INTRODUCTION
The fifth assessment report of the Intergovernmental Panel on Climate Change (IPCC, 2014) reported a global increase in average mean annual temperatures of + 0.85°C between 1880 and 2012, each decade being warmer than the previous one. This increase in temperature, associated with the melting of the northern ice cap and the rise of global sea level, constitutes the central concern of global climate change. Consequently, there is a need to predict the evolution of global climate through the use of numerical models ranging from simple energy-balance models to complex Earth system models (Flato et al., Reference Flato, Marotzke, Abiodun, Braconnot, Chou, Collins, Cox, Driouech, Emori and Eyring2013). Paleoclimatic data obtained from various proxies coming from sedimentary archives of interglacial stages are necessary to affix boundary conditions to such numerical models.
Paleontological, geochemical, and sedimentological records reveal climate variations between cold and warm stages for the last 3 Ma. For the last million years, since the mid-Pleistocene transition (Medina-Elizalde and Lea, Reference Medina-Elizalde and Lea2005), the cold stages lasted around 100,000 yr. The glacial and interglacial stages of the last million years, which are called Marine Isotope Stages (MIS), arouse much interest (e.g., Augustin et al., Reference Augustin, Barbante, Barnes, Barnola, Bigler, Castellano, Cattani, Chappellaz, Dahl-Jensen and Delmonte2004; Tzedakis, Reference Tzedakis2010; Robinson et al., Reference Robinson, Alvarez-Solas, Calov, Ganopolski and Montoya2017), because they could contribute to our understanding of the climate dynamics of the Holocene (MIS1) and, especially, the evolution of an interglacial stage devoid of any anthropic influence and its consequent impact upon ecosystems and coastal geomorphology. Many studies have been devoted to two interglacial stages, MIS5e (Oppo et al., Reference Oppo, McManus and Cullen2006; Jouzel et al., Reference Jouzel, Masson-Delmotte, Cattani, Dreyfus, Falourd, Hoffmann, Minster, Nouet, Barnola and Chappellaz2007) and MIS11c, the latter being usually considered as the best analog to Holocene climate change (Droxler et al., Reference Droxler, Alley, Howard, Poore and Burckle2003; Loutre and Berger, Reference Loutre and Berger2003). Indeed, MIS11 and MIS1 share the same configuration of orbital parameters: low eccentricity, low precession, and weak variations in insolation. MIS11c, which is an interglacial stage within the MIS11 warm isotope stage, lasted a long time (around 27,000 to 30,000 yr) compared with other interglacial stages such as MIS9e (≈12,000 yr) and MIS5e (≈13,000 yr) according to Shackleton (Reference Shackleton1967), Howard (Reference Howard1997), McManus et al. (Reference McManus, Oppo, Cullen and Healey2003), Augustin et al. (Reference Augustin, Barbante, Barnes, Barnola, Bigler, Castellano, Cattani, Chappellaz, Dahl-Jensen and Delmonte2004), and Prokopenko et al. (Reference Prokopenko, Bezrukova, Khursevich, Solotchina, Kuzmin and Tarasov2010). Candy et al. (Reference Candy, Schreve, Sherriff and Tye2014) performed a global review of the climate characteristics of the MIS11 warm isotope stage. In the framework of our study, it is worth noting that the record of δ2H in ice cores of Dome C in Antarctica (75°06′S, 123°20′E) (Augustin et al., Reference Augustin, Barbante, Barnes, Barnola, Bigler, Castellano, Cattani, Chappellaz, Dahl-Jensen and Delmonte2004; Jouzel et al., Reference Jouzel, Masson-Delmotte, Cattani, Dreyfus, Falourd, Hoffmann, Minster, Nouet, Barnola and Chappellaz2007) indicates that the air temperature optimum during MIS11c was around 3°C higher than today, although this surface temperature change refers to the high latitudes of the Northern Hemisphere and is higher than the global mean temperature change. The study of raised beaches located in tectonically stable regions and of ice cap dynamics during MIS11 revealed that the sea level was 6 to 13 m higher than today on average (de Vernal and Hillaire-Marcel, Reference de Vernal and Hillaire-Marcel2008; Elderfield et al., Reference Elderfield, Ferretti, Greaves, Crowhurst, McCave, Hodell and Piotrowski2012; Roberts et al., Reference Roberts, Karkanas, Jacobs, Marean and Roberts2012). Moreover, the analysis of terrestrial records corresponding to MIS11 has shown that this warm isotope stage was globally humid (Rousseau, Reference Rousseau2003; Prokopenko et al., Reference Prokopenko, Bezrukova, Khursevich, Solotchina, Kuzmin and Tarasov2010; Melles et al., Reference Melles, Brigham-Grette, Minyuk, Nowaczyk, Wennrich, DeConto, Anderson, Andreev, Coletti and Cook2012). The configuration of astronomical parameters during MIS11, especially the weak precession, induced weaker insolation variability than for other warm isotope stages (Berger and Loutre, Reference Berger and Loutre2002). Consequently, MIS11 is a warm isotope stage that is tricky to detect and quantify in the geologic record, as reflected in the relative lack of data for this stage compared with other marine isotope stages such as MIS5.
Here we provide measurements of the stable carbon and oxygen isotope ratios of skeletal carbonates from marine invertebrates sampled from two marine sequences from the Canary Islands that were deposited during MIS11. The oxygen isotope ratios are then used to calculate sea-surface temperatures (SSTs) using an oxygen isotope fractionation equation for the aragonite–water system. During the two periods of MIS11 studied, SSTs within tropical latitudes in the eastern Atlantic Ocean bracketed those documented during the present day, although the magnitude of seasonal variation was most likely weaker than at present.
GEOLOGIC SETTING AND MATERIAL STUDIED
The Canary archipelago is composed of seven volcanic islands located in the eastern Central Atlantic Ocean between 27°N and 30°N, west of Morocco (Fig. 1a). The formation of the archipelago is linked to the movement of the African plate over a magmatic hot spot (Carracedo et al., Reference Carracedo, Day, Guillou, Badiola, Canas and Torrado1998). Lanzarote, Fuerteventura, and Gran Canaria, the easternmost and oldest islands, share an arid and warm climate resulting from their latitudinal position, their orography, and their proximity to the Sahara Desert. El Hierro and La Palma, the westernmost and youngest islands, are under the influence of the humid northeast trade winds. Different methods have been used to estimate present-day mean annual temperatures (MATs) and SSTs for low latitudes such as 28°N. Borges et al. (Reference Borges, Hernández-Guerra and Nykjaer2004) analyzed time series of the southeastern North Atlantic sea surface obtained from the Advanced Very High Resolution Radiometer (AVHRR) and estimated an average SST close to 20.5°C. Comparable average SSTs close to 20.3°C were published by DeCastro et al. (Reference DeCastro, Gómez-Gesteira, Costoya and Santos2014) for the Moroccan subregion during the period 1982–2012. Such temperature estimates have been based on the National Oceanic and Atmospheric Administration's (NOAA) high-resolution analyses of daily SST. Gómez-Letona et al. (Reference Gómez-Letona, Ramos, Coca and Arístegui2017) estimated a SST of 20.3°C for the coastal waters of the Canary archipelago, using daily Reynolds analyses that combine AVHRR and in situ data. The Climate Atlas of the Spanish Met service (AEMET, 2012) indicates a MAT of 20°C for the Gran Canaria and Lanzarote islands. These temperatures are relatively low compared with those prevailing in the western part of the central Atlantic Ocean, such as the Caribbean Islands, which have temperatures close to 27°C–29°C (Winter et al., Reference Winter, Appeldoorn, Bruckner, Williams and Goenaga1998). Such low temperatures around the Canary archipelago are explained by the presence of a southward-flowing oceanic current, called the Canary Current, originating from the eastern boundary current of the subtropical North Atlantic gyre that is located along the northwest African coast. The current splits as it passes the Canary archipelago, with branches located east and west of Lanzarote. It is associated with a strong coastal upwelling regime along the northwest African coast, caused by the northeast trade winds. The coastal upwelling is permanent at a latitude of 28°N, with its strongest activity occurring in summer and early fall, which results in lower SSTs over the shelf (Navarro-Pérez and Barton, Reference Navarro-Pérez and Barton2001; Pardo et al., Reference Pardo, Padín, Gilcoto, Farina-Busto and Pérez2011).
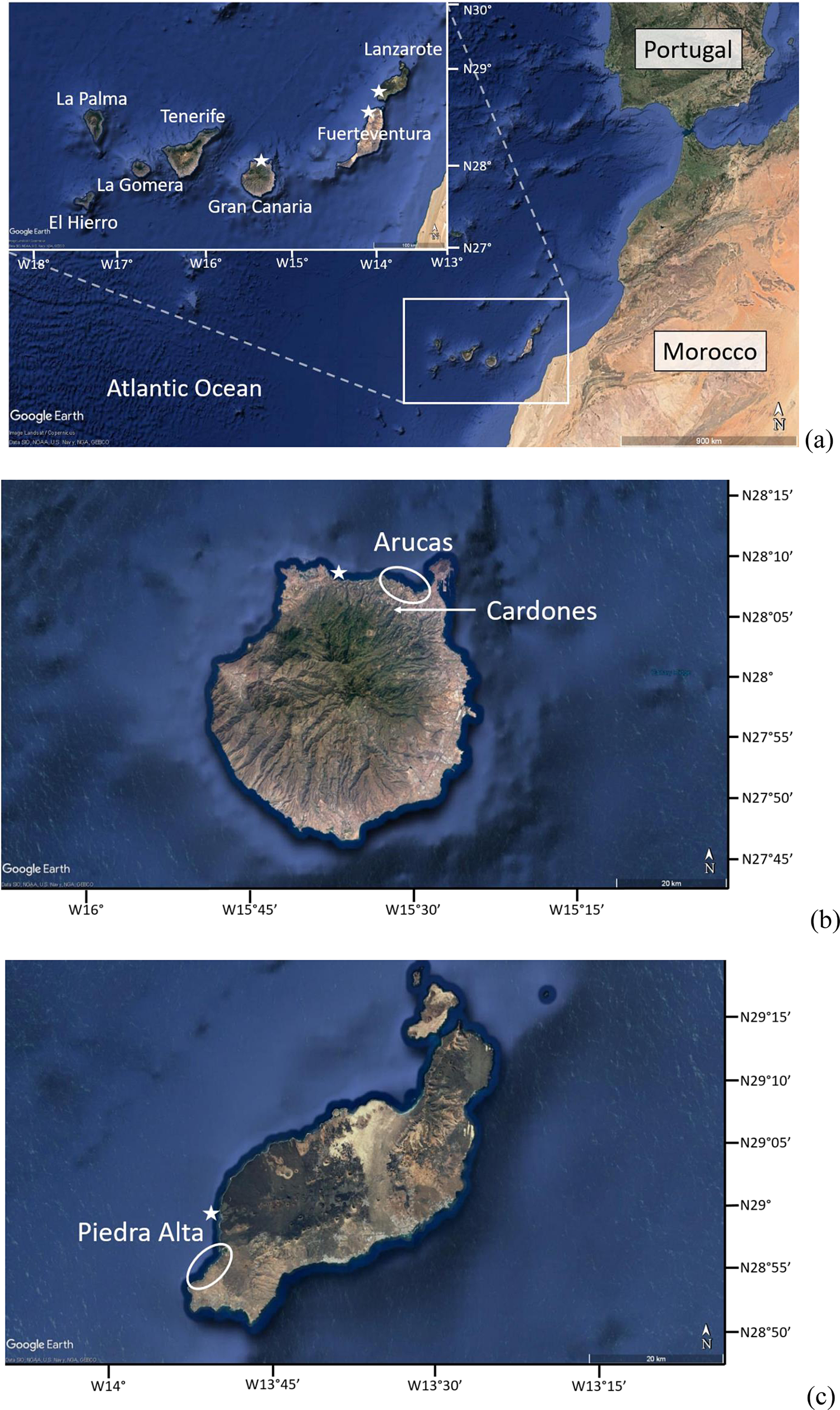
Figure 1. (color online) (a) Location of the Canary archipelago in the Atlantic Ocean; (b) Gran Canaria island, with the location of the Arucas marine deposit circled; and (c) Lanzarote island, with the location of the Piedra Alta tsunamite circled. The locations where coastal waters were sampled are represented by white stars. The datum used for latitude and longitude is WGS84. Pictures were taken from Google Earth.
Marine sedimentary deposits of Quaternary age crop out along the coasts of several islands in the Canary archipelago, namely Lanzarote, Fuerteventura, Gran Canaria, and Tenerife (Muhs et al., Reference Muhs, Meco and Simmons2014; Meco et al., Reference Meco, Lomoschitz, Rodríguez, Ramos, Betancort and Coca2018). This study focuses on two marine deposits attributed to MIS11, the first one being located on Arucas (Gran Canaria) and the second one being located in Piedra Alta (Lanzarote).
The first marine deposit crops out at Arucas on the northern side of the island of Gran Canaria (28°08′N, 15°29′W; Figs. 1b and 2a). First described by Benítez (Reference Benítez and Benítez1912), this sedimentary sequence extends to about 2 km in length from east to west and is deposited above a tephritic basaltic flow including some pillow lavas, most likely related to the former activity of the Cardones volcano. This lava flow was dated by the K-Ar method at 421 ± 20 ka (Meco et al., Reference Meco, Guillou, Carracedo, Lomoschitz, Ramos and Rodríguez-Yánez2002). This K-Ar age allowed Meco et al. (Reference Meco, Guillou, Carracedo, Lomoschitz, Ramos and Rodríguez-Yánez2002) to correlate this marine deposit with MIS11. Today, the base of the Arucas deposit crops out about 30 m above sea level as a result of a westward tilting of Gran Canaria, linked to the lithospheric flexure exerted by the growth of the younger island of Tenerife (Perez-Torrado et al., Reference Perez-Torrado, Cabrera, Carracedo, Gimeno Torrente, Schneider, Paris, Wassmer and Guillou2002; Menéndez et al., Reference Menéndez, Silva, Martín-Betancor, Pérez-Torrado, Guillou and Scaillet2008; Meco et al., Reference Meco, Muhs, Fontugne, Ramos, Lomoschitz and Patterson2011). An extralimital species such as Saccostrea cuccullata (Ostreidae) occurs in this deposit and suggests that SSTs were at least 4.2°C higher than those prevailing at present according to Montesinos et al. (Reference Montesinos, Ramos, Lomoschitz, Coca, Redondo, Betancort and Meco2014).
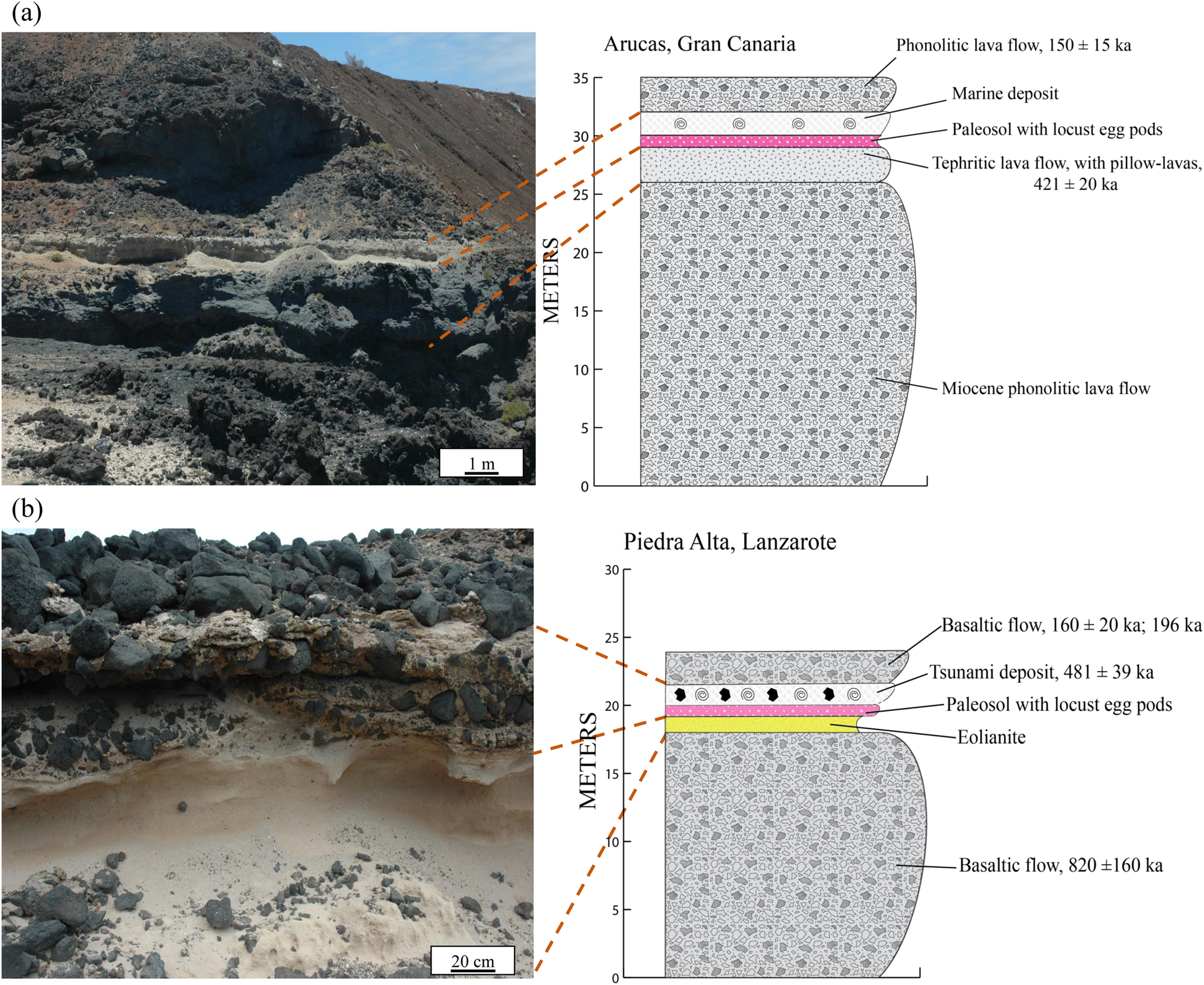
Figure 2. (color online) The Arucas marine deposit (a) and the Piedra Alta tsunamite (b) and their geologic interpretation with stratigraphic logs. Modified after Muhs et al. (Reference Muhs, Meco and Simmons2014) and Meco et al. (Reference Meco, Muhs, Fontugne, Ramos, Lomoschitz and Patterson2011).
The second marine deposit included in this study is exposed on the southwest part of the island of Lanzarote (28°53′N, 13°51′W; Figs. 1c and 2b). First described by Driscoll et al. (Reference Driscoll, Tinkler and Hendry1965), it crops out over 2 km along the coast and at least 7 km inland, while the base of the deposit occurs 20 m above the present-day sea level. Zazo et al. (Reference Zazo, Goy, Hillaire-Marcel, Gillot, Soler, González, Dabrio and Ghaleb2002) attributed the marine deposit to the Episode IX of their raised marine sequences with a sea level 8 to 10 m higher than today. In terms of chronology, they correlated this marine deposit to MIS11 (Zazo et al., Reference Zazo, Goy, Hillaire-Marcel, Gillot, Soler, González, Dabrio and Ghaleb2002). The marine deposit is interlayered between two basaltic flows that were dated by K-Ar at 820 ± 160 ka for the bottom flow (Meco and Stearns, Reference Meco and Stearns1981), while two ages were proposed for the top flow: 160 ± 20 ka by Zazo et al. (Reference Zazo, Goy, Hillaire-Marcel, Gillot, Soler, González, Dabrio and Ghaleb2002) and more recently 196 ka by Meco et al. (Reference Meco, Muhs, Fontugne, Ramos, Lomoschitz and Patterson2011). The marine deposit is poorly sorted, consists of abundant angular or subangular basaltic boulders, and includes large metric basalt boulders as well as finer-grained volcanic clasts. Some of the basaltic clasts are covered by a layer of marine sandstones, while the boulders are cemented with reddish limestones, generally rich in clays (Meco et al., Reference Meco2008). The sedimentary matrix is very fossiliferous, with abundant remains of marine invertebrate shells, none of which were observed in life position. Meco et al. (Reference Meco2008) noticed that the marine deposit contains a mixture of species with different ecology, such as the genus Patella, which is typical of the intertidal zone; species like Gadinia garnoti, which are intertidal and live in the high-tide zone; and fragments of the infralittoral coral Madracis pharencis and the circalittoral (at depths greater than 50 m) gastropod Bursa tenuisculpta.
These paleontological and sedimentological features allowed Meco et al. (Reference Meco2008) to consider that this deposit was not formed by a slow process of continuous sedimentation but rather by an instantaneous and catastrophic event at the geologic scale, such as a tsunami deposit, like the previously identified Agaete tsunami conglomerate in Gran Canaria and the northwestern coast of Tenerife (Paris et al., Reference Paris, Bravo, González, Kelfoun and Nauret2017, Reference Paris, Ramalho, Madeira, Ávila, May, Rixhon, Engel, Brückner, Herzog and Schukraft2018).
We selected 64 specimens of marine invertebrates from the Piedra Alta deposit and 77 specimens from the Arucas deposit for carbon and oxygen isotope measurements (see Table 1). In both cases, the faunal assemblage is composed of several species of molluscs, echinoderms, cnidarians, and bryozoans, whose habitats are infralittoral to intertidal and for which the taxonomy is reported in Table 1.
Table 1. Specimens collected from the Piedra Alta tsunamite and the Arucas marine deposit. The habitats data are extracted from Meco et al. (Reference Meco2008).

ANALYTICAL TECHNIQUES
Skeletons of marine invertebrates are mainly composed of either calcite or aragonite. Preservation of the initial mineralogy in a fossil is largely dependent on the temperature-sensitive solubility of these polymorphs in an aqueous phase. Indeed, at 25°C, the solubility products (Ksp) are quite high, with values of 10−8.48 and 10−6.40 for calcite and aragonite, respectively, according to data from the National Institute of Standards and Technology–Joint Army Navy NASA Air Force (NIST-JANAF) thermodynamic tables (Chase, Reference Chase1998). Consequently, because aragonite is less stable than calcite, it cannot be excluded that calcitic shells were aragonitic in origin before being diagenetically altered into secondary calcite. It has been known for decades (Land, Reference Land1967) that recrystallization operates at ambient temperature in the presence of water during the burial of fossils. Those aqueous fluids may be pore waters, in the case of early diagenesis, or crustal waters of meteoric or metamorphic origin during late diagenesis.
Raman spectroscopy
We used Raman spectroscopy to characterize the mineralogy of all the skeletal carbonates studied. The two polymorphs of CaCO3 are readily identified from the low-frequency part of the spectra, and especially from the position and splitting of the symmetric bending mode, which occurs as a single peak at 712 cm−1 in calcite and as a doublet at 701–704 cm−1 in aragonite (e.g., Unvros et al., Reference Unvros, Sharma and Mackenzie1991; Gillet et al., Reference Gillet, Biellmann, Reynard and McMillan1993). The Raman Spectrometer X'plora, hosted by the Laboratoire de Géologie de Lyon at the University Claude Bernard Lyon 1, was operated using a 1000× objective, an optical network of 1800 lines per mm, a monochromatic laser (532 nm wavelength) filtered by 10% with two acquisitions (60 s per acquisition) performed between 100 and 1600 cm−1.
Sampling strategy
Macroscopic criteria to select the skeletal carbonates for the measurement of stable isotope ratios are unbroken shells, the absence of mechanical erosion, and dissolution patterns. The selected skeletal carbonates were cleaned by repeated washing with deionized water in an ultrasonic bath to remove any remaining sedimentary particles. For most skeletal carbonates, we performed bulk analysis (hereafter “bulk” protocol), which consists, after mechanically removing the most juvenile parts of the shells (i.e., the umbo area), of powdering the remaining shells in an agate mortar. The juvenile parts are removed because they are biased by a kinetic fractionation linked to the fast growth of the shell at that moment. For two molluscs, Callista chione from Piedra Alta (PA-10-04) and Cerithium vulgatum from Arucas (ARU-18-02), shells were incrementally sampled with a Dremel micro-drill (hereafter “incremental” protocol) along the main growth axis from the apex of the shell to the aperture to measure the ontogenetic evolution of their carbon and oxygen isotope ratios.
Carbon and oxygen isotope analysis of skeletal carbonates
The carbonate analyses were carried out at the Laboratoire d’Écologie des Hydrosystèmes Naturels et Anthropisés (LEHNA) in Lyon using an isoFLOW system connected online in continuous-flow mode to a precisION mass spectrometer operated by visION software from Elementar UK Ltd. Sample powders were loaded in LABCO Exetainer 3.7 mL round-bottomed, nonevacuated soda-glass vials. For pure carbonate samples, aliquots of 300 to 500 μg for the bulk protocol and aliquots of 15 μg for the incremental protocol were reacted with anhydrous phosphoric acid prepared according to the protocol described by McCrea (Reference McCrea1950). Phosphoric acid was automatically introduced in contact with the sample by the use of an acid pump. The reaction took place at 70°C in a temperature-regulated sample tray. The CO2 gas generated during the acid digestion of the carbonate sample was then transferred to the mass spectrometer via the centrION interface. A calibrated CO2 gas was used as a monitoring gas. An acid fractionation factor α(CO2-aragonite) of 1.0085 was selected according to Kim et al. (Reference Kim, Mucci and Taylor2007a). The calibrated material used was Carrara marble (δ18OVPDB = −1.84‰; δ13CVPDB = +2.03‰) (Fourel et al., Reference Fourel, Martineau, Tóth, Görög, Escarguel and Lécuyer2016) and NBS18 (δ18OVPDB = −23.2‰; δ13CVPDB = −5.01‰) (Friedman et al., Reference Friedman, O'Neil and Cebula1982; Hut, Reference Hut1987; Stichler, Reference Stichler1995; Coplen et al., Reference Coplen, Brand, Gehre, Gröning, Meijer, Toman and Verkouteren2006). Aliquots of Carrara marble were placed at the beginning and at the end of each analytical batch to correct for potential instrument drift through time. Delta values are expressed with respect to VPDB. External reproducibility (2σ) was lower than ±0.1‰ for δ18O and ±0.05 ‰ for δ13C.
Oxygen isotope analysis of water
Coastal marine waters (1−5 m depth) were sampled along the coasts of Lanzarote (Piedra Alta, one sample), Fuerteventura (two samples), and Gran Canaria (Arucas, one sample), as depicted in Figure 1. The 18O/16O analyses from water samples were carried out at the LEHNA in Lyon using an isoFLOW system in water equilibration mode (Epstein and Mayeda, Reference Epstein and Mayeda1953; Horita et al., Reference Horita, Ueda, Mizukami and Takatori1989; McCarthy et al., Reference McCarthy, Pichler and Price2005) connected online in continuous-flow mode to a precisION mass spectrometer operated by a visION software from Elementar UK Ltd. Aliquots (200 μL) were loaded in LABCO Exetainer 3.7 mL round-bottomed, nonevacuated soda-glass vials. The sample vial headspace was automatically flushed with helium with the isoFLOW double-needle setup. The equilibration gas was a mixture of 10% CO2 in helium automatically introduced in contact with the sample through the isoFLOW. The equilibration reaction took place at 40°C in a temperature-regulated sample tray. The equilibrated CO2 gas generated was then transferred to the mass spectrometer via the centrION interface. A calibrated CO2 gas was used as a monitoring gas. Calibrated waters were used to anchor the results to the V-SLAP/V-SMOW scale. The calibrated waters used were EdL III (δ18OVSMOW = −9.34‰), EE1 (δ18OVSMOW = +6.442‰), and SSRW (δ18OVSMOW = −16.0‰). During the course of the experiments, the waters used as working standards were calibrated against waters from the Water Isotope Inter-Comparison intercalibration program (Wassenaar et al., Reference Wassenaar, Terzer-Wassmuth, Douence, Araguas-Araguas, Aggarwal and Coplen2018). Aliquots of EdL III were placed at the beginning and at the end of each analytical batch to correct for potential instrument drift with time. Delta values are expressed with respect to VSMOW. Typical external precision for δ18O analyses from water samples is 0.05‰. Samples were systematically duplicated. When necessary, in the case of oxygen isotope ratios, a correction of −0.27‰ was applied to convert δ18O of water from the VSMOW to VPDB scales according to Hut (Reference Hut1987). This correction is necessary to compare measured δ18O of CO2 produced by the reaction of the carbonate with H3PO4 and the CO2 equilibrated with H2O.
Calculation of SST for the marine deposits
The SSTs were calculated using an oxygen isotope fractionation equation for the aragonite–water system. Two main equations were available for this calculation. The first one (Grossman and Ku, Reference Grossman and Ku1986) was determined by analyzing modern molluscs and ambient water in the temperature range ≈ 3°C–15°C with δ18Oaragonite (δ18Oar) and δ18Oseawater (δ18Osw) relative to VPDB:
 $$T = {\rm \;} 20.6{\rm \;} \ndash {\rm \;} 4.34{\rm \;} (^{18}{\rm O}_{{\rm ar}}{\rm \;} - {\rm \;} ^{18}{\rm O}_{{\rm sw}})$$
$$T = {\rm \;} 20.6{\rm \;} \ndash {\rm \;} 4.34{\rm \;} (^{18}{\rm O}_{{\rm ar}}{\rm \;} - {\rm \;} ^{18}{\rm O}_{{\rm sw}})$$The second oxygen isotope fractionation equation (Kim et al., Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b) was obtained from laboratory experiments with δ18Oar and δ18Osw relative to VSMOW:
 $$1000{\rm \; l}{\rm n}_{{\rm ar} - {\rm sw}}{\rm \;} = {\rm \;} 17.88(\displaystyle{{{10}^3} \over T}){\rm \;} \ndash {\rm \;} 31.14$$
$$1000{\rm \; l}{\rm n}_{{\rm ar} - {\rm sw}}{\rm \;} = {\rm \;} 17.88(\displaystyle{{{10}^3} \over T}){\rm \;} \ndash {\rm \;} 31.14$$T being the temperature in kelvins and α the oxygen fractionation coefficient between aragonite and water:
 $$\alpha _{{\rm ar}-{\rm sw}} = \;\displaystyle{{{\left( {{{_\;^{18} {\rm O}} \bigg/ {_\;^{16} {\rm O}}}} \right)}_{ar}} \over {{\left( {{{_\;^{18} {\rm O}} \bigg/ {_\;^{16} {\rm O}}}} \right)}_{sw}}}$$
$$\alpha _{{\rm ar}-{\rm sw}} = \;\displaystyle{{{\left( {{{_\;^{18} {\rm O}} \bigg/ {_\;^{16} {\rm O}}}} \right)}_{ar}} \over {{\left( {{{_\;^{18} {\rm O}} \bigg/ {_\;^{16} {\rm O}}}} \right)}_{sw}}}$$The errors associated with the linear regression of empirical data (y = mx + b), which are not available in the original publications, have been computed as the mean standard error for the y value and are ±1.38°C for the Grossman and Ku (Reference Grossman and Ku1986) equation and ±1.15°C for the Kim et al. (Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b) equation. SSTs calculated from the δ18O of modern mollusc shells and δ18O of ambient seawater in Crete, Greece, best match those available from oceanic databases available for the Mediterranean Sea when using the Kim et al. (Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b) equation (Lécuyer et al., Reference Lécuyer, Atrops, Amiot, Angst, Daux, Flandrois, Fourel, Rey, Royer and Seris2018). Grossman and Ku (Reference Grossman and Ku1986) also acknowledged that their equation may overestimate temperature by about 2°C. Therefore, we chose to use the Kim et al. (Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b) equation to infer the SSTs that prevailed in the Canary Islands during MIS11.
Correction of SST related to sea-level changes
To correctly estimate the δ18O values of coastal seawaters in the Canary archipelago during MIS11, sea-level changes need to be taken into account. There is a long-standing debate about the sea-level range during MIS11. On the one hand, based on the study of raised shorelines in regions such as Bermuda (Hearty et al., Reference Hearty, Kindler, Cheng and Edwards1999; Olson and Hearty, Reference Olson and Hearty2009) or the United Kingdom (Bowen, Reference Bowen2003), the high sea-stand levels are estimated to range from 10 to 15 m above modern sea levels and even up to 20 m above modern sea levels according to Roberts et al. (Reference Roberts, Karkanas, Jacobs, Marean and Roberts2012). On the other hand, authors of some modeling studies, those devoted to the Red Sea record in particular, argue that sea level during MIS11 was comparable to current sea level (Rohling et al., Reference Rohling, Braun, Grant, Kucera, Roberts, Siddall and Trommer2010). For more information about this debate, see Candy et al. (Reference Candy, Schreve, Sherriff and Tye2014).
Muhs et al. (Reference Muhs, Meco and Simmons2014) calculated uplift rates for the Canary Islands by using the peak of the last interglacial period (LIG) and the dating of deposits from the LIG and proposed sea-level ranges associated with the different episodes of raised beaches in the Canary archipelago. Accordingly, based on a study of the Arucas deposit, Muhs et al. (Reference Muhs, Meco and Simmons2014) proposed a paleo–sea level of + 9 m to + 24 m relative to the present for Gran Canaria for MIS11. Muhs et al. (Reference Muhs, Meco and Simmons2014) also proposed a paleo–sea level of + 10 m to + 13 m relative to the present for Lanzarote, based on the Piedra Alta deposit, although the authors acknowledge being less confident concerning the latter estimate. Those two sea-level estimates are broadly consistent with each other and are similar to the range of sea-level estimates made for the island of Curaçao (Muhs et al., Reference Muhs, Pandolfi, Simmons and Schumann2012). We chose to consider the average value of the MIS11 sea-level range for each marine deposit, which is included in the average paleo–sea level range attributed to MIS11 (de Vernal and Hillaire-Marcel, Reference Meco, Guillou, Carracedo, Lomoschitz, Ramos and Rodríguez-Yánez2008; Elderfield et al., Reference Elderfield, Ferretti, Greaves, Crowhurst, McCave, Hodell and Piotrowski2012; Roberts et al., Reference Roberts, Karkanas, Jacobs, Marean and Roberts2012) and to integrate the range of uncertainty. Our final estimates correspond to sea-level ranges of + 16.5 ± 7.5 m for the Arucas marine deposit and + 11.5 ± 1.5 m for the Piedra Alta tsunamite. If we take an average 3700 m depth for the ocean (Charette and Smith, Reference Charette and Smith2010) coupled to an average isotopic composition of the polar caps of −40‰, a mass balance calculation indicates that a global sea-level rise of + 16.5 ± 7.5 m would correspond to a decrease of seawater δ18O by 0.18 ± 0.08‰, while a rise of + 11.5 ± 1.5 m would correspond to a decrease of 0.12 ± 0.02‰.
RESULTS
Mineralogical composition of the shells studied
All skeletal carbonates were analyzed by Raman spectroscopy to identify their mineralogy (Table 2). The inorganic fraction (>95%) of shells is entirely composed of either calcite or aragonite, with some exceptions, such as Patella depressa (PA-01), Stramonita haemastoma (ARU-02), Nucella lapillus (ARU-10), and Patella vulgata (ARU-19), which contain minute amounts (<5%) of calcite. In the Piedra Alta tsunamite, the shell of Patella aspera (PA-02) is made of calcite, while in the Arucas marine deposit, the shell of the same species (ARU-09) is made of aragonite. As aragonite is the original mineralogy of the inner layer of the shell of Patella aspera (Parker et al., Reference Parker, Yanes, Surge and Mesa-Hernández2017), calcite is thus interpreted as an alteration product in the case of sample PA-02. Consequently, all the species fully composed of aragonite were selected for stable carbon and oxygen isotope analysis. Moreover, the species Saccostrea cuccullata (PA-09/ARU-01) was also selected for isotopic analysis, as it is present in both marine deposits in sufficient number to allow the isotopic values to be compared. However, as Saccostrea cuccullata is composed of calcite, the oxygen isotope ratios were not used to calculate the SST attributed to the marine deposits.
Table 2. Mineralogy of the biogenic carbonates interpreted from Raman spectra. The terms marked with an asterisk (*) specify that the mineralogy of the shell is mainly aragonitic with less than 5% being calcitic.

Carbon and oxygen isotope compositions of skeletal carbonates
The isotopic data are available in the Supplementary Material. For the Piedra Alta tsunamite, specimens have δ18O values ranging from −1.40‰ to 1.60‰, with a mean value of 0.46‰, while δ13C values range from −2.71‰ to 2.95‰, with a mean value of 1.31‰. For the Arucas marine deposit, the δ18O values of skeletal carbonates are between 0.77‰ and 2.83‰, with a mean value of 1.55‰, while δ13C values are between 0.46‰ and 4.73‰, with a mean value of 2.42‰.
Two species, Callista chione from Piedra Alta (PA-10-04) and Cerithium vulgatum from Arucas (ARU-18-02), were sampled according to the incremental protocol. Callista chione has δ18O values ranging from 0.13‰ to 0.79‰, with a mean value of 0.35‰, while δ13C values are between 0.02‰ and 1.90‰, with a mean value of 0.92‰. Cerithium vulgatum has δ18O values ranging from 0.11‰ to 1.52‰, with a mean value of 1.07‰, while δ13C values are between 0.01‰ and 3.16‰, with a mean value of 1.98‰. In both cases, the δ13C and δ18O values show a pseudo-cyclic variation recorded during the ontogenetic evolution of these two specimens studied. However, these isotopic ratios do not show any significant correlation in the case of Callista chione (R 2 = 9 × 10−4), while they are significant in the case of Cerithium vulgatum (R 2 = 0.52).
A few corrections have been applied to the isotopic values in some outliers. Like many corals, Madracis pharensis (PA-11) is 18O- and 13C-depleted relative to coexisting molluscs. This isotopic pattern is related to the photosymbiotic relation between the coral and its zooxanthella (Land et al., Reference Land, Lang and Smith1975) and a kinetic isotope effect linked to a discrimination against 18O and 13C during the process of biomineralization, more precisely during the hydration and hydroxylation of CO2 (McConnaughey, Reference McConnaughey1989, Reference McConnaughey2003; Watkins et al., Reference Watkins, Hunt, Ryerson and DePaolo2014). Therefore, we excluded the isotopic compositions of this coral sample from our data set. All the remaining specimens studied are molluscs, which are known to precipitate their shell carbonate close to carbon and oxygen isotope equilibrium with seawater (Grossman and Ku, Reference Grossman and Ku1986; Lécuyer et al., Reference Lécuyer, Reynard and Martineau2004, Reference Lécuyer, Hutzler, Amiot, Daux, Grosheny, Otero, Martineau, Fourel, Balter and Reynard2012). We also subtracted 1.01‰ from each δ18O value from Patella vulgata (ARU-19) to account for the predictable vital effect of the species (Wang et al., Reference Wang, Surge and Mithen2012).
Once we made these modifications to our database, δ18O values of mollusc shells range from −0.26‰ to 1.60‰, with a mean value of 0.55‰, while the δ13C values are between −0.28‰ and 2.95‰, with a mean value of 1.47‰ for the Piedra Alta tsunamite. Concerning the Arucas marine deposit, δ18O values of mollusc shells range from 0.69‰ to 2.83‰, with a mean value of 1.49‰, while the δ13C values are between 0.46‰ and 4.73‰, with a mean value of 2.42‰. We also observed that the various mollusc species collected have clustered δ13C and δ18O values, which precludes any kind of identification of their distinct ecology within the littoral environment (Table 1).
Oxygen isotope ratios of seawater samples
The coastal marine waters sampled from Gran Canaria, Fuerteventura, and Lanzarote have δ18O values of 0.97 ± 0.05‰, 1.08 ± 0.07‰ (average between 1.09‰ and 1.06‰), and 1.09 ± 0.04‰, respectively. The values are quite homogenous among those islands, with a mean value of 1.05 ± 0.07‰. The oxygen isotope ratios of coastal waters from Gran Canaria and Lanzarote have been corrected according to a higher sea level during MIS11 (see subsection “Correction of SST related to sea-level changes”) and are 0.79 ± 0.01‰ and 0.97 ± 0.06‰, respectively.
Calculation of the global uncertainty range
There is a need to calculate a global uncertainty range integrating the several uncertainties produced by our calculations: the external reproducibility of the δ18O value of the skeletal carbonates (±0.1‰), errors associated with the linear regression of the oxygen isotope fractionation equations (±1.15°C for the Kim et al. [Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b] equation), and uncertainty linked to the correction for sea-level change during MIS11 (±0.06‰ for the Piedra Alta marine deposit and ± 0.01‰ for the Arucas marine deposit). Consequently, for the SST at the Arucas marine deposit, the total uncertainty range is ±1.4°C for the Kim et al. (Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b) equation. For the SST recorded at the Piedra Alta tsunamite, the total uncertainty range is ±1.5°C for the Kim et al. (Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b) equation.
SST calculated for the marine deposits
The calculation of SST has been made using the oxygen isotope fractionation equation (Eq. 2) for the aragonite–water system: the data from shells composed of calcite (PA-09 and ARU-01) have been excluded from this calculation. For the 36 specimens analyzed from the Piedra Alta tsunamite (Fig. 3a), we calculated an average SST of 21.2 ± 1.9°C according to Eq. 2. Similarly, for the 69 specimens analyzed from the Arucas marine deposit (Fig. 3b), we calculated an average SST of 15.9 ± 2.2°C according to Eq. 2.

Figure 3. (color online) Distribution of MIS11 SSTs calculated by using the δ18O of aragonitic mollusc shells from (a) the Piedra Alta tsunamite with a δ18Osw value of 0.97‰ and (b) the Arucas marine deposit with a δ18Osw value of 0.79‰. Those isotopic temperatures have been calculated according to the aragonite–water oxygen isotope fractionation equation given by Kim et al. (Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b).
The ontogenetic profiles, calculated using Callista chione from Piedra Alta (PA-10-04) and Cerithium vulgatum from Arucas (ARU-18-02), reveal SST sinusoidal-like patterns that can be assigned to seasonal temperature variations (Fig. 4).

Figure 4. (color online) SST inferred from the incremental δ18O of aragonitic mollusc shells from (a) Callista chione (PA-10-04) and (b) Cerithium vulgatum (ARU-18-02), using the aragonite–water oxygen isotope fractionation equation determined by Kim et al. (Reference Kim, Mucci and Taylor2007a, Reference Kim, O'Neil, Hillaire-Marcel and Mucci2007b).
DISCUSSION
Analysis of the SST calculated for the marine deposits
We consider that the SST values calculated from a sample of the population of each marine deposit can be considered as representative of the SST of coastal waters during MIS11. In fact, there are no strong differences, in both marine deposits, between the carbon and oxygen isotope ratios recorded by intertidal and infralittoral species (see Table 1 and Supplementary Material). In addition to comparable standard deviations between the two populations of fossil molluscs from MIS11, the SST values follow a normal distribution according to the use of normal probability plots (executed with the software Past3) in which the correlation coefficients, R 2, are always higher than 0.98 (Filliben, Reference Filliben1975). This means that molluscan assemblages sampled from both the Piedra Alta and Arucas marine deposits correspond to coeval populations, representative of their habitats at a given moment. Such an observation is of paramount importance in the case of the Piedra Alta tsunamite, considering that this catastrophic marine deposit may have included several diachronous populations mixed and intercalated between the lava flows. In the case of the Arucas marine deposit, there may exist time averaging (hundreds or thousands of years) associated with the formation of the marine deposit, but the normal distribution of SSTs indicates that there are no major changes in the SST during this period, and that the values calculated are representative of the SST during MIS11 in the coastal waters of Gran Canaria. The SSTs calculated for the MIS11 are, in the case of Piedra Alta, comparable to the present SST in the Canary archipelago. In the case of the Arucas marine deposit, the calculated SSTs are lower than present-day recorded SST.
These SST values are in good agreement with those obtained in the eastern Central Atlantic Ocean, such as ODP 958, which ranges from 15°C to 20°C using various methods such as planktonic foraminiferal abundances, alkenone ratios, and oxygen isotope ratios of planktonic and benthic foraminifera (Helmke et al., Reference Helmke, Bauch, Röhl and Kandiano2008; Kandiano et al., Reference Kandiano, Bauch, Fahl, Helmke, Röhl, Pérez-Folgado and Cacho2012). They are also in agreement with the general idea that MIS11c was characterized by moderate warmth (Jouzel et al., Reference Jouzel, Masson-Delmotte, Cattani, Dreyfus, Falourd, Hoffmann, Minster, Nouet, Barnola and Chappellaz2007; Lawrence et al., Reference Lawrence, Herbert, Brown, Raymo and Haywood2009) compared with other interglacial episodes such as MIS5e (see Candy et al. [2014] for a comparison of SST records between MIS11 and MIS5).
The exoskeleton growth rates for most mollusc species follow a Von Bertalanffy growth mode (Von Bertalanffy, Reference Von Bertalanffy1957) of the first order (Leveque, Reference Leveque1971; Cloern and Nichols, Reference Cloern and Nichols1978). Thus, the signal recorded during shell growth is stretched during the juvenile age when the growth is fast, and it contracts when the growth slows down as the organism approaches maturity. Moreover, growth is also dependent on the temperature of ambient seawater. Shell growth may stop under and above some species-dependent threshold seawater temperatures. Consequently, the input signal resulting from the seasonal temperature variations, which is basically sinusoidal, is then distorted in the output oxygen isotope record, with maxima and minima being possibly truncated as the result of a lack of mineralization (Cornu et al., Reference Cornu, Pätzold, Bard, Meco and Cuerda-Barcelo1993). Such potential bias in the SST record at the seasonal scale implies that the calculated annual amplitude is most likely underestimated in most cases. We note that the seasonal amplitude in our study was between 3°C and 4°C and is weaker than the current seasonal seawater temperature variation, which is estimated to be around 6°C in the Canary Islands (Borges et al., Reference Borges, Hernández-Guerra and Nykjaer2004).
Saccostrea cuccullata (PA-09/ARU-1; Table 1), which is an Ostreidae considered by Montesinos et al. (Reference Montesinos, Ramos, Lomoschitz, Coca, Redondo, Betancort and Meco2014) as an extralimital species, occurs in both marine deposits. Those authors used Saccostrea cuccullata to propose that the SSTs in the Canary archipelago were at least 4.2°C higher during MIS11 than today. When Eq. 2 is applied to fossil mollusc shells from both marine deposits, we notice that our SST estimates are lower than those proposed by Montesinos et al. (Reference Montesinos, Ramos, Lomoschitz, Coca, Redondo, Betancort and Meco2014). This means that Saccostrea cuccullata may be more tolerant to SST variations than previously thought and that this species might be able to live in colder environments.
Dating of the marine deposits
SSTs recorded in Arucas are around 5°C lower than those recorded in Piedra Alta and approximately 4°C lower than those measured during the last decades. The Arucas marine deposit is well dated, due to an interlayered basaltic flow dated by K-Ar at ca. 420 ka (Meco et al., Reference Meco, Guillou, Carracedo, Lomoschitz, Ramos and Rodríguez-Yánez2002, Reference Meco, Muhs, Fontugne, Ramos, Lomoschitz and Patterson2011). These dates need to be viewed in the light of the LR04 marine benthic δ18O stack record (Lisiecki and Raymo, Reference Lisiecki and Raymo2005). The astronomical parameter configuration during MIS11 (low eccentricity, low precession, and low insolation variations) places both the insolation peak and the temperature peak during the middle of the warm isotope stage, that is, around 400 ka BP (Candy et al., Reference Candy, Schreve, Sherriff and Tye2014). Such relatively low temperatures recorded in Arucas would be in accordance with the deposition of marine sediments during the end of the Termination V between MIS12 and MIS11.
Muhs et al. (Reference Muhs, Meco and Simmons2014) dated the marine deposit of Piedra Alta by using the uranium series applied to fragments of the scleratinian coral Madracis pharensis, and they obtained an age of 481±39 ka, although a possible bias may be linked to an initial lack of 234U/238U. According to Muhs et al. (Reference Muhs, Meco and Simmons2014), the Piedra Alta marine deposit could be correlated to either MIS11 or MIS13. However, the co-occurrence in the Piedra Alta tsunamite of the gastropods Patella depressa and Patella aspera, which have not been observed in the Canary archipelago before MIS11 and in the Mediterranean basin before the mid-Pleistocene (Meco et al., Reference Meco2008), suggests that this deposit was more or less contemporaneous with MIS11. The occurrence of a similar paleosol (Fig. 2a and b) that contains innumerable fossilized egg pods from orthopters and terrestrial gastropods in both the Piedra Alta and Arucas marine deposits constitutes an argument to correlate them (Meco et al., Reference Meco, Muhs, Fontugne, Ramos, Lomoschitz and Patterson2011). These egg pods are usually linked to humid periods that correspond to the transitions between glacial and interglacial episodes in the Canary Islands (Meco et al., Reference Meco, Muhs, Fontugne, Ramos, Lomoschitz and Patterson2011). This paleosol is directly overlain by the tsunamite at Piedra Alta and by the marine deposit at Arucas (Meco et al., Reference Meco, Muhs, Fontugne, Ramos, Lomoschitz and Patterson2011). The paleo–sea level of + 10 m to + 13 m associated with the Piedra Alta tsunamite (Muhs et al., Reference Muhs, Meco and Simmons2014) is also inconsistent with its correlation to MIS13, as most sea-level modeling outputs indicate the maximum sea level during MIS13 to have been about 16 m (Miller et al., Reference Miller, Kominz, Browning, Wright, Mountain, Katz, Sugarman, Cramer, Christie-Blick and Pekar2005, Reference Miller, Mountain, Wright and Browning2011) to 28–30 m (Bintanja and Van de Wal, Reference Bintanja and Van de Wal2008) below present sea level. Moreover, the presence of species such as Saccostrea cuccullata and the SST values inferred from mollusc shells from Piedra Alta exclude its correlation with MIS13, which is considered a cold interglacial stage, as witnessed by global air temperatures deduced from ice core isotopic records (Augustin et al., Reference Augustin, Barbante, Barnes, Barnola, Bigler, Castellano, Cattani, Chappellaz, Dahl-Jensen and Delmonte2004; Jouzel et al., Reference Jouzel, Masson-Delmotte, Cattani, Dreyfus, Falourd, Hoffmann, Minster, Nouet, Barnola and Chappellaz2007) and SSTs calculated using the alkenone record in the Atlantic Ocean (Ho et al., Reference Ho, Mollenhauer, Lamy, Martínez-Garcia, Mohtadi, Gersonde, Hebbeln, Nunez-Ricardo, Rosell-Melé and Tiedemann2012), which are consistently lower than SST values for MIS11 inferred from the study of marine sediments (Past Interglacials Working Group of PAGES, 2016).
Considering the absolute dating and the paleontological, sedimentological, and paleoclimatic data of the two marine deposits, we propose that the Piedra Alta deposit is younger than the Arucas deposit. This hypothesis is indeed in agreement with the absolute dating of the Arucas marine deposit at 421 ± 20 ka (Meco et al., Reference Meco, Guillou, Carracedo, Lomoschitz, Ramos and Rodríguez-Yánez2002) and the SST values recorded in Piedra Alta mollusc shells, which are around 5°C higher than those calculated in Arucas. Consequently, the Piedra Alta marine deposit could correspond to the MIS11c interglacial stage, while the Arucas marine deposit could correspond to Termination V, between MIS12 and MIS11 (Fig. 5).

Figure 5. (color online) The LR04 marine benthic δ18O record normalized and plotted on the LR04 time scale, with the proposed chronology of the Piedra Alta tsunamite and the Arucas marine deposit with respect to MIS11, MIS12, and MIS13. After Lisiecki and Raymo (Reference Lisiecki and Raymo2005).
δ13C of marine species
Carbon in the atmosphere is mostly present as carbon dioxide (CO2), whereas more than 90% of the carbon in the deep ocean is present as bicarbonate (HCO3−), referred to as “dissolved inorganic carbon” (DIC). The reaction for precipitation of carbonates in the seawater, known as the “carbonate pump,” is:
 $${\rm C}{\rm a}^{2 +} + 2{\rm \; HCO}_3^ - {\rm \;} \leftrightarrow {\rm CaC}{\rm O}_3 + {\rm C}{\rm O}_2 + {\rm \;} {\rm H}_2{\rm O}$$
$${\rm C}{\rm a}^{2 +} + 2{\rm \; HCO}_3^ - {\rm \;} \leftrightarrow {\rm CaC}{\rm O}_3 + {\rm C}{\rm O}_2 + {\rm \;} {\rm H}_2{\rm O}$$This chemical reaction involves little carbon isotopic fractionation compared with that taking place between carbon dioxide and bicarbonate ions, which constitute the main reservoir of DIC in the oceans. Thus, the δ13C of inorganically and biologically precipitated carbonate in the oceans is very close to that of DIC in the oceans (Maslin and Swann, Reference Maslin and Swann2006). The species Saccostrea cuccullata (PA-09/ARU-01) and Bolma rugosa (PA-03/ARU-04) are common in the two marine deposits studied (Table 1), even though the number of specimens analyzed is more important for the Piedra Alta tsunamite (11 specimens of Bolma rugosa and 8 specimens of Saccostrea cuccullata) than for the Arucas marine deposit (1 specimen of Bolma rugosa and 3 specimens of Saccostrea cuccullata). As only one specimen of Bolma rugosa from the Arucas marine deposit has been analyzed, which prevents a comparison between the two marine deposits, we compared the δ13C values of Saccostrea cuccullata for Piedra Alta and Arucas. This comparison allows us to dismiss the species-dependent carbon isotope fractionation between calcium carbonate and DIC. Consequently, a δ13C difference between the shells of Saccostrea cuccullata from the two marine deposits studied should represent a similar δ13C difference in seawater DIC of these marine deposits that could be attributed to a difference in interrelated variables: seawater temperature, the atmospheric pCO2, and the productivity of surface waters (Maslin and Swann, Reference Maslin and Swann2006). We observed that specimens of Arucas mollusc shells have δ13C values about 0.6‰ higher on average than those present in Piedra Alta, which we consider to reflect a similar difference in the δ13C values of DIC of the Canary Islands coastal waters. Such an isotopic difference could be the consequence of SST being 6°C lower according to the gradient of carbon isotope fractionation between atmospheric CO2 and DIC of 0.1‰ per 1°C, although this equilibrium is not achieved anywhere, because surface waters are replaced too quickly (Saltzman and Thomas, Reference Saltzman and Thomas2012). A higher atmospheric pCO2 prevailing during the Piedra Alta tsunamite deposition event could have lowered the δ13C values of DIC subsequent to equilibration with seawater. Another scenario could involve stronger productivity during the Arucas marine deposit than during the MIS11c interglaciation, when the upwelling may have been diminished, resulting in colder waters that allowed more CO2 to be dissolved.
The two mollusc shells were selected for obtaining isotopic ontogenetic profiles provide complementary information at the seasonal scale between SST and the δ13C values of DIC. In the case of the mollusc Cerithium vulgatum (ARU-18-02), the δ13C values of the shell and the SST estimates inferred from the δ18O values of the shell are linearly correlated, with a slope of −0.31 and an R 2 of 0.52. As we already proposed, this correlation may also indicate that there is a temperature-dependent isotopic fractionation between air CO2 and dissolved bicarbonate in the case of the Arucas marine deposit (Bojar et al., Reference Bojar, Hiden, Fenninger and Neubauer2004). At the seasonal scale, it means that during summer, when SSTs are the highest (Borges et al., Reference Borges, Hernández-Guerra and Nykjaer2004), δ13C values of DIC are at their lowest. The variation in δ13C values of DIC can be assigned, at the seasonal scale, to a change in the activity of the upwelling of the Canary Current, which brings to the surface ancient and 13C-depleted waters relative to surface waters. Therefore, the Canary Current may have been stronger during the warm season in the Canary Islands during MIS11, which is compatible with the observations made by Mittelstaedt (Reference Mittelstaedt1991), Navarro-Pérez and Barton (Reference Navarro-Pérez and Barton2001), and Pardo et al. (Reference Pardo, Padín, Gilcoto, Farina-Busto and Pérez2011) concerning the activity of the Canary Current.
CONCLUSION
MIS11 is a warm isotope stage considered to be the best reference for understanding the evolution of the MIS1 climate. We have studied two sedimentary marine deposits located in two eastern islands of the Canary archipelago, Lanzarote (Piedra Alta tsunamite) and Gran Canaria (Arucas sedimentary deposit). These marine deposits contain many biogenic skeletal remains of marine origin, which we have studied using Raman spectroscopy to select only the aragonitic skeletal remains for the stable isotope analysis.
Using the bulk oxygen isotope analysis of the aragonitic mollusc shells, SST estimates for MIS11 are 21.2 ± 1.9°C for the Piedra Alta tsunamite (Fig. 3a) and 15.9 ± 2.2°C for the Arucas marine deposit (Fig. 3b) according to available oxygen isotope fractionation equations. The normal distribution of SSTs associated with similar standard deviations for both sites means that coeval populations of molluscs were sampled in each sedimentary marine deposit. These SST estimates, combined with absolute chronological dating and sedimentary and paleontological observations, argue in favor of the deposition of the Arucas marine deposit at the end of Termination V and the formation of the Piedra Alta tsunamite during MIS11c, which is the warmest period of the warm isotope stage MIS11. The SSTs we calculated are consistent with other data suggesting warm temperatures in the central Southern Ocean during MIS11, although these temperatures are not exceptional compared with SSTs recorded for MIS5e.
The carbon isotope measurements for Saccostrea cuccullata and Bolma rugosa, which are present in both marine deposits, show that species from the Arucas deposit are 13C-enriched relative to those from Piedra Alta. This isotopic difference may be linked to temperature, atmospheric pCO2, or marine productivity variations that took place between the formation of these two deposits.
The incremental oxygen isotope measurements for Callista chione and Cerithium vulgatum (Fig. 4) revealed sinusoidal-like signals that we interpreted as seasonal variations of SST between 3°C and 4°C, an amplitude slightly weaker than the present-day SST documented in the Canary Islands. The carbon isotope measurements performed on Cerithium vulgatum (ARU-18-02) are linearly and negatively correlated to the SST variations, which highlights a stronger activity of the Canary upwelling current during the warm season.
The paleoclimatological data characterizing the early and mid-Pleistocene isotope stages are commonly derived from ice cores and marine and lacustrine sediments, with high latitudes sampled in most cases. However, this study shows that, during MIS11c, the global warming also significantly impacted low-latitude marine regions.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2019.65.
ACKNOWLEDGMENTS
This study was funded by the Centre National de la Recherche Scientifique and the Institut Universitaire de France (CL). AL and J-FB were supported by PAMEV project (Paleontología de la Macaronesia, Espacio Virtual) ULPGC PEJ-2014-A-83608.









