All US nursing homes (NHs) are required to implement antibiotic stewardship programs by November 2017, 1 due to the increasing prevalence of multidrug-resistant organisms (MDROs) and research demonstrating that as many as 75% of NH antibiotic prescriptions are unnecessary or are for the wrong drug, dose, or duration. 2 , Reference Daneman, Gruneir and Bronskill 3 A suspected urinary tract infection (UTI) is the most common reason NH antibiotics are prescribed,Reference Zimmerman, Sloane and Bertrand 4 , Reference Eikelenboom-Boskamp, Cox-Claessens, Boom-Poels, Drabbe, Koopmans and Voss 5 and UTI prescriptions are key contributors to the emergence of MDROs.Reference Nace, Drinka and Crnich 6 – Reference Trautner and Grigoryan 8 Approximately half of these prescriptions are not for a UTI but for asymptomatic bacteriuria,Reference Phillips, Adepoju and Stone 9 a condition for which treatment does not improve health outcomes but does increase drug resistance and may lead to serious adverse events.Reference Nicolle, Bradley, Colgan, Rice, Schaeffer and Hooton 10 , Reference Cai, Nesi and Mazzoli 11
Thus, an important component of NH antibiotic stewardship must be improved evaluation and diagnosis of UTIs. Urine testing often drives prescribing; so a key component of antibiotic stewardship around NH UTIs must be the ordering and interpretation of urine tests.Reference Nace, Drinka and Crnich 6 , Reference Eke-Usim, Rogers, Gibson, Crnich and Mody 12 Evidence-based guidelines for urine testing have been developed; the most explicit are the 2008 update by the Infectious Diseases Society of America (IDSA),Reference High, Bradley and Gravenstein 13 the consensus criteria of Loeb, et al,Reference Loeb, Bentley and Bradley 14 , Reference Loeb, Brazil and Lohfeld 15 and the updated criteria of Genao and Buhr.Reference Genao and Buhr 16 All of these rely heavily on focal urinary tract symptoms as a prerequisite of testing.
Despite the high level of interest in changing NH practice around UTIs, little information exists about decision making in community-based NHs or the areas of practice that might be most amenable to change. To address this gap, we identified and reviewed 254 cases from 31 community NHs in which urine cultures were ordered by medical providers. Our goals were (1) to describe the decision-making process around urine testing, including documentation, prescribing decisions, and outcomes, and (2) to identify areas amenable to quality improvement efforts aimed at reducing unnecessary antibiotic prescribing.
METHODS
Setting and Study Population
As part of a research study regarding infection management and antibiotic stewardship in NHs, we enrolled 31 community-based NHs in North Carolina, and within each study NH, we audited a sample of cases in which urine cultures had been ordered. To promote NH buy-in and thereby assure a more representative sample, NHs were approached through either a for-profit regional NH chain or a regional long-term-care medical group. A total of 35 NHs were approached for participation; 4 refused. In each participating NH, a team of geriatricians and research staff made a site visit between November 2014 and March 2015 to conduct medical record audits. From a list of all urine cultures reported in each NH, the study team randomly selected up to 10 cases from the previous month; in NHs having fewer than 10 cases, all available cases were audited, yielding a total of 254 cases. Study methods were approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
Measures
For each case, medical and nursing records were systematically audited to record signs and symptoms during the 48 hours before a test was ordered, guided by the published literature on signs and symptoms associated with UTIs in older persons.Reference High, Bradley and Gravenstein 13 – Reference Sloane, Kistler and Mitchell 18 The following data were also collected: the reason for the culture (if stated); urinalysis and culture results, including type and quantity of bacteria as well as antibiotic resistance patterns; antibiotic treatment decisions on the day the test was ordered and during the subsequent 5 days; signs and symptoms during days 2–5 after a test was ordered (ie, when a urine culture result would have become available); and whether or not the patient had an emergency department visit, hospitalization, or death during the 7 days after the culture was ordered. Because only 1 of the 31 NHs performed on-site urine testing, dipstick and urinalysis results were not included in these analyses. To adjust for difference in the method of measuring temperature, we subtracted 1.35°C (0.75°F) from rectal and tympanic readings and added 1.35°C (0.75°F) to axillary readings to estimate an oral temperature equivalent. 19
To better estimate the concordance of empirical antibiotic selection with NH-specific antibiotic resistance patterns, the study team gathered all urine culture reports for the 31 study NHs between December 1, 2014, and August 31, 2015, for a total of 2,985 cultures reported by 21 different laboratories. Because large samples are needed to construct stable antibiograms, these results are reported at the aggregate level and not for specific NHs.
Statistical Analysis
Analyses included descriptive statistics, a comparison of empirical antibiotic choices with aggregate data on sensitivity and resistance patterns of commonly cultured bacteria, and the calculation of the proportion of cases consistent with the Loeb criteria for initiation of antibiotics for presumed UTIs and the modified IDSA criteria for surveillance of UTIs.Reference Loeb, Brazil and Lohfeld 15 , Reference Stone, Ashraf and Calder 17 In applying the IDSA criteria, “constitutional criteria” other than fever were omitted because no NHs routinely assessed residents using the 28-point functional status index or the quantitative confusion assessment method, as required by these criteria.Reference Stone, Ashraf and Calder 17 The statistical significance of differences in the proportion of residents treated with empirical antibiotics was tested using Pearson’s χ2 test, and Fisher’s exact test was used when any cell count was ≤10.
RESULTS
Of the 31 community NHs that participated in this study, 81% were for-profit facilities. The mean bed size was 113; the mean occupancy rate was 87%; the mean work hours per resident for licensed nurses and certified nursing assistants, respectively, were 1.5 and 2.2; and the mean quality rating on Nursing Home Compare was 3.3. None of these values were statistically different from the mean of all NHs nationally. 20
Clinical Features and Treatment Decisions at the Time a Urine Culture Was Ordered
Urine cultures were obtained using a variety of methods. More than one-third (36%) of NH records did not document the method by which the culture was obtained; the remainder included 26% collected by straight (in-and-out) catheterization, 20% as voided specimens, and 19% from an indwelling device.
On the day of or before a urine culture was ordered, 32% of patients whose urine was cultured were reported to have an acute mental status change; 17% were reported to have a change in the urine color, odor, or sediment; 14% had dysuria; 14% had a body temperature documented at ≥37.2°C (99°F); and 74% lacked documentation of any urinary-tract–specific signs or symptoms. Only 22% of these patients met the Loeb criteria for ordering a urine culture (Table 1).
TABLE 1 Presentation, Initial Treatment, and Culture Results Among 254 Nursing Home Residents Who Had Urine Cultures
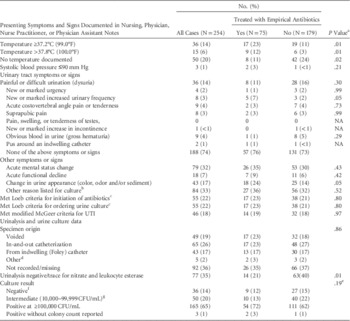
NOTE. NA, not appropriate; UTI, urinary tract infection; CFU, colony-forming units.
a Pearson χ2 test. Fisher’s exact test used where any cell count is ≤10.
b The most common “other” reasons documented for urine collection were fall and/or syncope (14.3%), “history of” chronic or recurrent UTIs (9.5%), “urinary retention” (9.5%), agitation/behavior change (8.3%), and increased white blood cell count (8.3%).
c Loeb 2005.
d The 5 specimens collected using “other” methods included ileal conduit (N=1) and suprapubic catheter (N=4).
e P value for 3 categories, with the 3 patients with an organism reported without a colony count excluded.
f 32 cultures showed no growth and 4 cultures showed <10,000 CFU/mL.
g Includes 22 “polymicrobial” with no organism reported.
In almost one-third of cases (30%), empirical treatment with antibiotics was started before the culture report was available. Only temperature elevation, increased urinary frequency, and change in urine appearance were significantly associated (P≤.05) with empirical antibiotic prescribing; a negative urine dipstick for nitrite and leukocyte esterase was significantly associated with a decision to withhold treatment. Treated cases were no more likely than untreated cases to meet the 2005 Loeb criteria for empirical initiation of antibiotics for presumed UTI in NH residents or the modified McGeer surveillance criteria for UTIs (Table 1).
In the 75 cases treated empirically with antibiotics, the most commonly prescribed agents were ciprofloxacin (31%), trimethoprim-sulfamethoxazole (20%), nitrofurantoin (11%), ceftriaxone (11%), and levofloxacin (7%). When the most commonly prescribed antibiotics were compared with the antibiogram we constructed from 2,985 consecutive urine cultures from study NHs, high levels of resistance were observed, with E. coli (the most common pathogen) being resistant to ciprofloxacin in 57% of cases and to trimethoprim-sulfamethoxazole in 42% of cases (Table 2).
TABLE 2 Relationship Between the Antibiotics Most Commonly Prescribed Empirically for a Presumed Urinary Tract Infection and Resistance Patterns Among Positive Urine Cultures From 31 Nursing HomesFootnote a

NOTE. NR, not reported.
a The study included 75 empirical antibiotic prescriptions. The antibiotic resistance antibiogram is based on 1,580 positive urine cultures (ie, ≥100,000 CFU/mL) from 31 nursing homes.
b Not reported because resistance is known to be very high.
Clinical Features and Treatment Decisions When a Urine Culture Became Available
Results from reporting laboratories indicated that, of these 254 specimens, 36 (14%) had no bacteria, 50 (20%) had intermediate colony counts (≤100,000 CFU/mL or polymicrobial results with no organism reported, ie, “contaminated” specimens), 165 (65%) were positive with ≥1 bacteria at ≥100,000 CFU/mL, and 3 (1%) had an organism reported but no colony count report. Of the positive cultures, the majority (84%) yielded a single organism, 15% yielded 2 bacteria, and 1% reported 3 bacteria (Table 1).
Our evaluation of prescribing decisions made during days 2–5 after a culture had been ordered (and the result would have been available in the NH) indicates that a positive culture in a patient who was not on antibiotics (N=111 cases) led to initiation of an antibiotic in 89% of cases. Treated patients with positive urine cultures did not, during the days when the culture results became available, differ significantly from untreated patients in temperature, frequency of urinary signs and symptoms, or presence of Loeb criteria for initiating antibiotics, and only a small minority (13%) met the modified McGeer criteria for having a UTI (Table 3).
TABLE 3 Symptoms and Treatment Decisions During Days 2–5 After a Urine Culture among 178 Nursing Home Residents Who Were Not Treated Empirically Before the Culture Result Was AvailableFootnote a

NOTE. UTI, urinary tract infection; CFU, colony-forming units.
a Among the 179 patients who did not initially receive antibiotics, 1 patient had no colony count reported and was excluded.
b A positive culture result was defined as having a colony count ≥100,000 CFU/mL; an intermediate result as between 10,000 and 99,000 CFU/mL or polymicrobial with no organism identified; and a negative culture as no growth or <10,000 CFU/mL.
c Pearson χ2 test (Fisher’s exact test used where any cell count is ≤10).
d Among these cases, 13 had an organism reported at <100,000 CFU/cm3; 2 were reported as “polymicrobial”; and 1 reported no bacteria.
Among the 9 patients who had been initially prescribed antibiotics and had negative urine culture results, none had their antibiotic stopped, and the prescription was changed for 1 patient despite a colony count <10,000 CFU/mL. Among the 10 patients who were initially prescribed antibiotics whose colony counts were intermediate, antibiotics were stopped in only 2. Among the 67 patients who had not been prescribed antibiotics and who had cultures with colony counts <100,000 CFU/mL, 16 (24%) were started on antibiotics. Of these, 13 cultures had bacteria at colony counts between 10,000 and 99,000 CFU/mL, while 2 had polymicrobial results (without sensitivities reported), and only 1 was completely devoid of bacteria. Overall, 191 (75%) urine cultures were associated with at least 1 antibiotic prescription, and 189 (74%) of all subjects completed a full course of antibiotics (Figure 1).

FIGURE 1 Flow chart demonstrating prescribing decisions among the 251 cases in which urine cultures and colony counts were ordered in 31 nursing homes (3 cases were excluded that reported organisms but no colony counts). While only 30% of the cases were associated with an initial antibiotic prescription, 74% ultimately received a full course of antibiotics. Negative=<10,000 CFU/mL; Intermed (intermediate)=10,000–99,000 CFU/mL; Positive=>100,000 CFU/mL.
Adverse Patient Events
During the 7 days after the 254 urine cultures were ordered, 2 study participants died and 2 others were hospitalized with a diagnosis of “sepsis” or “bacteremia.” Of the 2 deaths, 1 patient had a positive urine culture for Pseudomonas that had been ordered the day before she died, at which time she was on hospice care, was afebrile, and was experiencing functional decline. The other had recent hip replacement surgery, a Foley catheter, and a culture that reported 90,000 CFU/mL of Staphylococcus spp.; 7 days after the urine culture was ordered her incision dehisced and drained pus, after which she was transferred to the hospital and subsequently died. Of the 2 patients who were hospitalized and returned to the NH with discharge diagnoses that included “sepsis” both had negative urine cultures at the NH.
DISCUSSION
In this study of 254 urine cultures ordered in 31 community NHs, we found that, in the face of little documentation of acute UTI, the act of ordering a culture led to an antibiotic prescription in the vast majority of cases. Empirical antibiotics were started in 30% of patients, often with antibiotics that have a moderate-to-low likelihood of being effective due to antibiotic resistance patterns of common urinary pathogens in these NHs, thereby risking alteration in normal bowel and other flora without treating the presumed pathogen.Reference Rafii, Sutherland and Cerniglia 21 When urine culture reports became available, although most patients lacked constitutional or urinary-tract–specific symptoms, the presence of bacteria usually led to an antibiotic prescription in persons who had not been treated empirically, and a negative culture rarely led to the discontinuation of empirically prescribed antibiotics. As a result, 74% of urine cultures were associated with completion of a course of antibiotics.
Why many of these cultures were ordered is unclear. Most patients lacked documentation of either a temperature ≥37.2°C (99.0°F) (86%) or of any urinary tract signs or symptoms (74%), and only 22% satisfied criteria for ordering a urine culture that were developed to reduce unnecessary urine cultures.Reference Loeb, Brazil and Lohfeld 22 The result was overtreatment of asymptomatic bacteriuria, a common NH condition that is not associated with increased morbidity or mortality,Reference Trautner and Grigoryan 8 , Reference Boscia, Abrutyn and Kaye 23 , Reference Wagenlehner, Naber and Weidner 24
Mental status change was the symptom or sign most commonly documented in the 48 hours before this sample of NH residents had urine cultures obtained, and this observation has been noted previously regarding catheterized NH residents.Reference Eke-Usim, Rogers, Gibson, Crnich and Mody 12 The likely reason for obtaining cultures in these patients is concern that any change in cognition could represent incipient delirium, a common and dangerous condition in acutely ill hospital patients.Reference Inouye 25 However, most NH residents have dementia, which in the absence of acute illness is frequently associated with fluctuation in cognitive status, including affective, hyperactive, and delusional symptoms.Reference Borsje, Wetzels, Lucassen, Pot and Koopmans 26 Thus, although a recent systematic review found a modest association between delirium and urine infection in older persons, no study meeting review criteria was NH based, and all had methodological flaws.Reference Balogun and Philbrick 27 Therefore, little evidence supports ordering a urine culture when the only symptom is mental status change.
The second most common sign or symptom documented in these patients was a change in urine appearance. Alteration in urine color, odor, or sediment is associated with a positive urine culture,Reference Juthani-Mehta, Quagliarello, Perrelli, Towle, Van Ness and Tinetti 28 but the literature emphasizes that most people with cloudy or odorous urine are not sick.Reference Midthun, Paur and Lindseth 29 , Reference Vaisman, Gold and Leis 30 Furthermore, bacteriuria in asymptomatic NH patients is highly prevalent, and nonbacterial factors can alter the odor or appearance of voided urine.Reference Pandey, Kim, Choi, Sa and Oh 31 , Reference Nicolle, Bradley, Colgan, Rice, Schaeffer and Hooton 32 Subanalyses of these 43 cases verified this hypothesis; patients with changes in urine appearance did not differ significantly from the group overall: 12% had a temperature ≥37.2°C (99°F); 9% had dysuria documented; and 63% had no urinary tract-specific symptoms or signs documented. Thus, belief that a change in urine color, odor, or sediment indicates infection is likely to have influenced overprescribing of empirical antibiotics in our sample.
As these findings illustrate, addressing the complex interplay of factors that result in overprescribing for presumed UTIs will require multifaceted educational and quality improvement efforts.Reference Wald 33 To date, the majority of antibiotic stewardship activities have involved guidelines aimed at reducing urine testing, with mixed results.Reference Loeb, Brazil and Lohfeld 22 , Reference Pettersson, Vernby, Mölstad and Lundborg 34 – Reference Zabarsky, Sethi and Donskey 36 Instead, we recommend that urinary-tract–oriented antibiotic stewardship include all of the following measures: (1) guidelines for urine testing; (2) tracking of urine culture results, and where volume of studies permits, creation of antibiograms to guide empirical treatment; (3) strict guidelines for what to do when urine culture results are reported; and (4) guidelines for initiating antibiotics, including recommended treatment duration. Observation (“watchful waiting”) orders should also be implemented for use in patients with nonspecific or mild symptoms who do not meet guidelines for empirical antibiotics. These should include hydration; medication review; orientation procedures; measures to encourage restful sleep; evaluation for new or worsening cardiac, renal, or metabolic problems and for depressive symptoms; and regular documentation of symptoms and vital signs.Reference Nace, Drinka and Crnich 6 , Reference Juthani-Mehta, Datunashvili and Tinetti 37 Guidelines for responding to urine culture results should include an admonition to prescribe antibiotics only if the patient has clear signs of illness (eg, meets the modified McGeer criteria)Reference Stone, Ashraf and Calder 17 plus discontinuation of antibiotics in persons with negative cultures. Guidelines should be endorsed by all medical providers and communicated clearly to all nursing staff, with room provided for discussion and protocol deviation in unusual situations.
ACKNOWLEDGMENTS
The following laboratories contributed urine culture results to one or more of our study nursing homes, which were included in our aggregate antibiogram: LabCorp, Solstas, Moore Regional Hospital, Betsy Johnson Regional Hospital, Columbus Regional Healthcare, Vidant Bertie Hospital, Halifax Regional Medical Center, Sampson Regional Medical Center, New Hanover Regional Medical Center, Meridian Laboratory, Pathologists Diagnostic Laboratory, NC Baptist Hospital, Johnston Medical Center, Rowan Medical Center, Lake Norman Regional Medical Center, Novant Health Medical Center, Caromont Regional Medical Center Laboratory, Wake Forest Baptist Health, Wayne Memorial Hospital, Harnett Health System Laboratory, and Gaston Memorial Hospital.
Financial support: This research was conducted by the Program on Aging, Disability, and Long-Term Care of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill with support from the United States Agency for HealthCare Research and Quality (grant no. R18 HS022846-01).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.






