Introduction
The process whereby seeds or spores sprout and begin to grow is a fundamental and sometimes limiting process in the life cycle of a plant species. Knowledge about seed germination is therefore essential to understand ecological adaptations (Albert et al., Reference Albert, Iriondo and Pérez-García2002; Tobe et al., Reference Tobe, Li and Omasa2004; Giménez-Benavides et al., Reference Giménez-Benavides, Escudero and Pérez-García2005), reproductive strategies (Doussi and Thanos, Reference Doussi and Thanos1997; Meyer and Witmer, Reference Meyer and Witmer1998; Traveset et al., Reference Traveset, Riera and Mas2001) or the level of plant establishment in natural environments (Quilichini and Debussche, Reference Quilichini and Debussche2000). Information about seed germination and requirements for dormancy break is also important for restoration projects, where there is often a limited supply of seeds (Middleton, Reference Middleton1999).
Although seed germination can be regulated by several genetic factors (Nambara et al., Reference Nambara, Hayama, Tsuchiya, Nishimura, Kawaide, Kamiya and Naito2000; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008), environmental factors are major determinants in promoting germination under suitable conditions (Beardsell and Richards, Reference Beardsell and Richards1987). Among these determinants, temperature and light play a crucial role in seed germination (Baskin and Baskin, Reference Baskin and Baskin1988; Meyer et al., Reference Meyer, Carlson and Garvin1998; Marone et al., Reference Marone, Horno and Solar2000). Temperature controls germination by influencing the loss of seed dormancy and the rate of germination, or by inducing secondary seed dormancy (Bewley and Black, Reference Bewley and Black1994), whereas light is one of the main determinants for the existence of a persistent soil seed bank (Baskin and Baskin, Reference Baskin and Baskin1989; Pons, Reference Pons1991).
Environmental factors should have important implications for germination and survival of Mediterranean plants since the climate is well characterized by its seasonality in temperature and precipitation (Thompson, Reference Thompson2005). For instance, seedlings of most plant species emerge in spring and/or in the autumn season (Baskin and Baskin, Reference Baskin and Baskin1998; Thompson, Reference Thompson2005). Nevertheless, survival of spring-germinated plants is usually higher than that of autumn-germinated plants, at least in species that germinate in both seasons (Masuda and Washitani, Reference Masuda and Washitani1992; Picó, Reference Picó2012), which suggests the existence of some constraints in their life cycles.
Autumn-flowering geophytes can be considered as a suitable phenological model since species exhibit a markedly phenological strategy: most geophytes avoid the hot and dry summer season and the cold winter through dormancy (Dafni, Reference Dafni1996), while a few bloom in early autumn producing seeds during the late-autumn season (Marques and Draper, Reference Marques and Draper2012). However, despite various studies concerning the germination of Mediterranean species, information about geophytes remains scarce (but see Doussi and Thanos, Reference Doussi and Thanos2002; Vandelook and Van Assche, Reference Vandelook and Van Assche2008).
Geophytes exist in a variety of habitats in Mediterranean-climate zones, including fire-prone ecosystems, and although fire has a promoting effect on flowering of many geophytes (Lamont and Downes, Reference Lamont and Downes2011), its effects on seed germination vary from a lack of fire-stimulated germination (Keeley, Reference Keeley1987; Doussi and Thanos, Reference Doussi and Thanos2002) to a significant decrease in germination of heated seeds in comparison with controls (Keeley, Reference Keeley1987, 1995). Nonetheless, there is a consensus that after a fire there is an increase in seedling recruitment (Doussi and Thanos, Reference Doussi and Thanos2002). In this sense, the existence of a persistent soil seed bank with viable seeds through time would be an important strategy in this type of plants. However, to our knowledge, there is no information about studies related to the soil seed bank and seed longevity of Mediterranean geophytes.
Thus, this work aims to provide information about the germination behaviour of geophytes. In order to achieve this, five autumnal-flowering Mediterranean species were selected: Leucojum autumnale L. (Amaryllidaceae), Narcissus cavanillesii A. Barra & G. López (Amaryllidaceae), N. serotinus L. (Amaryllidaceae), Scilla autumnalis L. (Asparagaceae) and Urginea maritima (L.) Baker (Asparagaceae). Generally, these five geophytes are widely distributed in the Mediterranean basin (Tutin et al., Reference Tutin, Heywood, Burges, Moore, Valentine, Walters and Webb1980) except N. cavanillesii, which is endemic in the south-western Iberian Peninsula and north Africa (Rosselló-Graell et al., Reference Rosselló-Graell, Marques and Draper2003). These geophytes occur in a wide range of Mediterranean habitats, showing no particular habitat preferences. All species flower during early autumn while the seeds are released during the late autumn season. Thus, we expect some strategies that might favour germination in these conditions or some mechanism of delayed germination, postponing germination until early spring to prevent mortality during winter.
In the present work, we study the germination behaviour of these five Mediterranean geophytes to understand the patterns of germination during the autumn season. The specific questions were: (1) What are the temperature requirements to promote germination? (2) Does light have an effect on the germination of these geophytes? (3) Is the germination fire-dependent? and (4) Does seed viability change with time? All these questions allow us to reveal whether these co-blooming species share the same germination patterns or if they have specific requirements for germination.
Materials and methods
Seed collection
Seed samples were collected in October–November 2003 in the province of Alto Alentejo (Portugal) in four populations: (1) Ajuda (AJU; Elvas, longitude − 7.1633, latitude 38.7749; collected 25 October), mainly composed of holm oak woods and Mediterranean riparian communities dominated by Nerium oleander L. galleries and thickets; (2) Montejuntos (MJT; Alandroal, longitude − 7.3186, latitude 38.5053; collected 30 October), mainly composed of open scrub communities dominated by Cistus ladanifer, Genista hirsuta and Ulex argenteus; (3) Outeiro do Pombo (OUT; Alandroal, longitude − 7.3038, latitude 38.5336; collected 1 November), largely composed of open scrubs of Retama monosperma and C. ladanifer; and (4) Monte Fidalgo (FID; Elvas, longitude − 7.28 576, latitude 38.7131; collected 15 November), dominated by Mediterranean scrub communities of C. ladanifer. Following the classification of Rivas-Martínez et al. (Reference Rivas-Martínez, Lousa, Díaz, Fernández-González and Costa1990), the climate is sub-humid Mediterranean with an annual mean temperature of 16°C and precipitation of 670 mm (Instituto Nacional de Meteorologia e Geofísica, 1961–1990). In the region where the seeds were collected, the annual temperature varies between a maximum of 25°C and a minimum temperature of 9°C, although during the studied months (October–November) the minimum temperature only reaches 13.9°C and the maximum temperature, 23.1°C. Relative humidity (RH) has a mean value of 60% during these months.
Small sets of seeds were kept in paper bags, stored in the dark for 1 month in a drying chamber set at 10°C and RH 10%, and weighed until equilibrium to determine the dry seed mass of each species. The mean (n = 100) air-dry seed mass was 1.29 ± 0.09 mg for L. autumnale, 1.30 ± 0.08 mg for N. cavanillesii, 1.09 ± 0.07 mg for N. serotinus, 0.89 ± 0.03 mg for S. autumnalis and 2.70 ± 0.11 mg for U. maritima (mean ± SD considering all populations; no significant differences were found between them at P = 0.05). All species have black seeds, and they are usually small (less than 4 mm) and round, with the exception of U. maritima that has a fattened, winged testa. Embryos of all species were small in comparison to the rest of the seed but fully developed at the time of collection. The remaining seeds were kept in paper bags under laboratory conditions (darkness, 21 ± 1°C) until germination tests began c. 2 weeks after collecting the seeds (considering the time of collection of each population). Visibly deficient seeds were excluded from the experiments.
Germination tests
Seeds were placed in Petri dishes (7 cm in diameter) lined with two filter paper discs and moistened with 3 ml of distilled water. Petri dishes were kept in four different incubators set at 15, 20, 25 or 25/15°C (under darkness and light conditions) in order to simulate those conditions prevailing in the study area during the selected months (see above). Darkness was obtained by wrapping the dishes in aluminium foil, while in the experiments testing the effects of light, seed incubation occurred under a 16-h light/8-h dark photoperiod. Light was provided by cool white fluorescent tubes with an irradiance of 35 μmol m− 2 s− 1 (Osram Lumilux Cool white). When the alternating temperature was used, the lower temperature coincided with the dark period. Dishes under conditions of light and darkness were monitored every 2 d over a period of 30 d and germinated seeds were removed. The dark seeds were examined in a darkroom under a dim green safelight (Doussi and Thanos, Reference Doussi and Thanos2002). Radicle emergence was the criterion for deciding if germination had taken place (Come, Reference Come1970). Each value is the mean ± standard deviation (SD) of four replicates of 25 seeds. In addition, to study seed longevity, the seeds collected in AJU were used after 6, 12 and 18 months of storage and the results were compared with those obtained at 1 month of age. In this case, seeds were stored in a dry, dark chamber (during the study periods of time), set at 5°C and 60% RH (RH at the time of seed collection in natural populations). At the end of all experiments, non-germinated seeds were checked for viability through the tetrazolium test (2,3,5-triphenyl-tetrazolium chloride solution, 1%, 6 h at 25°C in darkness; Freeland, Reference Freeland1976). In live tissues, this colourless salt is reduced to a non-diffusible red-coloured formozan by cellular dehydrogenases (Moore, Reference Moore1962). To overcome the possible interpretational errors of a staining test we compared the results with two control samples. We used fresh collected seeds (less than 1 month old) as a positive control and dead seeds (heated in a furnace at 180°C for 48 h) as a negative control.
Fire-dependent treatments
To determine whether germination of these species is fire-dependent, seeds collected in AJU were submitted to three different treatments. (1) Seeds were heated to temperatures of 100°C for 5 min using a furnace with stable temperatures. The temperature selected is within the range occurring during wildfires in the upper layer of the soil (Valbuena and Vera, Reference Valbuena and Vera2002). (2) As a comparison to understand the role of thermal treatment, a second set of seeds was mechanically scarified to remove dormancy (Thanos and Georghiou, Reference Thanos and Georghiou1988). Scarification was achieved by abrasion between two pieces of sandpaper. (3) Finally, in order to understand the influence of post-fire conditions, another set of seeds was sown in natural ashes. This material was collected 1 d after a wildfire, in a population close to AJU ( < 3 km) with the same type of vegetation. Germination conditions and criteria were the same as described above, but Petri dishes were kept in incubators set at 15°C since results showed that this was the optimum temperature for the germination of these species (Draper et al., Reference Draper, Rosselló-Graell, Marques, Magos Brehm, Leitão Serra and Correia2003).
Statistical analysis
The following parameters were used to compare germination behaviour of each species across treatments: (1) final germination percentage; and (2) rate of germination defined by T50, time taken to reach 50% of maximum germination value. The cumulative germination curves of all treatments and species were plotted in CurveExpert 1.3 (http://curveexpert.webhop.net/), considering a sigmoidal model. Correlation coefficients were always superior to 0.95. For each species, the selected parameters were evaluated using a general linear model (GLM) with temperature, light and population as predictor variables. A one-way analysis of variance (ANOVA) was used to analyse the effect of the different fire-dependent treatments. For comparison of means, the Scheffe post hoc multiple comparison test or a t-test was used (P < 0.05). Data of final germination needed an arcsin transformation. All remaining statistical analyses were performed with SPSS package v. 12.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Effect of temperature
The geophytes studied always showed some level of germination at all temperatures tested under conditions of light and darkness, with the exception of the narrowly endemic N. cavanillesii, which only germinated at 15°C (Fig. 1). For L. autumnale, S. autumnalis and U. maritima, final germination percentages showed significant differences between temperatures (Table 1). These three geophytes showed the highest percentage of germination at 15°C, which dropped significantly with the rise of temperature and even under alternating temperature conditions (Fig. 1). N. serotinus was the only species to germinate equally well at all the temperatures tested (Table 1, Fig. 1). Germination was faster at the lower temperatures (15, 20°C), usually reaching 50% of the final germination percentage within 1 week except in the case of L. autumnale and N. cavanillesii where T50 values were always higher than 10 d (Fig. 2). Significant differences were found between temperatures except in the case of N. cavanillesii, which only germinated at 15°C (Table 2). Non-germinated seeds were not stained by tetrazolium test, thus revealing that these seeds were non-viable.

Figure 1 Final percentage of germination of Leucojum autumnale (Laut), Narcissus cavanillesii (Ncav), N. serotinus (Nser), Scilla autumnalis (Saut) and Urginea maritima (Umar) after 30 d of incubation under darkness (black bars) and light conditions (white bars). Mean values ± standard deviation across all populations. Superscripts with different letters indicate significant differences between seeds at different temperatures, for each species (Scheffe test). Values within boxes indicate results of a t-test comparing the effect of light versus dark conditions on the final percentage of germination for each species and temperature. **, P < 0.01; ***, P < 0.001.
Table 1 Results of the general linear model assessing the effects of temperature (temp), light and populations (pop) on the final germination percentage of five geophyte species: Leucojum autumnale (Laut), Narcissus cavanillesii (Ncav), N. serotinus (Nser), Scilla autumnalis (Saut) and Urginea maritima (Umar). Significant values (P<0.05) are indicated in bold

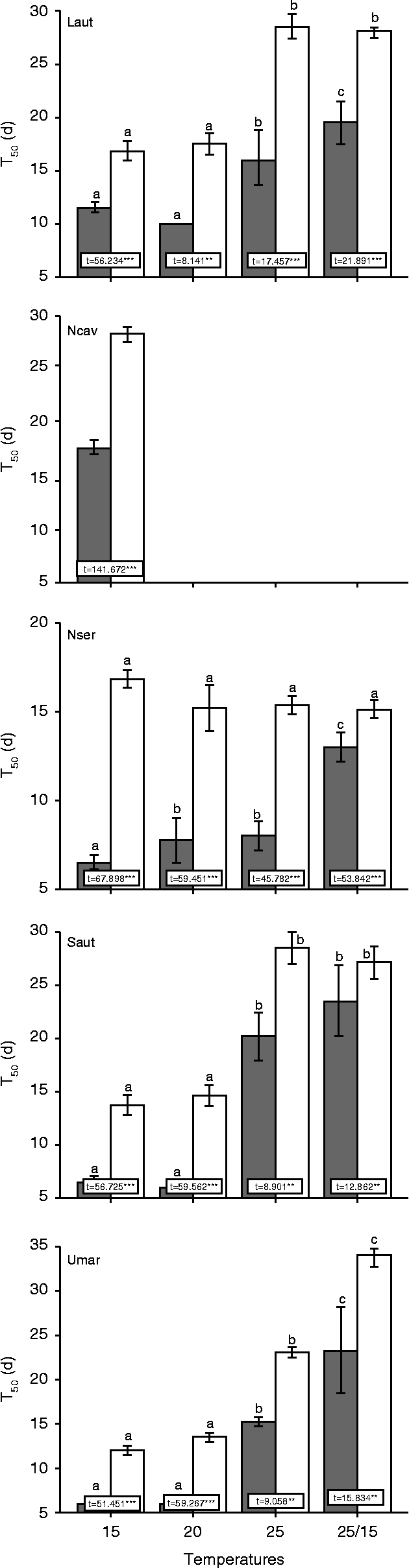
Figure 2 Germination rates, T50 (d) of Leucojum autumnale (Laut), Narcissus cavanillesii (Ncav), N. serotinus (Nser), Scilla autumnalis (Saut) and Urginea maritima (Umar) after 30 d of incubation under darkness (black bars) and light conditions (white bars). Mean values ± standard deviation across all populations. Superscripts with different letters indicate significant differences between seeds at different temperatures, for each species (Scheffe test). Values within boxes indicate results of a t-test comparing the effect of light versus dark conditions on the final percentage of germination for each species and temperature. **, P < 0.01; ***, P < 0.001.
Table 2 Results of the general linear model assessing the effects of temperature (temp), light and populations (pop) on the germination rate (T50) of five geophyte species: Leucojum autumnale (Laut), Narcissus cavanillesii (Ncav), N. serotinus (Nser), Scilla autumnalis (Saut) and Urginea maritima (Umar). Significant values (P<0.05) are indicated in bold

Effect of light
Light had a marginal effect on the final percentage of germination of the five geophyte species (Fig. 1, Table 1). Interactions between light and populations showed no significant differences (Table 1). Nevertheless, T50 values were always higher under light than under darkness, implying that germination was significantly slower under light (Fig. 2). Therefore, for T50 values, significant differences were found between light and dark treatments although the remaining interactions showed no differences (Table 2).
Intraspecific variation in seed germination
No differences in the final percentage of germination and in the rate of germination were found between populations of L. autumnale, N. cavanillesii and S. autumnalis (Tables 1 and 2). Nevertheless, a marginal significance was found between populations of N. serotinus and U. maritima, either considering the final percentage of germination or the rate of germination (Tables 1 and 2). No differences were found in the interactions between populations and temperature or between populations and light (Tables 1 and 2).
Fire-dependent treatments
All fire-dependent treatments had a negative impact on the germination of the five geophyte species (Table 3). Viable seeds that were heated to 100°C for 5 min always showed a decrease in the final percentage of germination, ranging from a minimum of 63% in N. serotinus to a maximum decrease of 86% in N. cavanillesii. Similar results were found in viable seeds that were mechanically scarified (Table 3). Seeds that were sown in natural ashes never germinated. All fire-dependent treatments enhanced T50 values, ranging from a minimum of 9.24 d (N. cavanillesii) and a maximum increase of 12.83 d (U. maritima) in seeds that were heated and from 9.14 d (N. cavanillesii) to 12.87 d (S. autumnalis) in seeds that were mechanically scarified (Table 3). Non-germinated seeds were not stained with tetrazolium.
Table 3 Effects of fire-dependent treatments (heat shock of 100°C for 5 min and mechanical scarification of seed coat) on final germination (%) and germination rate (days; indicated between brackets) of Leucojum autumnale (Laut), Narcissus cavanillesii (Ncav), N. serotinus (Nser), Scilla autumnalis (Saut) and Urginea maritima (Umar). As a comparison, seeds without any treatment (control) are also show in the table. Mean values ± standard deviation. F, ANOVA F ratio value. Superscripts with different letters indicate significant differences between treatments within each species (Scheffe test). ***, P<0.001

Seed longevity
The level of germination dropped significantly as viable seeds were getting older (Fig. 3A). After 18 months, germination of viable seeds decreased by 57% in L. autumnale (F 3,12 = 230.667, P = 0.0001), 75% in N. cavanillesii (F 3,12 = 302.902, P = 0.0001), 67% in N. serotinus (F 3,12 = 236.907, P = 0.0001), 96% in S. autumnalis (F 3,12 = 437.652, P = 0.0001) and 66% in U. maritima (F 3,12 = 328.429, P = 0.0001). Germination was also delayed in older seeds since values of T50 were always enhanced with time (Fig. 3B). Non-germinated seeds did not stain with tetrazolium.

Figure 3 Effect of seed age (1, 6, 12 and 18 months) on the germination of Leucojum autumnale (Laut), Narcissus cavanillesii (Ncav), N. serotinus (Nser), Scilla autumnalis (Saut) and Urginea maritima (Umar) after 30 d of incubation in darkness. (A) Final germination values (%). (B) Rate of germination (T50). Mean values ± standard deviation. Superscripts with different letters indicate significant differences between seeds of different ages, for each species (Scheffe test).
Discussion
What factors affect germination of autumnal geophytes?
Our results show that the five selected species, L. autumnale, N. cavanillesii, N. serotinus, S. autumnalis and U. maritima, produce a high number of water-permeable seeds per population that germinate to high percentages and rapidly (under optimal conditions) without the need for any dormancy-breaking treatments (Figs 1 and 2). Several species within Liliales and Asparagales produce seeds with an underdeveloped embryo at the time of seed dispersal (Kondo et al., Reference Kondo, Sato, Baskin and Baskin2006), which implies that the embryo still has to grow inside the seed before germination can occur (Baskin and Baskin, Reference Baskin and Baskin2004). Although these seeds (termed as morphologically or morpho-physiologically dormant) are quite common in the families studied here, they were not found in these five autumnal geophytes, since embryos were fully developed at the time of seed dispersion. Further, seedlings of these species emerged shortly after seed germination and at the same temperatures, and, therefore, although specific studies in natural conditions are needed, we can exclude the presence of epicotyl dormancy, since there is no apparent physiological block preventing the growth of the epicotyl (Barton, Reference Barton1933).
Temperature seems to play an important ecological role in controlling the germination of Mediterranean geophytes (Thanos and Doussi, Reference Thanos and Doussi1995; Baskin and Baskin, Reference Baskin and Baskin1998; Doussi and Thanos, Reference Doussi and Thanos2002) and this is in agreement with the results obtained in this study. Three different patterns of germination were observed: (1) species adapted for late autumn germination (L. autumnale, S. autumnalis and U. maritima), which germinate at the coolest temperature tested (15°C, 20°C); (2) species with specific temperature requirements (N. cavanillesii) that germinate only at the coolest temperature tested (15°C); and (3) species that have an opportunistic behaviour (N. serotinus), germinating equally well in all temperatures tested. Thus, with the exception of the latter species, temperature is an important driving force for germination to occur in these autumnal Mediterranean geophytes. T50 values indicate that seeds can germinate at the same time and usually in the first few days ( < 7 d) with optimum temperatures. This strategy can be useful for the rapid colonization of space but it also implies some level of interspecific competition. Therefore, it seems important that these five co-occurring geophytes exhibit some differences in their patterns of seed germination, since they can explore environmental conditions and available resources in different ways. Furthermore, the temperature range of germination of these species may explain some differences in species distribution. For instance, the narrowly endemic N. cavanillesii has a specific temperature requirement, while its wide congener, N. serotinus, germinates evenly in all temperatures tested, even in alternating temperatures. A positive effect of alternating temperatures on germination has been hypothesized as an important ecological strategy because species might be able to germinate when daily temperatures rise but also under night temperatures that are usually lower (Baskin and Baskin, Reference Baskin and Baskin1998; Albert et al., Reference Albert, Iriondo and Pérez-García2002).
Artificial white light had a negative impact on the germination of the five geophyte species, as final germination was lower and significantly slower under light than under dark conditions (Figs 1 and 2). In other geophyte species, white light even leads to the induction of secondary dormancy (Muscari neglectum, Doussi and Thanos, Reference Doussi and Thanos2002; Allium staticiforme, Thanos et al., Reference Thanos, Georghiou, Douma and Marangaki1991; Bellevalia brevipedicellata and Pancratium maritimum, Delipetrou, Reference Delipetrou1996). Although this was not observed in the present study, and the fluorescent white light generally used in germination tests is different from natural light conditions (see Thanos, Reference Thanos1993), these results suggest that germination of seeds directly exposed to sunlight might be severely restricted under natural conditions. Other factors not studied here might also affect the germination of geophyte species. For instance, the availability of nutrients (Allison, Reference Allison2002), abiotic stresses, such as flooding (Clevering, Reference Clevering1995), or even seed size (Andersson, Reference Andersson1996) may also affect seed germination, as revealed in other species. The presence of more competitive plants, such as grasses and shrubs, might also influence the patterns of germination of these geophytes.
How variable is germination between autumn- and spring-flowering Narcissus?
In our study we found that the germination of autumn-flowering species of Narcissus occurs quickly, is usually higher at the lowest temperatures tested and there are no signs of delayed germination. Although there are few studies on the germination of Narcissus seeds, our results contrast sharply with the previously published studies, which were all directed at spring-flowering species of Narcissus. For instance, Thompson (Reference Thompson1977) found that freshly collected seeds of N. bulbocodium var. conspicuous were dormant and failed to germinate in a wide range of temperatures (3–38°C) unless a warm pre-treatment was applied. Vandelook and Van Assche (Reference Vandelook and Van Assche2008) reported that seeds of N. pseudonarcissus are dispersed in spring, although the underdeveloped embryo grows continuously until seeds germinate in the following autumn or winter. Therefore, a sequence of high summer temperatures followed by lower autumn and winter temperatures is necessary to stimulate germination and seedling emergence (Vandelook and Van Assche, Reference Vandelook and Van Assche2008). More recently, Copete et al. (Reference Copete, Herranz, Ferrandis, Baskin and Baskin2011) also reported that fresh seeds of N. hispanicus have an underdeveloped embryo at the time of dispersal in late spring. The embryo grew inside the seed during the warm summer temperatures and became fully elongated at the cool autumn temperatures, when germination began to occur (Copete et al., Reference Copete, Herranz, Ferrandis, Baskin and Baskin2011). However, these seeds have deep, simple, epicotyl, morpho-physiological dormancy, since shoot growth only occurs in the next spring, after the germinated seeds have been stimulated by the low winter temperatures, and in some cases the embryo can even re-enter dormancy (Copete et al., Reference Copete, Herranz, Ferrandis, Baskin and Baskin2011). Clearly, even though species belong to the same genus, they may not have the same germination requirements or even the same type and level of dormancy.
How variable is the pattern of germination across populations?
Several studies have demonstrated that a completely non-dormant seed has the ability to germinate over the widest range of environmental factors possible for the genotype (Baskin and Baskin, Reference Baskin and Baskin1998) and can even present different seasonal patterns of germination. For example, populations of Arabidopsis thaliana can express winter or spring life history and some populations can even express both patterns (Donohue et al., Reference Donohue, Dorn, Griffith, Kim, Aguilera, Polisetty and Schmitt2005). Therefore, some variation across populations is expected for seed germination (Pérez-García et al., Reference Pérez-Garcia, Hornero and Gonzalez-Benito2003). However, that was not the case in our study since we did not find any significant differences between populations for most species and the significance was only marginal in the case of N. serotinus and U. maritima (Tables 1 and 2). This result may be explained by the fact that all populations grow in the same biogeographical area (the Guadiana basin), sharing similar climate conditions and possibly having evolved under the same selective abiotic factors.
Is germination fire-dependent?
Fire-stimulated germination is found in several species from Mediterranean fire-prone ecosystems (Keeley, Reference Keeley1995). However, in this study, all fire-dependent treatments had a negative impact on the germination of the selected geophytes. Seeds that were heated or mechanically scarified showed a decrease in the percentage of germination, while T50 values were enhanced, indicating a delay in the onset of germination (Table 3). The positive contribution of this thermal process probably comes from other causes, such as the elimination of stronger competitive plants (Noble and Slatyer, Reference Noble and Slatyer1977). In addition, seeds sown in ashes did not germinate, which suggests that post-fire conditions have a negative impact on the germination of these species. Nevertheless, smoke compounds were not tested and these may also stimulate germination. For instance, in south-western Australia, seeds of several herbaceous species have a very strong response to the chemicals in smoke (Dixon et al., Reference Dixon, Roche and Pate1995; Keeley and Fotheringham, Reference Keeley and Fotheringham2000; Light et al., Reference Light, Daws and Van Staden2009). In the Mediterranean region, the role of smoke is less clear, but some studies have revealed that this factor is an important germination cue in a wide range of woody species (Thanos et al., Reference Thanos, Georghiou, Kadis and Pantazi1992; Pérez-Fernández and Rodríguez-Echeverría, Reference Pérez-Fernández and Rodríguez-Echeverría2003; Crosti et al., Reference Crosti, Ladd, Dixon and Piotto2006; Moreira et al., Reference Moreira, Tormo, Estrelles and Pausas2010).
Does seed viability change with time?
Seed germination of the five geophytes was relatively high immediately after collection. However, germinability diminished progressively with age and the rate of germination was also delayed in older seeds (Fig. 3). When the tetrazolium test was applied, non-germinated seeds did not stain, implying a loss of viability over time. Many factors can contribute to seed ageing, namely the inactivation of enzymes (McDonald, Reference McDonald1999; Murthy et al., Reference Murthy, Kumar and Sun2003), genetic damage (McDonald, Reference McDonald2004; Lehner et al., Reference Lehner, Mamadou, Poels, Côme, Bailly and Corbineau2008) and the loss of seed moisture content (McDonald, Reference McDonald1999, Reference McDonald2004). In natural populations this loss of viability should occur even sooner since RH decreases during spring and summer while in our artificial experiment RH was constant and set at the level registered in natural populations during autumn. These results suggest that under natural conditions, seeds die very quickly and most seeds will not overcome the summer season. Although the causes explaining the loss of viability in the studied geophytes are not known, these results highlight the importance of ex situ seed conservation if seeds are to be used in restoration projects. Several species of geophytes present problems of conservation (namely Narcissus) and therefore this study helps us to understand how to conserve seeds and grow plants from seeds in case it is necessary to reinforce populations. On the other hand, our data also reveal that seed longevity, a trait that is an essential condition to form a soil seed bank, is very low and, therefore, these species do not form a permanent soil seed bank. The decrease in seed germinability, 6–18 months after seed collection, suggests that germination occurs shortly after seed dispersion otherwise seeds will start to die. In accordance, the high germination of seeds in the dark reported in this study might explain the absence of a permanent soil seed bank (Baskin and Baskin, Reference Baskin and Baskin1989; Pons, Reference Pons1991). However, studies of seed viability need to be performed on natural populations to understand the dynamics of seed viability under different field conditions. Although vegetative propagation through bulb multiplication occurs frequently in these geophytes, the formation of seeds has an important role in their propagation and especially in maintaining the genetic diversity of populations.
Acknowledgements
The authors thank three anonymous referees and the Associate Editor, for their useful comments on a previous version of this manuscript. This work was supported by the Empresa de Desenvolvimento e Infra-estruturas do Alqueva, SA and co-funding by the Empresa de Desenvolvimento e Infra-estruturas do Alqueva, SA and European Regional Development Funds.








