INTRODUCTION
There is a perennial need to monitor marine ecosystems, where marine mammals are considered useful indicators of ecosystem change (Hammond et al., Reference Hammond, Berggen, Benke, Borchers, Collet, Heide-Jørgensen, Heimlich, Hiby, Leopold and Øien2002). Marine mammal populations in European waters are protected under several international agreements (e.g. the EU Habitats Directive, ASCOBANS, the Marine Strategy Framework Directive, and the Common Fisheries Policy). These require regular monitoring of their conservation status through estimations of abundance and distribution, as well as the identification of present and emerging pressures and threats (Hoyt, Reference Hoyt2005; Hammond et al., Reference Hammond, Macleod, Berggren, Borchers, Burt, Cañadas, Desportes, Donovan, Gilles, Gillespie, Gordon, Hiby, Kuklik, Leaper, Lehnert, Leopold, Lovell, Øienm, Paxton, Ridoux, Rogan, Samarra, Scheidat, Sequeira, Siebert, Skov, Swift, Tasker, Teilmann, Van Canneyt and Vázquez2013). A successful monitoring programme should therefore be able to assess changes in a population's condition and conservation status and help inform management goals and objectives (Bubb et al., Reference Bubb, Jenkins and Kapos2005).
Monitoring marine mammal populations through the collection of long-term, fine scale at-sea data is financially costly (Peltier et al., Reference Peltier, Baagoe, Camphuijsen, Czeck, Dabin, Danie, Deaville, Haelters, Jauniaux, Jensen, Jepson, Keijl, Siebert, van Canneyt and Ridoux2013), and logistically challenging due to the temporal and spatial heterogeneity of the marine environment and the wide range and mobility of the species of interest. As a consequence, information on significant pressures and fine scale estimates of abundance and distribution are generally unavailable for most geographic regions. Large scale decadal live surveys, such as SCANS I and SCANS II (Hammond et al., Reference Hammond, Macleod, Berggren, Borchers, Burt, Cañadas, Desportes, Donovan, Gilles, Gillespie, Gordon, Hiby, Kuklik, Leaper, Lehnert, Leopold, Lovell, Øienm, Paxton, Ridoux, Rogan, Samarra, Scheidat, Sequeira, Siebert, Skov, Swift, Tasker, Teilmann, Van Canneyt and Vázquez2013) offer a way of investigating abundance and distribution, but produce low resolution outputs with limited temporal or spatial precision and thereby remain unable to detect all but the most catastrophic population changes and declines (Taylor et al., Reference Taylor, Martinez, Gerrodett, Barlow and Hrovat2007; Williams et al., Reference Williams, Gero, Bejder, Calambokidis, Kraus, Lusseau, Read and Robbins2011).
Stranded individuals provide a sample of the living population and offer a relatively low cost method of surveillance on the incidence of disease and causes of death. Strandings monitoring is encouraged by multiple intergovernmental organizations and agreements (e.g. International Whaling Commission Scientific Report, 2016, and through various agreements under the Convention for Migratory Species), yet has been relatively underutilized as an indicator to assess population management objectives as well as metrics of wider marine ecosystem conservation. This is in part due to the uncertainty around the ecological value of the stranding record in informing on the at-sea population, caused by the complexity in quantifying the multiple components of the stranding process (Hart et al., Reference Hart, Mooreside and Crowder2006; Williams et al., Reference Williams, Gero, Bejder, Calambokidis, Kraus, Lusseau, Read and Robbins2011; Peltier et al., Reference Peltier, Baagoe, Camphuijsen, Czeck, Dabin, Danie, Deaville, Haelters, Jauniaux, Jensen, Jepson, Keijl, Siebert, van Canneyt and Ridoux2013). Strandings are a function of biological, physical and social processes that influence stranding rates. The biological aspect is of primary interest in the context of conservation as this holds information on the parameters of concern, such as cause of death, population health, and species abundance and distribution. However this biological signal can be confounded by physical and social factors which influence the incidence of reported beach cast carcasses (Epperly et al., Reference Epperly, Braun, Chester, Cross, Merriner, Tester and Churchill1996; Flint & Fowler, Reference Flint and Fowler1997), complicating the interpretation of stranding data at a population level. Nevertheless the accumulation of stranding data allows analysis of historical trends and patterns, providing a means to define a baseline for stranding rates as well as potentially biological and ecological metrics of the stranded population. These include cause of death (e.g. Kirkwood et al., Reference Kirkwood, Bennet, Jepson, Kuiken, Simpson and Baker1997), nutritional condition (e.g. Lockyer, Reference Lockyer1995), disease burden (e.g. Dailey & Stroud, Reference Dailey and Stroud1978), diet (e.g. Santos et al., Reference Santos, Pierce, Learmonth, Reid, Ross, Patterson, Reid and Beare2004; Leopold, Reference Leopold2015), life history (e.g. Lockyer & Kinze, Reference Lockyer and Kinze2003) and environmental contaminant levels (e.g. Bennett et al., Reference Bennett, Jepson, Law, Jones and Kuiken2001; Jepson et al., Reference Jepson, Deaville, Barber, Aguilar, Borell, Murphy, Barry, Brownlow, Barnett, Berrow, Cunningham, Davison, ten Doeschate, Esteban, Ferreira, Foote, Genov, Gimenez, Loveridge, Llavona, Martin, Maxwell, Papachlimitzou, Penrose, Perkins, Smith, de Stephanis, Tregenza, Verborgh, Fernandez and Law2016). These metrics can reveal vital clues about the processes that generated these data and assist in identifying change, pressures and threats; both in terms of acute impacts and long-term loss of population viability (McFee et al., Reference McFee, Hopkins-Murphy and Schwacke2006; Leeney et al., Reference Leeney, Amies, Broderick, Witt, Loveridge, Doyle and Godley2008).
Strandings of marine mammals in Scotland have been recorded since the early 1900s (Fraser, Reference Fraser and Harrison1977) and have been actively monitored since 1992 by the Scottish Marine Animal Stranding Scheme (SMASS). This dedicated research and reporting scheme collates spatial and temporal information on each reported stranding and a subset of reported cases is necropsied by specialist veterinary pathologists (Jepson, Reference Jepson2005). As a result, the Scottish stranding database has become one of the most extensive sources of information for many cetaceans along the Scottish coastline and holds the potential to be used as a population monitoring tool.
Using harbour porpoise (Phocoena phocoena) strandings on the east coast of Scotland as an example, we demonstrate how examination of baseline patterns can facilitate the detection of unusual variability in stranding rates. The stranding record gathered by SMASS between 1992 and 2014 was used to assess seasonal variation in the incidence of strandings of harbour porpoises along the east coast of Scotland. We show and discuss how this improved understanding of baseline variation can contribute to the interpretation of strandings data, thereby exploring the potential of the strandings record as a monitoring tool and its expediency to management.
MATERIALS AND METHODS
Data collection
Strandings in Scotland are discovered opportunistically and reported by members of the public to the Scottish Marine Animal Stranding Scheme. The amount and quality of information can vary across stranding events, therefore only those cases that included data on stranding date and location were included in the analysis. Animals that were reported floating at sea were excluded. Data were used from the start of the stranding scheme in January 1992 up to and including December 2014.
Data analyses
Temporal variation in stranding numbers was modelled to examine and quantify seasonal patterns in stranding frequencies. Data exploration was applied by examining covariates for outliers, missing values and collinearity, prior to analysis.
To examine temporal patterns in the total numbers of strandings, a Generalized Additive Mixed Model (GAMM) was applied to the stranding data, using the nlme and mgcv packages available in R (Venables & Ripley, Reference Venables and Ripley2002; Wood, Reference Wood2006; Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009; R Core Team, 2014). A count of number of strandings was modelled as a function of month and year. The model was fitted using a Poisson error distribution with a log-link function and including an AR1 correlation structure. The appropriate level of smoothness was found by utilizing the integrated smoothness estimation and cross validation function available within the mgcv library. A new variable comprising a continuous count of months since the beginning of the study period was created, and fitted to the correlation structure to account for continuous autocorrelation. Model selection was carried out by comparing different forms of inclusion of the variables month and year, and identifying the most appropriate model structure through examination of residuals, parameter estimates, and evaluation of the Akaike Information Criterion (AIC, Akaike, Reference Akaike1974). The AIC is however approximate in GAMM and provides only an indication of the model fit. GAMM procedures generate no overall goodness of fit measure and r 2 values are also approximations. Model validation was therefore carried out primarily by evaluating diagnostic plots and residual variance using normalized residuals. The residual scaled deviance to the residual degrees of freedom-ratio (rdev/rdf-ratio) was calculated to examine possible overdispersion in the model as a control for over-fitting.
RESULTS
A total of 964 harbour porpoise strandings were reported along the east coast of Scotland (Figure 1) throughout the 23 year study period. The total number of strandings per year ranged from a minimum of 22 in 1999, to a maximum of 93 in 2013, with a mean of 42 strandings (SD ± 16.57) per year.
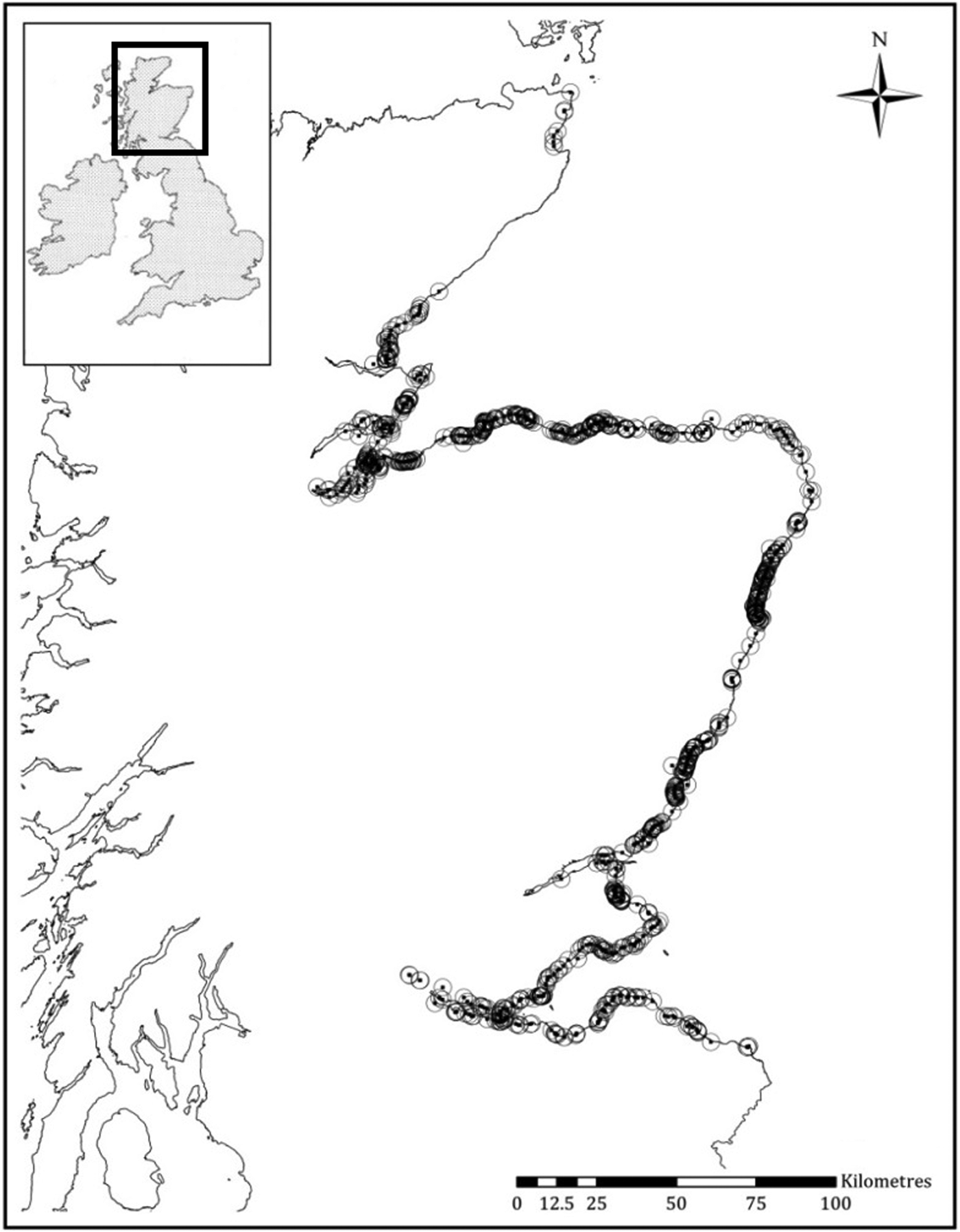
Fig. 1. Map of the Scottish east coast. Circled dots indicate harbour porpoise stranding locations from 1992–2014.
The optimal model incorporated month of stranding fitted as a smoother (circular cubic spline), and included year as a random effect. The degrees of freedom of the smoother was estimated to be 4.7 and was significant (Table 1), providing evidence for a non-linear effect of month, and seasonality in number of strandings. Evaluation of the smoothing curve shows an increasing incidence of strandings between February with a peak in March and April, after which numbers decrease throughout the summer months to a constant low between September and January (Figure 2). There was no evidence of serial correlation in the residuals, which demonstrates that using a continuous correlation structure correctly modelled the autocorrelation present in the data. Its parameter (φ) was estimated to be 0.221, indicating that the level of autocorrelation is rather weak. The rdev/rdf ratio was calculated at 0.585, meaning the model fitted the data well and was not overdispersed. The variance of the random intercept for year was estimated at 0.238 suggesting that whilst there are differences in numbers per year, the between year variation was small and there is no apparent inter-annual trend present in the data.
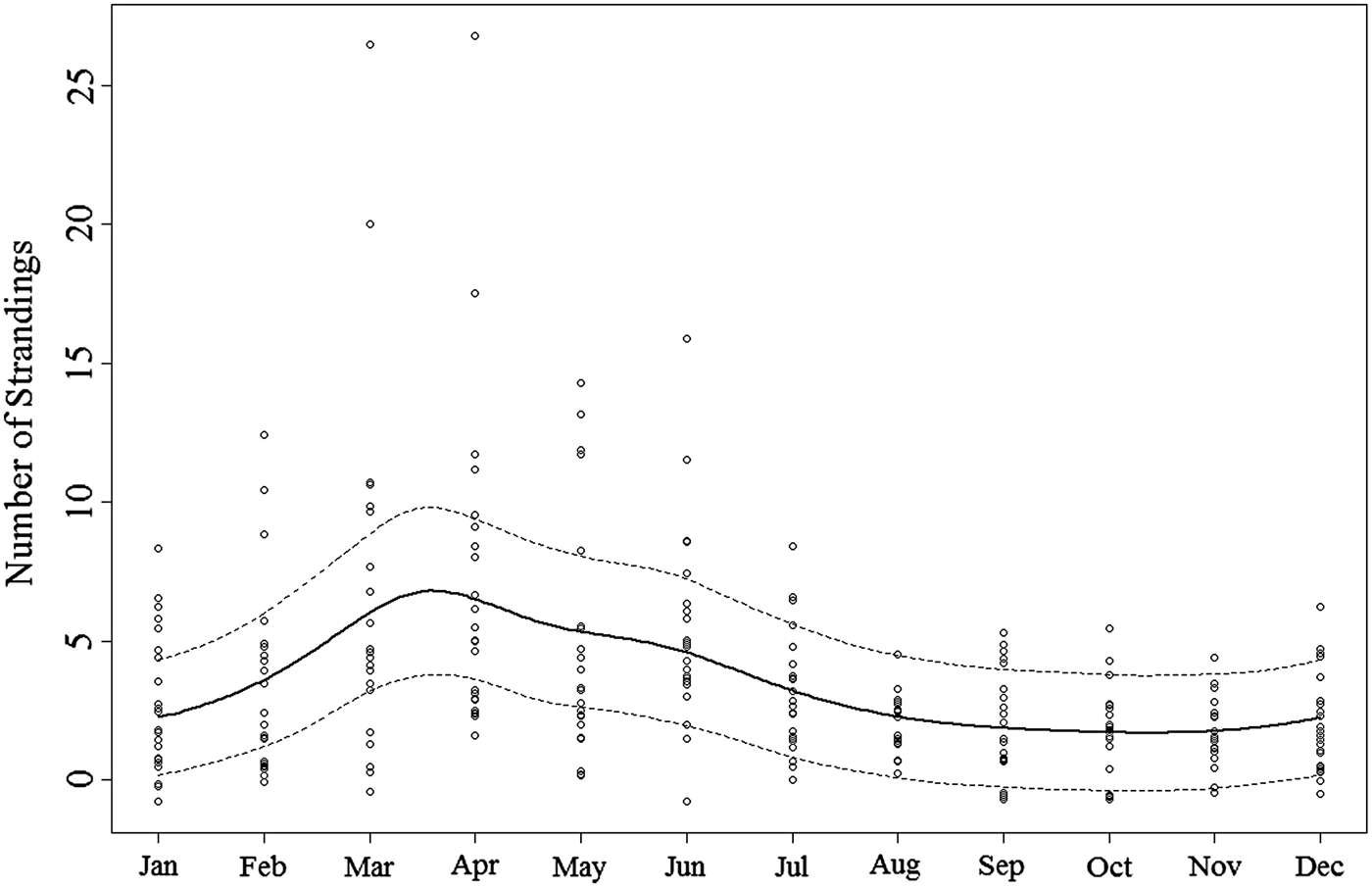
Fig. 2. Solid line for predicted number of strandings for a typical year as informed by the final GAMM model. Dotted lines represent 95% confidence intervals scaled according to the rdev/rdf ratio to control overfitting of the model. Points are vertically jittered to facilitate graph interpretation and represent a count of actual (raw) monthly observations.
Table 1. Results of the final GAMM model for the number of strandings modelled as a smooth function (circular cubic spline) of month with a random intercept for year.
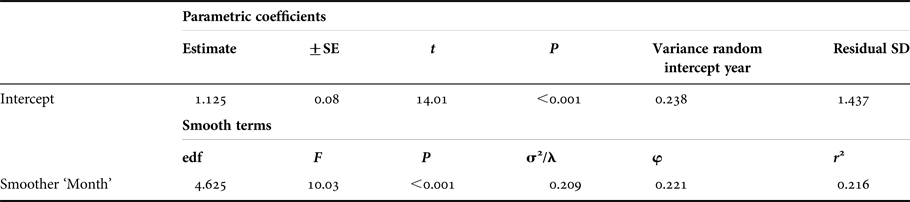
Table values show parametric coefficients, the estimated degrees of freedom of the smoother (edf), and results from an F test of the significance of the smoothing effect. The smoothing parameter is given under σ 2/λ. The r 2 value is an adjusted value indicating the approximate variance explained by the model. Phi (φ) is the autocorrelation parameter.
DISCUSSION
Cetaceans are low resilience organisms that can take decades to recover from low population levels (Magera et al., Reference Magera, Mills Flemming, Kaschner, Christensen and Lotze2013) hence detecting trends is a major goal of any monitoring strategy for these species. Identifying unusual population mortality requires a good knowledge of the existing seasonal pattern, and understanding baseline variation is therefore essential when trying to increase the ecological value of strandings data as a population indicator. This analysis of 23 years of stranding data has identified a strong seasonal trend in harbour porpoise strandings along the east coast of Scotland, which have remained consistent throughout the study period. While knowing the seasonal pattern of a process is crucial to detect any unusual event in the short term, understanding potential multi-decadal trends is equally desirable to be able to detect and interpret possible long-term changes in the population. Results from this study showed that while there were differences in total stranding numbers per year, there was no significant between-year variation detected as indicated by the low estimate for the variance of the random intercept. This means that there has not been a large-scale change or long-term trend in strandings during the 23 year study period.
Modelling seasonality as done here is a robust means to identify significant deviations from expected strandings numbers, with Generalized Additive Mixed Models allowing the examination of non-linear relationships and nested data structures while being able to account for temporal auto-correlation commonly associated with time series data (Wood, Reference Wood2004; Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009). The model permits for any change in the incidence of strandings to be detected and quantified, providing a regional framework for monitoring unusual mortality events that can be used for future reference. By evaluating the normalized model residuals, which for each year should be centred around a zero mark, increases and decreases can be observed whilst taking into account between-year variation. As a standard, the residual variation for every individual year exceeding the area between −1.5 and 1.5 could potentially be evaluated as unusual. This makes it possible to not only identify unusual mortality, but additionally assess the order of magnitude of the event. Similar modelling can be done for a variety of frequently stranded species and could additionally be applied to larger geographic areas, and theoretically anywhere in the world where carcasses of cetaceans can become stranded. Implementation of this approach would be an improvement to more basic measures currently used elsewhere, by for example Hohn et al. (Reference Hohn, Rotstein and Byrd2013) who defined an increase in number of strandings to be unusual if it exceeded a historical mean plus 2 SD based on 7 years of stranding data collected along the coast of North Carolina, USA.
Nevertheless unusual variations should be interpreted with caution. Where an exceptional increase in strandings could indicate increased mortality or increased abundance (biological variation), it may well be a function of unusual variation in environmental conditions (referring to physical aspects) or observer efforts (social components). Detecting the elevation in stranding rates therefore is just the beginning; further examination of the underlying cause is then essential in order to establish if an observed increase is real and whether mitigation is required. Full characterization of any unusual deviations in stranding rates would likely require the identification and integration of multiple data sources. These would need to inform on a number of biological, physical and social factors likely to affect the observed strandings rate. Establishing the baseline variation of these metrics, in similar fashion to the work described here, would further quantify and reduce the biases associated with strandings data.
Population indicators should function as informative low-cost simplifications of direct measurements of the population parameters of interest (Bubb et al., Reference Bubb, Jenkins and Kapos2005). This study has demonstrated that when analysed correctly, a described baseline in stranding frequencies can be informative in assessing variances in stranding rates and identify and quantify unusual mortality events. As with other opportunistic wildlife data, information derived from strandings usually cannot be gathered by other means, thus tools to improve and expand the utility of these data will be of significant benefit. An extended more detailed baseline with regards to the composition of the stranded population would further contribute to this, and additionally facilitate the detection of substantial variations in the biological components of a population such as age or sex structure of affected animals, and any altered exhibition of pathological findings, clinical signs, or physical condition of stranded individuals. These are all factors that can be used as indicators of change in the status and health of a population (Norman et al., Reference Norman, Bowlby, Brancato, Calambokidis, Duffield, Gearin, Gornall, Gosho, Hanson, Hodder, Jeffries, Lagerquist, Lambourn, Mate, Norberg, Osborne, Rash, Riemer and Scordino2004; McFee et al., Reference McFee, Hopkins-Murphy and Schwacke2006; Nemiroff et al., Reference Nemiroff, Wimmer, Daoust and McAlpine2010; Hohn et al., Reference Hohn, Rotstein and Byrd2013). This is likely to further improve the qualitative and quantitative applicability of strandings as a population indicator for future monitoring.
ACKNOWLEDGEMENTS
Significant thanks are due to the many people involved with data collection; in particular everyone who reported, photographed and collated strandings reports over the years. Special thanks to Eileen Hesse, Jessica Wingfield and Dr Ewan Edwards for all useful discussions and support during the first stages of development of this study.
FINANCIAL SUPPORT
Data collection and post-mortem investigations were carried out under the aegis of the UK Cetacean Strandings Investigation Programme, which is jointly funded by the Department for Environment Food and Rural Affairs (Defra) and the Scottish Government.





