INTRODUCTION
Numerous studies have been published on the oncospheres in various cyclophyllidean cestodes, but very little information is available on cellular organization and functional ultrastructure of infective oncospheres of anoplocephalid cestodes. The transmission electron microscope (TEM) studies on anoplocephalid egg differentiation and ultrastructure have been impeded by numerous technical difficulties in getting the egg content fixed and infiltrated with embedding media, and in cutting thick and hard egg structures such as outer coats or shell and the pyriform apparatus of embryophores. Such technical difficulties are particularly evident in TEM studies of species belonging to the subfamily Anoplocephalinae such as Anoplocephaloides dentata (Galli-Valerio, 1905) (see Świderski et al. Reference Świderski, Ndiaye, Tkach, Miquel, Marchand, Chomicz, Grytner-Ziecina and Sereda2001a , Reference Świderski, Ndiaye, Tkach, Miquel, Marchand, Chomicz, Grytner-Ziecina and Sereda b ) or Neoctenotaenia ctenoides (Railliet, 1890), described by us previously in several papers as Mosgovoyia ctenoides (see Młocicki et al. Reference Młocicki, Świderski, Eira and Miquel2005, Reference Młocicki, Świderski, Miquel, Eira and Conn2006).
Our earlier examination of the ultrastructure of eggs and hexacanths of Inermicapsifer madagascariensis (Davaine, 1870) (see Świderski and Tkach, Reference Świderski and Tkach2002), greatly facilitate our interpretation of the present TEM results on Thysanotaenia congolensis Dronen et al. 1999, as both belong to the subfamily Inermicapsiferinae. Comparisons of our earlier results on A. dentata and N. ctenoides, which belong to the subfamily Anoplocephalinae, with those of I. madagascariensis indicate great ultrastructural variety of eggs and hexacanths among these taxa of anoplocephalid cestodes.
New ultrastructural data on oncospheres have the potential to be useful criteria in phylogenetic analyses of cestodes (Świderski, Reference Świderski1975, Reference Świderski, Clark and Adams1981; Beveridge, Reference Beveridge, Littlewood and Bray2001). In addition, ultrastructural data of cestode larvae are essential to our understanding of cestode biology, parasite–host interactions and prevention as was demonstrated most recently by Jabbar et al. (Reference Jabbar, Świderski, Młocicki, Beveridge and Lightowlers2010a , Reference Jabbar, Crawford, Młocicki, Świderski, Conn, Jones, Beveridge and Lightowlers b ). Little information is available on the ultrastructure of anoplocephalid cestodes representing the subfamily Inermicapsiferinae.
The purpose of the present TEM study is to describe the functional ultrastructure of mature eggs and the cellular organization of the hexacanth larvae of T. congolensis and compare them with data on the eggs and hexacanths of other cyclophyllideans to suggest potential phylogenetic relationships. Cestodes of the genus Thysanotaenia Beddard, 1911 have traditionally been classified within the subfamily Inermicapsiferinae, which has usually been considered as part of the family Anoplocephalidae (Spasskii, Reference Spasskii1951; Beveridge, Reference Beveridge, Khalil, Jones and Bray1994). However, there is considerable morphological evidence to suggest that the four currently recognized subfamilies of Anoplocephalidae (Anoplocephalinae, Linstowiinae, Inermicapsiferinae, Thysanosomatinae) represent a polyphyletic assemblage (Voge, Reference Voge and Schmidt1969; Beveridge, Reference Beveridge, Khalil, Jones and Bray1994). Thysanotaenia congolensis and other inermicapsiferines are morphologically similar to the cestodes of the family Davaineidae, the former evidently differing from the latter only in lacking rostellum and hooks. Following this observation (Baer and Fain, Reference Baer and Fain1955), Baer (Reference Baer1956) transferred the inermicapsiferine genera Inermicapsifer Janicki, 1910 and Thysanotaenia to the subfamily Davaineinae, then regarded as part of Linstowiidae (see also Beveridge, Reference Beveridge, Khalil, Jones and Bray1994). However, this action has not been generally accepted in subsequent classifications.
To evaluate the phylogenetic position of T. congolensis we compare its new ultrastructural data on eggs and hexacanths with similar data on anoplocephalines, linstowines and other cyclophyllidean cestodes. Unfortunately, there are no previous data for any other inermicapsiferines with the exception of I. madagascariensis.
MATERIALS AND METHODS
Live specimens of T. congolensis were isolated from the intestine of naturally infected black rats Rattus rattus from São Domingos and Orgãos (Santiago Island, Cape Verde) in December 2009.
Methods applied in ultrastructural studies
Adult tapeworms were immediately rinsed with a 0·9% NaCl solution. Later, they were fixed in cold (4 °C) modified Karnovski fixative (4% paraformaldehyde and 0·25% glutaraldehyde) in a 0·1 m sodium cacodylate buffer at pH 7·4 for a minimum of 2 h, rinsed in 0·1 m sodium cacodylate buffer at pH 7·4, post-fixed in cold (4 °C) 1% osmium tetroxide with 0·9% potassium ferricyanide [K3Fe(CN)6] in the same buffer for 1 h, rinsed in Milli-Q water (Millipore Gradient A10), dehydrated in an ethanol series and propylene oxide, embedded in Spurr's resin and polymerized at 60 °C for 72 h.
Ultrathin sections (60–90 nm thick) were obtained with a Reichert-Jung Ultracut E ultramicrotome. Sections were placed on 200-mesh copper grids and double-stained with uranyl acetate and lead citrate.
The hexacanths of T. congolensis were reconstructed from the consecutive semithin serial sections, stained with 1% methylene blue in borax solution, by means of light microscopy (LM), and partially from the ultrathin sections, by means of TEM.
The grids were examined in a JEOL 1010 TEM operated at 80 kV, in the ‘Centres Científics i Tecnològics’ of the University of Barcelona (CCiTUB).
Methods for TEM cytochemistry for glycogen and other polysaccharides
For cytochemistry of glycogen and other polysaccharides, such as membrane-bound glycoproteins, the ultrathin sections were collected on gold grids. The periodic acid–thiosemicarbazide–silver proteinate (PA–TSC–SP) technique of Thiéry (Reference Thiéry1967) was applied to determine cytochemical localization of glycogen and other polysaccharides at the ultrastructural level. These grids were also examined in a JEOL 1010 TEM operated at an accelerating voltage of 80 kV.
RESULTS
General topography of gravid proglottids and parenchymatous capsules
In T. congolensis, the wall of the uterus forms a branched sac in the medullary parenchyma of mature proglottids, then rapidly breaks down in the gravid proglottids. The egg masses that are released into the parenchyma become arranged inside parenchymatous egg capsules which completely fill the entire gravid proglottids. The individual capsules appear polyhedral due to their compressed packing. There are about 120–150 egg capsules per proglottid and each contains 6–12 eggs. Mature eggs measure about 43 µm in diameter, with the enclosed oncosphere about 25 µm in diameter. The embryonic envelopes surrounding the mature hexacanths are reduced to a very thin residual layer of the membranous embryophore with several long, and much infolded cytoplasmic processes. Protection and all other functions of the embryonic envelopes, which appear briefly in the earlier stages of embryonic development observed in immature and mature proglottids, are taken over by the parenchymatous capsules in the gravid proglottids.
The parenchymatous egg capsules originate from medullary parenchyma of immature proglottids, and then undergo differentiation into the three layers observed in the parenchymatous capsules of gravid proglottids (Figs 1 and 2): a thin outer filamentous layer; a middle thick metachromatic layer; and an inner compact cellular layer.

Fig. 1. Diagram of the entire parenchymatous capsule surrounding several mature eggs in the gravid proglottid of Thysanotaenia congolensis. CC, calcareous corpuscles; CT, outer filamentous layer; db, dense bodies; Em, embryophore; EP, embryophore processes; ICL, inner compact layer; ML, middle metachromatic layer; OM, oncospheral membrane; Onc, oncosphere; UE, uterine wall remnants.

Fig. 2. General topography of the oncosphere surrounded by the parenchymatous capsule and its ultrastructural details. (A) Low-power electron micrograph showing fully developed oncosphere surrounded by the highly reduced membranous embryophore and additional protective structures represented by the remnants of the uterine wall and parenchymatous capsule. (B) The region situated between the middle metachromatic layer (upper part of micrograph) and inner compact cellular layer (lower part of micrograph). (C) Higher magnification of the inner compact cellular layer showing numerous calcareous corpuscles embedded in the cytoplasm of lipid-containing ‘vacuolated’, ‘spongiform’ cells owing its nature to a large number of small non-osmiophilic, saturated lipid droplets. CC, calcareous corpuscles; CT, outer filamentous layer; db, dense bodies; Em, embryophore; EP, embryophore processes; ICL, inner compact layer; MF, muscle fibres; ML, middle metachromatic layer; OM, oncospheral membrane; Onc, oncosphere; UE, uterine wall remnants.
The outer filamentous layer (Figs 1 and 2B) is composed of long, delicate filaments of unknown chemical nature embedded in a granular extracellular matrix.
The middle metachromatic layer (Figs 1 and 2A and B) appears as an accumulation of large, closely packed mucous droplets containing spherical electron-dense bodies of different sizes. They are intensely metachromatic after toluidine staining, indicating that acid mucopolysaccharides are the main chemical components of this layer.
The inner compact layer (Figs 1 and 2A and C) is composed of three cell types: lipid-containing cells; cells containing calcareous corpuscles; and muscle cells. The cytoplasm of lipid-containing cells is vacuolated owing to a large number of very small non-osmiophilic saturated lipid droplets. The second cell type, involved in calcareous corpuscle formation, usually shows a variety of sizes, shapes and stages of corpuscle formation. In the early stage, the cell usually contains dilated Golgi complexes and small corpuscles within Golgi vesicles. The corpuscles grow by accretion of their two structural components: a homogeneous matrix of low electron density and a granular substance of high density rich in calcium carbonate. The accumulation of a large number of mature mineralized corpuscles is accompanied by progressive degeneration of the entire cell with the disappearance of the plasma membrane and compression of the cytoplasm and nucleus.
General topography and cellular organization of the infective hexacanth
The fully developed hexacanths of T. congolensis are armed with three pairs of oncospheral hooks (Figs 1, 3, 4C–E and 5). The oncospheral terminology was proposed by Ogren (Reference Ogren1971) and Conn and Świderski (Reference Conn and Świderski2008). Such terms as ‘anterior pole’ and ‘posterior pole’ of the oncosphere are used in this paper with respect to hexacanth's invasive activity. It uses its hooks, generally in conjunction with penetration gland secretion, to penetrate through host tissue with the hooks oriented in the direction of movement. Therefore, the hook region, directed forward during movement, is considered as the anterior part of the larva and functionally as the ‘somatophore’ (Figs 3 and 4). The opposite part, containing germinative cells, is considered as posterior and functionally as ‘germatophore’ (Figs 3A, 6A and 7). Six oncospheral hooks (Figs 3 and 4C–E), one pair of median hooks and two pairs of lateral hooks, are interconnected by a complex hook muscle system, responsible for coordination of their synchronized hook movements. The fully formed hooks, when observed at cross-, oblique and longitudinal sections, show a heterogeneous structure and are composed of three or four layers of different electron densities in some regions, depending on the section level (Figs 3B and 4C–E). In T. congolensis the infective oncosphere consists of numerous cells which are arranged symmetrically (Figs 3 and 5). They also include two multinucleated structures: the bi-nucleate perikaryon of the epithelium and the hexa-nucleate penetration gland (Fig. 5). Therefore, within the infective hexacanth larva the following cell types were distinguished: (1) bi-nucleate subepithelial cell; (2) U-shaped, hexa-nucleate penetration gland (PG1); (3) second type of penetration gland (PG2); (4) two nerve cells; (5) two types of somatic cells, including the myocytons of both somatic and hook musculature and numerous degenerating micromeres with pycnotic nuclei; and (6) about 12 germinative cells (two groups of six cells, localized in the posterior pole of the hexacanth (=germatophore), with characteristic prominent nucleoli in their large spherical nuclei, surrounded by very thin layers of granular cytoplasm.

Fig. 3. Somatophore and germatophore regions of the hexacanth. (A) Longitudinally oblique section through the middle part of the oncosphere showing a bilateral symmetry of the hexacanth. Note the somatophore and germatophore regions situated at the opposite poles of the larva and bilaterally symmetrical localization of germinative cells and six oncospheral hooks in cross-section, and their hook muscles. (B) Higher magnification of the somatophore region showing three-layered nature of hook material at different levels of their cross- and oblique sections and complex structure of somatic musculature interdigitating with hook muscle system and several zones of their attachments. Em, embryophore; EP, embryophore processes; GC, germinative cell; H, hook; HM, hook muscles; n, nucleolus; N, nucleus; OE, oncospheral syncytial epithelium; OM, oncospheral membrane.

Fig. 4. Cytochemical localization of β-glycogen in the embryophore and hook muscles; ultrastructural details of the oncospheral hooks. (A,B) Cytochemical test of Thiery showing a heavy accumulation of glycogen in the cytoplasm of flattened, degenerating embryophore (A) and showing accumulation of β-glycogen particles in the oncospheral hook and somatic musculature (B). (C) Section through the somatophore region of the hexacanths showing two obliquely sectioned hooks, their accompanying musculature and zones of hook-muscle attachments. (D,E) Longitudinally oblique sections through the blades and shanks of oncospheral hooks. Note the heterogeneous, tripartite nature, even four-partite nature (in some regions) of the hook material which continues through its blade, shank and base. Note the hook attachment zones around the enlarged collar of the hook shank, surrounded by powerful bundles of hook muscles shown in D. Enlarged details of the hook protruding from oncosphere (compare D and E) are showing the circular septate junction above the collar region and two highly electron-dense rigid rings situated on each side of the junction. Bl, hook blade; D, circular septate desmosome; DR, desmosome rings; Em, embryophore; gl, glycogen; H, hook; HM, hook muscles; HMA, hook attachment zones; m, mitochondria; N, nucleus; nsg, neurosecretory granules; OE, oncospheral syncytial epithelium; OM, oncospheral membrane; SC, somatic cell; Sh, hook shank.

Fig. 5. Diagram showing localization of three secretory regions: two types of penetration glands PG1 and PG2 and two neurosecretory cells. Note the oncospheral epithelium composed of its peripheral anucleated layer and submerged subepithelial perikaryon or bi-nucleate medullary centre and the hook region membrane surrounding the somatophore pole of the hexacanth. BMC, bi-nucleate medullary centre; H, hooks; HRM, hook region membrane; N, nucleus; NC, nerve cells; OE, oncospheral syncytial epithelium; PG1, type 1 penetration gland; PG2, type 2 penetration gland.

Fig. 6. Oncospheral secretory regions: comparison of the three types of their secretory granules. (A) Electron micrograph showing the germatophore region of hexacanth with a large germinative cell on the left side and a few secretory regions containing three types of secretory granules: sg1, sg2 and nsg granules. Two types of penetration glands, PG1 and PG2, show evidently different types of their secretory granule sg1 and sg2. (B–D) Ultrastructural details of three types secretory granules. sg1 (or sg2) represents secretory granules of the first (or second) type of penetration glands and nsg, neurosecretory granules of neurosecretory cells. Em, embryophore; GC, germinative cell; n, nucleolus; N, nucleus; nsg, neurosecretory granules; OM, oncospheral membrane; sg1, secretory granules of the first type penetration glands; sg2, secretory granules of the second type penetration glands.

Fig. 7. Ultrastructure of the germinative cells and enlarged details of the oncospheral surface. (A) Two enlarged germinative cells (GC) surrounded by hook muscle bundles and the elongated processes of the penetration gland PG1 containing characteristic disc-shaped secretory granules sg1. (B) Low-power electron micrograph showing the germatophore region of hexacanth with two large germinative cells on the upper right corner of left side. Em, embryophore; GC, germinative cell; HM, hook muscles; m, mitochondria; n, nucleolus; N, nucleus; OM, oncospheral membrane; SC, somatic cell; sg1, secretory granules of the first type penetration glands; sg2, secretory granules of the second type penetration glands.
Oncospheral surface: its three tightly connected structures
The oncospheral surface is formed by three closely adjacent and tightly connected structures: (1) irregularly shaped layer of rudimentary embryophore with very long, much infolded cytoplasmic processes; (2) very thin and highly electron-dense oncospheral membrane; and (3) a thin anucleated layer of oncospheral epithelium (Figs 4A–C, 5, 6A and 7). The degenerating, much reduced volume of embryophore cytoplasm still contains a few flattened nuclei, heavy accumulations of β-glycogen particles and several mitochondria (Figs 4A–C, 6A and 7). The hook region membrane, in form of a cap or ‘calotte’, surrounds only the somatophore pole of hexacanth, including hook blades, and is attached directly to the oncospheral surface (Fig. 5).
Penetration glands
Two types of penetration glands, PG1 and PG2, with evidently different types of secretory granules, sg1 and sg2, were observed in T. congolensis (Figs 5, 6A–C and 7). The first, classical type of oncospheral penetration gland (PG1) forms a U-shaped, hexa-nucleate syncytium with two large cytoplasmic processes or penetration gland arms (Fig. 5) containing characteristic type secretory granules of discoid shape and moderate electron density, sometimes forming rouleau-shaped assemblages including several granules. Gland exits open into the cytoplasmic layer of the epithelium in the hook region of the oncosphere (Fig. 5). The irregularly shaped nuclei of the penetration gland contain numerous large heterochromatin islands and prominent nucleoli (Fig. 7A). They are surrounded by a granular syncytial cytoplasm, rich in free ribosomes, Golgi complexes, well-developed profiles of endoplasmic reticulum, numerous mitochondria and characteristic secretory granules of different shapes and sizes, but of similar electron density (Fig. 7). The second type (PG2) represents two additional pairs of glandular regions, apparently joined by a narrow isthmus, and situated inside a deep invagination, between the arms of the typical U-shaped penetration gland (PG1). The secretory granules (sg2) of this PG2 are spherical and show a high electron density. They are markedly larger than the vesicles observed in nerve cells and evidently different from the discoid-shaped secretory granules of the penetration gland (compare ultrastructure of secretory granules of Fig. 6). The gland exits of PG2 were not clearly observed in our material.
Nerve cells
The nerve cells are situated in the central part of the larva, usually in the invagination of the penetration gland (Figs 5 and 6A and D). Their electron-lucent nuclei contain only a few heterochromatin islands dispersed in the karyoplasm and adjacent to the nuclear membrane. The nerve cells are characterized by the presence, in their cytoplasm, of characteristic neurosecretory-like granules (Fig. 6A and D). As shown on the high-magnification Fig. 6D, these granules are always membrane-bound and dense-cored. This type of granule was observed not only in the nerve cell perikaryon but also in the nerve processes distributed within the hexacanth body and frequently adjacent to the penetration gland arms and oncospheral musculature, in particular near the hook muscles.
Somatic cells
In T. congolensis the somatic cells comprise two cell types: (1) the typical somatic cells observed in all oncospheres, which functionally represent the myocytons of both somatic and hook musculature; and (2) the small degenerating micromeres with characteristic ‘pycnotic’ nuclei undergoing apoptosis.
Myocytons are situated mainly in the somatophore or anterior pole of the infective larvae (Figs 3A and 4B–E). They are characterized by a thin layer of perinuclear sarcoplasm with direct connection with muscle fibres composed of myofibrils. The rather thin layer of cytoplasm surrounding the nuclei contains numerous ribosomes, mitochondria and β-glycogen particles, localized mainly near the muscle boundaries (Fig. 7B). Highly electron-dense hook attachment zones occur between different muscle fibres as well as between hooks and their hook musculature (Figs 3B and 4C–E).
The second type of somatic cells is degenerating micromeres, which appear as electron-dense shrunken nuclei resembling cells undergoing apoptosis. Their presence was observed usually during the early and advanced stages of preoncospheral differentiation, but, in the fully formed hexacanths of T. congolensis they were noticed very seldom.
Germinative cells
The large germinative cells are localized in the germatophore or posterior pole of the oncosphere, arranged symmetrically in two groups (Fig. 3A). These cells are characterized by the presence of large nuclei containing prominent electron-dense nucleoli of heterogeneous type showing regions of different electron densities (Fig. 7). The nucleoplasm of germinative cells contains several heterochromatin islands of irregular shapes, frequently adjacent to the nuclear membrane (Figs 6A and 7). The thin layer of their granular cytoplasm is rich in free ribosomes, a few mitochondria and short profiles of endoplasmic reticulum (Fig. 7B).
DISCUSSION
Comparative ultrastructure of the oncospheres in two subfamilies of Anoplocephalidae
The characteristic type of eggs of T. congolensis, without a pyriform apparatus, shows a great similarity to the eggs of another inermicapsiferine cestode, I. madagascariensis (see Świderski and Tkach, Reference Świderski, Tkach and Vaucher2002). Results of this comparison clearly indicate that there exist obvious differences in the ultrastructure of eggs of inermicapsiferine cestodes in comparison with those of other anoplocephalids belonging to subfamily Anoplocephalinae, e.g. Moniezia expansa (Rudolphi, 1810) (see Rybicka, Reference Rybicka1964), A. dentata (see Świderski et al. Reference Świderski, Ndiaye, Tkach, Miquel, Marchand, Chomicz, Grytner-Ziecina and Sereda2001a , Reference Świderski, Ndiaye, Tkach, Miquel, Marchand, Chomicz, Grytner-Ziecina and Sereda b ) and N. ctenoides (see Młocicki et al. Reference Młocicki, Świderski, Eira and Miquel2005, Reference Młocicki, Świderski, Miquel, Eira and Conn2006) and Monoecocestus americanus (Stiles, 1895) (see Conn, Reference Conn1985a ). The similarities and differences which occur both in morphology and ultrastructure of egg envelopes and cellular organization of hexacanths of these two subfamilies of Anoplocephalidae provides strong ultrastructural evidence for the polyphyletic character (assemblage) of the currently recognized four subfamilies of Anoplocephalidae. Regarding the great resemblance between eggs of T. congolensis and I. madagascariensis (see Świderski and Tkach, Reference Świderski and Tkach2002), both species show a characteristic arrangement of mature eggs in gravid proglottids grouped within the parenchymatous egg capsules, containing about 6–12 eggs in each egg capsule. Great similarity also exists both in the ultrastructure of egg envelopes and the cellular organization of hexacanths; the most characteristic feature for both species is progressive degeneration of their typical outer and inner embryonic envelopes, which are formed in the earlier stages of embryogenesis, but undergo apoptosis rapidly and are reduced to only a very thin membranous layer of embryophore. Simultaneously, the protective, nutritive and possibly other functions of oncospheral envelopes are taken over by the parenchymatous egg capsules. In both species, numerous much elongated embryophoral processes were observed around mature oncospheres. Their exact functions, however, are still unclear and should be elucidated experimentally, among others by molecular methods. At this stage, we can only suppose that the presence of these processes may greatly extend the surface area, thus increasing its absorptive surface area, which is important for transport of nutrients and other substances from gravid proglottids via parenchymatous capsule tissues. Our preliminary results on the ultrastructure of the parenchymatous capsules of I. madagascariensis and T. congolensis were published only in the form of congress abstract or a preliminary paper (Świderski, Reference Świderski, Imura, Maruse and Suzuki1986a ; Świderski et al. Reference Świderski, Miquel, Feliu and Conn2015). Reduction in embryonic envelope number and/or thickness has been described for several cestode species that use special maternal structures for protecting the oncospheres within intact gravid proglottids (Conn et al. Reference Conn, Etges and Sidner1984; Conn and Etges, Reference Conn and Etges1984; Conn, Reference Conn1985b , Reference Conn1988, Reference Conn1999; Jones, Reference Jones1988; Świderski and Tkach, Reference Świderski and Tkach1997a , Reference Świderski and Tkach b ).
In both A. dentata and N. ctenoides, in the advanced preoncospheral phase, the inner envelope undergoes differentiation into three sublayers: (1) a thick extraembryophoral cytoplasmic layer; (2) an electron-dense embryophore, as a rigid pyriform apparatus; and (3) a thin intra-embryophoral cytoplasmic layer containing mesomere nuclei. The oncosphere is located in the extended cupule-like part of the pyriform apparatus. The two embryophoral horns elongate and fuse, thus forming a rigid cone. Four oncospheral envelopes surround the fully mature infective hexacanth of A. dentata and N. ctenoides: (1) a thick capsule; (2) the outer envelope; (3) the inner envelope with a characteristic embryophore, in the form of the pyriform apparatus; and (4) the oncospheral membrane. The differentiation and ultrastructure of the oncospheral envelopes of A. dentata and N. ctenoides are essentially similar to those described in other cestode species of the subfamily Anoplocephalinae, e.g. M. expansa (see Rybicka, Reference Rybicka1964; Caley, Reference Caley1975), M. americanus (see Conn, Reference Conn1985a ) and Neoctenotaenia variabilis (Stiles, 1895), described before as Cittotaenia variabilis (see Coil, Reference Coil1979); all of them show the differentiation of the inner envelope embryophore into the characteristic pyriform apparatus.
Regarding linstowiines, published data exist only on the protective parenchymal and uterine structures and embryonic envelopes, but the ultrastructure of their oncospheres and cellular organization of hexacanths has not been described. In mature eggs of Oochoristica anolis (see Conn, Reference Conn1985b ) and Oochoristica agamae (see Świderski and Subilia, Reference Świderski, Subilia and Mangclaviraj1988) the embryonic envelopes persist, but in much reduced form, as their protective function is taken over by the parenchymatous egg capsules, in a manner very similar to that of Inermicapsiferinae. Ultrastructure of various types of parenchymatous capsules in representatives of different cyclophyllidean families such as paruterine organs of Nematotaeniidae and Mesocestoididae, uterine and parenchymatous egg capsules in Anoplocephalidae (Linstowiinae and Inermicapsiferinae, respectively) was compared by Świderski and Tkach (Reference Świderski and Tkach1997a ).
Oncospheral nerve cells
In all early LM studies (Rybicka, Reference Rybicka1966; Ogren, Reference Ogren1968a , Reference Ogren b , Reference Ogren1971), it was generally believed that in the cestode hexacanth larvae the oncospheral nerve cells are absent. Rybicka (Reference Rybicka1967), in her LM histochemical study demonstrated acetylcholinesterase activity in the mature oncospheres of Hymenolepis diminuta (Rudolphi, 1819). She was, however, unable to find specific nerve cells or oncospheral nerve centre. She suggested then that since the oncospheral muscular system is quite extensive, the presence of acetylcholinesterase activity is not surprising if this activity is indeed associated with hexacanth myofibres. Until now only a single type of dense-cored vesicle has been detected in cestode oncospheres, and Świderski and Mackiewicz (Reference Świderski and Mackiewicz2004) stated that consequently, it can be presumed that acetylcholine does not function as a neurotransmitter in the hexacanth larvae. At the ultrastructural level the oncospheral nerve cells were described originally by Fairweather and Threadgold (Reference Fairweather and Threadgold1981). More recently, their presence has been demonstrated in some other oncospheres such as those of Echinococcus granulosus (Batsch, 1786) (see Świderski, Reference Świderski, Le Poole and Zeitler1982, Reference Świderski1983), Echinococcus multilocularis Leuckart, 1863 (see Świderski, Reference Świderski, Jouffrey and Colleix1994), Gulyaevilepis tripartita (Zarnowski, 1956), described by us previously as Ditestolepis tripartita (see Świderski and Tkach, Reference Świderski and Tkach1997c ), Pseudhymenolepis redonica Joyeux & Baer, 1936 (see Tkach and Świderski, Reference Tkach and Świderski1997), I. madagascariensis (see Świderski and Tkach, Reference Świderski and Tkach2002) and in N. ctenoides (see Młocicki et al. Reference Młocicki, Świderski, Miquel, Eira and Conn2006). The nerve cells in the coracidia of three species of bothriocephalideans and diphyllobothriideans have been described by Wikgren (Reference Wikgren1986) in Diphyllobothrium dendriticum (Nitzsch, 1824), by Korneva (Reference Korneva1994) in Triaenophorus nodulosus (Pallas, 1781) and in Bothriocephalus clavibothrium Ariola, 1899 by Świderski and Mackiewicz (Reference Świderski and Mackiewicz2004). The two cells containing the granules of neurosecretory type in the central part of T. congolensis are very similar to those described as neurosecretory cells in oncospheres of several species of cyclophyllidean cestodes (for review see: Świderski and Tkach, Reference Świderski and Tkach2002; Młocicki et al. Reference Młocicki, Świderski, Miquel, Eira and Conn2006). It should be emphasized, however, that the classification of these cells as neurosecretory is based on cytological criteria; therefore more specific histochemical and immunocytochemical information is needed to confirm their exact classification and to elucidate their function. The typical oncospheral nerve cells with characteristic dense-cored vesicles observed in I. madagascariensis, are similar to those reported previously in hexacanths of other cestodes (Fairweather and Threadgold, Reference Fairweather and Threadgold1981; Świderski, Reference Świderski and Menezes da Silva1997; Tkach and Świderski, Reference Tkach and Świderski1997; Świderski and Tkach, Reference Świderski and Tkach1997d , Reference Świderski and Tkach1999). It should be emphasized, however, that in the oncospheres of some other cyclophyllidean species, e.g. Catenotaenia pusilla (Goeze, 1782) (see Świderski, Reference Świderski1972) and Hepatocestus hepaticus (Baer, 1932) (see Świderski et al. Reference Świderski, Tkach and Vaucher2000), the nerve cells were absent.
Penetration glands
Reid (Reference Reid1948) introduced the term ‘penetration glands’, hypothesizing that the secretion of these oncospheral glands may help hexacanths to penetrate the tissues of the intermediate host. Initially, these glands have been described as comprising two cells located symmetrically behind the hooks and joined together by an isthmus to give a U-shaped syncytial structure. The nuclei lie at the base of the U and the cytoplasm contains numerous secretory granules (Reid, Reference Reid1948). Later, in some light and electron microscopical studies of cestode oncospheres, the penetration glands were described as bi-nucleated, four-nucleated, multicellular or unicellular glands (for review see: Świderski and Tkach, Reference Świderski and Tkach2002). The most common, however, is the U-shaped syncytial bi-nucleated penetration gland. Świderski (Reference Świderski1983) observed three different types of glandular regions in oncospheres of E. granulosus. He described two additional pairs of glandular regions between the arms of the typical U-shaped penetration gland (=glandular region PG1) of which one pair contained electron-lucent secretory granules while the other possessed small membrane-bound granules of high electron density. Based on the studies published by Fairweather and Threadgold (Reference Fairweather and Threadgold1981) concerning nerve cells in Hymenolepis nana (Siebold, 1852), the third type of secretory granules of the so-called ‘glandular regions’ (Świderski, Reference Świderski1983) are also considered to be neurosecretory granules of nerve cells in E. granulosus and E. multilocularis (Świderski, Reference Świderski and Menezes da Silva1997). Hence, there seems to be a consensus of opinion that there are two different types of penetration glands PG1 and PG2 in E. granulosus, based on differences in the shape and electron density of their secretory granules: sg1 and sg2, localized, respectively, in PG1 and PG2. The mechanism of penetration gland secretion was classified as merocrine, apocrine or holocrine (for reviews see Lethbridge and Gijsbers, Reference Lethbridge and Gijsbers1974; Lethbridge, Reference Lethbridge1980; Świderski and Tkach, Reference Świderski and Tkach1997c , Reference Świderski and Tkach d ; Młocicki et al. Reference Młocicki, Świderski, Bruňanská and Conn2010). In spite of the above hypotheses, the mechanism of secretion from the penetration glands still remains unclear and indeed there is neither direct evidence for the contents of the cells being secreted in vivo nor any direct evidence that they play a role in penetration.
In an earlier paper on I. madagascariensis (see Świderski and Tkach, Reference Świderski and Tkach2002), we described only one type of penetration gland and the so-called ‘additional type of secretory cells’ containing secretory granules different from those characteristic for the penetration glands and nerve cells. Now, however, after a careful re-examination and comparison of the ultrastructural details of the three types of oncospheral secretory regions in both T. congolensis and I. madagascariensis, we came to the conclusion that as in E. granulosus (see Świderski, Reference Świderski1983) both species contain two types of penetration glands PG1 and PG2, with evidently different types of their secretory granule sg1 and sg2. This inconsistency between our earlier observations on I. madagascariensis and their present verification may be due to differences in the methodologies used in the two studies. In addition, the hexacanths of T. congolensis and I. madagascariensis represent a unique example of the oncospheres having hexa-nucleated penetration glands; so far this has not been reported in other cestodes. In T. congolensis as I. madagascariensis (see Świderski and Tkach, Reference Świderski and Tkach2002), most of the gland cytoplasm is filled with accumulation of disc-shaped moderately electron-dense membrane-bound secretory granules, arranged in some parts in parallel stacks or rouleau. Recent TEM studies based on ultrastructural reconstruction of Taenia ovis (Cobbold, 1869) oncospheres from serial sections (Jabbar et al. Reference Jabbar, Świderski, Młocicki, Beveridge and Lightowlers2010a , Reference Jabbar, Crawford, Młocicki, Świderski, Conn, Jones, Beveridge and Lightowlers b ) also confirmed the presence of two types of penetration glands in the hexacanths of this cestode species.
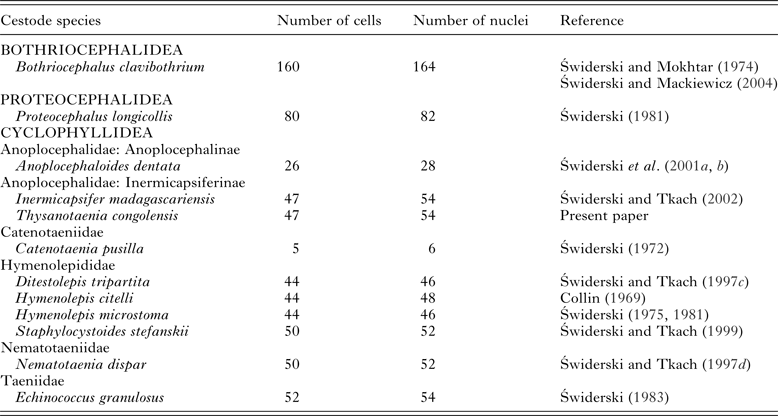
Number of oncospheral cells
As indicated in our results, the approximate number of oncospheral cells in T. congolensis is 47 (54 nuclei), including two syncytial structures, namely the bi-nucleate epithelial perikaryon and six-nucleate penetration gland. A similar number of oncospheral cells were reported in several hymenolepidids (Table 1). The number of oncospheral cells in proteocephalideans is about 80 and the highest number of oncospheral cells was observed in the lower cestode, bothriocephalidean B. clavibothrium – about 160 (for references see Table 1). The comparison of the number of oncospheral cells determined by means of TEM may suggest that the progressive reduction in the number of oncospheral cells is a general trend in cestode evolution and may represent one of their ontogenetic adaptations. Our results on cellular composition of I. madagascariensis oncospheres provide additional comparative data and support the above working hypothesis. Detailed examination of oncosphere cellular organization of more cestode taxa is necessary in order to provide ground for more robust conclusions on this subject.
Table 1. Number of oncospheral cells in some cestode species.
Phylogenetic implications
Our new ultrastructural data presented here provide strong evidence that T. congolensis is firmly positioned within the family Davaineidae, rather than within the anoplocephalid subfamilies Anoplocephalinae or Linstowiinae. The implication is that Thysanotaenia, and perhaps all Inermicapsiferines (Świderski and Tkach, Reference Świderski and Tkach2002), are actually davaineid cestodes that have secondarily lost their rostellum and hooks (Świderski, Reference Świderski, Imura, Maruse and Suzuki1986a , Reference Świderski, Imura, Maruse and Suzuki b , Reference Świderski, Imura, Maruse and Suzuki c ; Khalil et al. Reference Khalil, Jones and Bray1994). The present ultrastructural data, in fact, support the phylogenetic distinction between Inermicapsiferinae and Anoplocephalinae. New ultrastructural data would also be needed for other davaineids (e.g. Raillietina) to confirm these patterns. Overall, our ultrastructural results provide a significant revision to the understanding of the phylogenetic relationships among these cyclophyllidean families.
ACKNOWLEDGEMENTS
We wish to thank the ‘Centres Científics i Tecnològics’ of the University of Barcelona (CCiTUB) for their assistance in the preparation of samples.
FINANCIAL SUPPORT
This study was financially supported by the Spanish grants from AGAUR (no. 2014 SGR 1241) and from ‘Ministerio de Economía y Competitividad’ (no. CGL2015-65138-C2-1-P) and by the ‘Comissió de Recerca’ of the Pharmacy Faculty of the University of Barcelona, all three to J.M.
DEDICATION
This paper is dedicated to the memory of Professor Jean-Georges Baer (1902–1975), the distinguished Swiss parasitologist, the author of the excellent revision on morphology, taxonomy and systematics of Inermicapsifer madagascariensis, see: Baer, Reference Baer1956, on the occasion of the 40th anniversary of his death.











