanes imperialis (Baird and Girard, 1859), Coniophanes quinquevittatus (Duméril, Bibron and Duméril, 1854), Leptodeira septentrionalis (Kennicott, 1859) and Thamnophis sp.] from Mexico (Thatcher, Reference Thatcher1964; Sey, Reference Sey1991; Paredes-León et al., Reference Paredes-León, Garcia-Prieto, Guzman-Cornejo, Leon-Regagnon and Perez2008). In the current study, four new definitive hosts (L. macrosternum, Pseudopaludicola sp., B. raniceps and P. platensis) are reported for C. marinholutzi, including the first record in hylid anurans. Environments that present seasonal floods, i.e. water expansion and retraction in annual cycles, such as the Pantanal wetlands and our sampling area, promote ecological opportunities via ecological fitting (Janzen, Reference Janzen1985) for new host–parasite associations (see D'Bastiani et al., Reference D'Bastiani, Campião, Boeger and Araújo2020 and references therein). This process may have boosted the encounters of C. marinholutzi with a diverse array of aquatic and semiaquatic anuran hosts. Regardless of environmental conditions, these findings are not unexpected given that Catadiscus species are known to have broad host specificity. Besides amphibians and non-avian reptiles, adult forms of Catadiscus spp. have been found in the golden apple snail Pomacea canaliculata (Ampullariidae) and the annual fishes Austrolebias spp. (Rivulidae) in Argentina and Uruguay, respectively (Hamann, Reference Hamann1992; Vettorazzi et al., Reference Vettorazzi, Norbis and Martorelli2020).
The morphometric data obtained here are compatible with the description of C. marinholutzi. Also, first SEM images of a Catadiscus species demonstrated more clearly the presence of a constriction in the acetabulum of C. marinholutzi, a typical trait of the genus Catadiscus but described as being relatively poorly developed in this species (Freitas and Lent, Reference Freitas and Lent1939). The presence of a small genitalium bearing a prominent genital papillae is evidenced for the first time in species of Catadiscus by the aid of SEM. Histological sections of this structure are broadly used to characterize different groups of paramphistomoids (see Sey, Reference Sey1991), but likely due to their small size, diplodiscid taxa have never been evaluated by means of this approach. Therefore, further comparative studies of the terminal genitalium among members of the Displodiscidae may be useful to support or refute the current classification scheme of the family. Morphologically, C. marinholutzi most closely resembles C. propinquus. Both species possess vitelline follicles confluent anteriorly (Sey, Reference Sey1991); however, they can be distinguished by the pharynx/acetabulum ratio (higher than 1:3 in C. marinholutzi vs lower than 1:3 in C. propinquus). Moreover, the presence of larger vitelline follicles (larger than eggs) is clearly the main differential feature between C. marinholutizi and C. propinquus. In fact, in C. propinquus, the vitelline follicles are smaller [28–56 μm × 28–42 μm, as obtained from line drawings published by different authors (Travassos et al., Reference Travassos, Freitas and Kohn1969; Hamann, Reference Hamann2004)] than those in C. marinholutzi studied here (71–142 μm × 63–170 μm) or calculated from the drawings in the original description (82–163 μm × 82–112 μm).
In the samples of C. marinholutzi evaluated in this study it was verified the presence of worms at different stages of maturation, including ovigerous juveniles and mature worms. Similar results were also found in C. propinquus from Argentina (Hamann, Reference Hamann2004). We consider that the presence of worms at different stages of maturation can contribute to the overestimation of the Catadiscus diversity as some species were described based on few apparently not complete mature specimens [e.g. Catadiscus pygmaeus (Lutz, 1928)]. Moreover, it is not possible to know if juvenile ovigerous worms were considered in species descriptions. Thus, we recommend that comparative morphometric studies should be based on fully developed parasites. Further studies to evaluate the impact of the differences in parasite maturation, fixation techniques as well as host-induced phenotypic plasticity are also required. That being said, to overcome any obstacle for the reliable identification of Catadiscus spp., the combining of different sources of information (e.g. morphology and DNA) are required.
In the current study, the link between larval and adult stages of C. marinholutzi was based on experimental and molecular approaches. Both larval stages (cercariae and metacercariae) have a constricted acetabulum, as observed in their adult counterparts. Interestingly, in other genera of the Paramphistomoidea, including in some species of Diplodiscidae, the shape of acetabulum and arrangement of intestinal caeca in adults and cercariae are similar (Yamaguti, Reference Yamaguti1975; Besprozvannykh et al., Reference Besprozvannykh, Rozhkovan, Ermolenko and Izrailskaya2018). Such similarities between larvae and adults are thought to be common among ‘primitive’ trematodes (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). Despite the necessary caution related to identification of larval stages, it is possible that the study of cercariae can support the identification at least at the generic level of amphistomes from anurans.
Regarding the first intermediate host, species of the South American genus Drepanotrema (Planorbidae) are confirmed to harbour larval stages of a species of Catadiscus. Ostrowski de Núñez (Reference Ostrowski de Núñez1979) described the putative cercariae of C. uruguayensis based on herpetological and malacological findings recovered in a single pond in Uruguay. The cercariae of C. marinholutzi described in our study are larger than those identified as C. uruguayensis. It is important to note that both line drawing and description of cercariae of C. uruguayensis by Ostrowski de Núñez (Reference Ostrowski de Núñez1979) do not show the acetabulum medially constricted, a feature easily observed in the described adults of C. uruguayensis. Given the lack of experimental or molecular confirmation, cercariae suggested by Ostrowski de Núñez (Reference Ostrowski de Núñez1979) as being C. uruguayensis may belong to another amphistome group, which deserves new studies.
Most species of Diplodiscus with known life cycles have planorbids of the subfamily Planorbinae as intermediate hosts. The finding of Drepanotrema spp. as host of C. marinholutzi is an additional evidence of the involvement of this host group in the transmission of amphistomes from anurans. Aspects related to specificity to the first intermediate host require more in-depth studies on paramphistomoids, especially in Diplodiscidae, since populations of the same species were reported infecting phylogenetically distant planorbid genera (Sey, Reference Sey1991). In the case of C. marinholutzi, it appears to be specific to species of Drepanotrema, given the other planorbid examined (1013 specimens of Biomphalaria occidentalis) were not found infected with this parasite. Regarding the infection of the definitive host, it probably occurs by the ingestion of encysted metacercariae present in food, as already elucidated to several other paramphistomoids, including amphibian parasites (Yamaguti, Reference Yamaguti1975). However, new studies are required to test the possibility of infection by ingestion of cercariae, which is a real possibility considering anuran tadpoles, as verified to Diplodiscus (Besprozvannykh, et al., Reference Besprozvannykh, Rozhkovan, Ermolenko and Izrailskaya2018)
The phylogenetic reconstructions based on 28S rDNA sequence data revealed that representatives of Catadiscus and Diplodiscus (type genus of Diplodiscidae) did not clustered in a well-supported clade. Hence the monophyly of the family Diplodiscidae was not supported in our molecular phylogeny. In addition to that, unusually high intergeneric divergence among these two diplodiscid taxa, much higher than in other monophyletic assemblages recovered from our analyses, provides further evidence for the non-monophyly of the group that will likely require a revaluation of species composition and the proposal of a new family for species of Catadiscus. Nevertheless, there are no sequences to species of other genera currently included in the family Diplodiscidae [Progonimodiscus Vercammen-Grandjean, 1960 (in frogs from Africa), Dermatemytrema Price, 1937 (in turtles from North America), Pseudodiplodiscus Manter, 1962 (in fish from South America), and Australodiscus Sey, Reference Sey1983 (in frogs from Oceania – Australia)] (Sey, Reference Sey1991; Jones, Reference Jones, Jones, Bray and Gibson2005b).
Morphologically, members currently included in Diplodiscidae can be separated into two groups: with (Progonimodiscus, Australodiscus and Diplodiscus) and without (Catadiscus, Dermatemytrema and Pseudodiplodiscus) central peduncle or sucker in acetabulum (Jones, Reference Jones, Jones, Bray and Gibson2005b); in the latter case all species were described from the Americas. Acetabulum morphology may be phylogenetic informative and therefore useful for higher-level classification of these amphistomes from the New World, but, as above-mentioned, the relative taxonomic value of such characters should also be assessed in further phylogenetic studies. Even though Yamaguti (1971, p. 393) proposed the subfamily Catadiscinae to include amphistomes from amphibians and reptiles having a constriction in the acetabulum, this taxon was not considered in any subsequent revision (Sey, Reference Sey1983, Reference Sey1991, Jones, Reference Jones, Jones, Bray and Gibson2005b). Whether further studies confirm the distinctive molecular signature of Catadiscus species as suggest by the 28S rDNA gene for C. marinholutzi, we might consider elevating the Catadiscinae sensu Yamaguti (Reference Yamaguti1971) to the familial level in order to accommodate all members of this genus.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021000330
Acknowledgements
We thank the reviewers for the constructive comments and suggestions, which significantly improved the quality of the manuscript.
Author contributions
M.S.Q., L.A.A. and H.A.P. conceived and designed the study. M.S.Q. and L.A.A. conducted fieldwork. D.L.H. and P.V.A. performed molecular and phylogenetic analyses. M.S.Q., P.V.A., D.L.H., L.A.A. and H.A.P. wrote the article.
Financial support
We thank the National Council for the Improvement of Higher Education (CAPES, Brazil) (scholarship to D.L.H. and P.V.A.) and the National Council for Scientific and Technological Development (CNPq, Brazil) (research scholarship to H.A.P. and L.A.A.).
Conflict of interest
None.
Ethical standards
The capture of anurans was authorized by the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA) (research permit SISBIO-58746-4). The experimental procedures were carried out in this study following the recommendation of the local committee of ethics in animal experimentation (FEIS/UNESP – protocol CEUA/FEIS – 12/2018).