Introduction
The HIV epidemic has been raging in sub-Saharan Africa since the early 1980s. By 2017, UNAIDS estimated that some 25 million people were living with HIV in the continent (UNAIDS, 2017; UNAIDS/WHO/UNICEF, 2017). The dynamics of the epidemic have been impressive, with a fast rise in the 1980s and 1990s and stabilization followed by a slow decline thereafter in most countries, with a few exceptions where the number of cases is still increasing (UNAIDS/WHO/UNICEF, 2017). The recent dynamics in the past 15 years (the decline in prevalence and incidence) are relatively well documented and well understood, and can be reproduced by computer simulation. They were due to changing sexual behaviour (less risky behaviour, more condom use), as well as using anti-retroviral therapies (ARVs), which not only dramatically reduce AIDS mortality but also have an impact on incidence because they lower the viral load and therefore reduce transmission. However, ARVs leave the size of the reservoir almost intact, therefore perpetuating the risk of transmission.
An additional tool for controlling HIV/AIDS transmission was recommended in 2007 by the World Health Organization (WHO): Voluntary Medical Male Circumcision (VMMC) (UNAIDS/WHO, 2007, 2011). This recommendation was based on results from three randomized controlled clinical trials conducted in three African countries, which showed a decrease in HIV incidence in the 18–24 months following VMMC, most likely due to a decrease in female-to-male transmission rates after the surgical procedure (Auvert et al., Reference Auvert, Taljaard, Lagarde, Sobngwi-Tambekou, Sitta and Puren2005; Bailey et al., Reference Bailey, Moses, Parker, Agot, Maclean and Krieger2007; Gray et al., Reference Gray, Kigozi, Serwadda, Makumbi, Watya and Nalugoda2007). However, the long-term population impact of VMMC has been seriously questioned, because in demographic surveys there are hardly any differences in HIV prevalence between circumcised groups and non-circumcised groups (Garenne, Reference Garenne2006, Reference Garenne2007, Reference Garenne2008, Reference Garenne, Denniston, Hodges and Milos2010; Garenne et al., Reference Garenne, Giami, Perrey, Giles-Vernick and Webb2013). The net effect of VMMC has also been questioned in observational studies (Van Howe, Reference Van Howe1999, Reference Van Howe2015; Van Howe & Storms, Reference Van Howe and Storms2011). In addition, in order to promote VMMC, activists claim that circumcision provides ‘partial protection’ against HIV, a claim which remains controversial. There is no doubt that circumcision reduces HIV transmission through a variety of mechanisms, which have been recently explored: the foreskin drives immune activation in adjacent foreskin tissues, facilitating HIV infection (Prodger & Kaul, Reference Prodger and Kaul2017); penile inflammation increases susceptibility to HIV and is reduced by circumcision (Prodger et al., Reference Prodger, Gray, Shannon, Shahabi, Kong and Grabowski2016); HIV induces a dynamic process of immune cell relocation in the inner foreskin that is associated with specific chemokine secretion, which favours HIV entry at this site (Zhou et al., Reference Zhou, Barry de Longchamps, Schmitt, Zerbib, Vacher-Lavenu, Bomsel and Ganor2011). However, firstly these mechanisms only reduce HIV transmission but do not stop it, and secondly there are other target cells located on glans penis and in the urethra on which circumcision has no effect. Furthermore, circumcision being associated with lower susceptibility could induce more risky behaviours because it can suggest a sense of being ‘protected’ or ‘no longer susceptible’. Lastly, at population level, fully circumcised groups (such as Xhosa and Shangan in South Africa) have the same level of HIV infection and the same dynamics of the epidemic as uncircumcised groups (such as Zulu and Tswana in South Africa).
Since the 2007 WHO recommendation, large-scale VMMC campaigns were promoted in several southern and eastern African countries, generously financed by the international community. By 2017, some 14.5 million men had been circumcised in fourteen target countries, with large variations among them (United Nations Population Division, 2016; UNAIDS/WHO/UNICEF, 2017).
Zambia provides an opportunity to test the impact of VMMC campaigns on HIV infection at population level. Zambia is a country with high levels of HIV prevalence (from 13.3% to 15.3% in Demographic and Health Surveys [DHS]), where traditional circumcision is rare, and where VMMC campaigns have reached a significant proportion of the population. The country provides ample opportunity for in-depth analysis of the complex dynamics between HIV and circumcision. Zambia is a medium-sized country (13 million inhabitants at the 2010 census), partly urbanized (39% urban in 2010), partly industrialized because of the presence of copper mines in the Copperbelt province, has a relatively high level of education, is primarily of Christian faith, with tiny minorities of Muslims and other groups, and has great diversity in ethnic composition, with some 61 ethnic groups identified in the DHS. Among these groups, only eight groups, representing about one-tenth of the total population, used to practise traditional circumcision (see below for details). The HIV epidemic has been raging since the early 1980s: the first case was officially identified in 1984, and the number of infected persons has been increasing rapidly, to reach a peak of some 900,000 infected persons by 2001 (Central Statistical Office of Zambia, 2011). After this date, HIV prevalence has been declining slowly, but the number of infected persons remained high in the following years. The reasons for the large epidemic have been well documented in several of the DHS: high prevalence of risky behaviour (multiple partnerships, extramarital sex, commercial sex) and low condom use in the early years. The dynamics of the epidemic, given what was known from sexual behaviour surveys, have been reproduced with a two-sex micro-simulation mathematical model, which was able to reproduce the age and sex pattern of HIV prevalence in 2001 (Leclerc & Garenne, Reference Leclerc and Garenne2008; Leclerc et al., Reference Leclerc, Matthews and Garenne2009).
Numerous health education programmes have been conducted since the early 1990s that have had an impact on sexual behaviour, well documented in a series of surveys (Central Statistical Office of Zambia et al., 1993, 1997, 1999, 2003, 2004, 2009, 2010, 2014). As a result, HIV prevalence among adults declined slowly over the years, from the 2001 DHS to the 2013 DHS, but not for all age groups, as will be seen below. The decline in HIV prevalence among pregnant women was documented earlier using data from antenatal care (ANC) sentinel sites, as well as from sexual behaviour surveys (Fylkesnes et al., Reference Fylkesnes, Musonda, Kasumba, Ndhlovu, Mluanda, Kaetano and Chipaila1997, Reference Fylkesnes, Musonda, Sichone, Ndhlovu, Tembo and Monze2001; Michelo et al., Reference Michelo, Sandøy, Dzekedzeke, Siziya and Fylkesnes2006a, b; Sandøy et al., Reference Sandøy, Kvåle, Michelo and Fylkesnes2006, Reference Sandøy, Michelo, Siziya and Fylkesnes2007). In addition, a large programme of VMMC was launched in June 2007, aiming at circumcising the 15–29 male adult population. Lastly, an extensive programme of ARV treatment was also launched at about the same time, reaching about two-thirds of the infected population by 2016, with a significant impact on adult mortality (Central Statistical Office of Zambia, 2017).
The aim of this study was to assess the changing dynamics of the HIV epidemic, and the role of VMMC in Zambia. The expectation was that VMMC could contribute to reducing HIV prevalence among young men, and possibly also among young women who are their partners. This will be confronted with the observations made in demographic surveys on trends in HIV prevalence and male circumcision among young men. This analysis addresses several levels: a national level (trends in HIV prevalence), a social group level (differentials by socioeconomic group), and an individual level – that is, whether VMMC is associated or not with HIV infection in both univariate and multivariate analysis.
Methods
Demographic data
Zambia enjoys a wealth of demographic surveys, as well as other statistical data, crucial to understand the dynamics of the HIV epidemic and the role of VMMC. Three Demographic and Health Surveys (DHS), conducted in 2001, 2007 and 2013, contain information on HIV prevalence among adults (women aged 15–49 and men aged 15–59), and the last two surveys also have information on male circumcision, both traditional and medical. All three DHS contain a variety of socio-demographic variables (urbanization, education, marital status, ethnicity, religion, etc.), and information on sexual behaviour and condom use. In particular, they provide details about the last three sexual partners in the 12 months preceding the survey, whether these were marital partners or casual partners, and for men use of commercial sex and of condoms. All details on these variables and their coding can be found on the DHS programme website.
Statistical methods
Methods of analysis of the demographic surveys were straightforward: trend analysis at national level; correlation analysis at group level; cross-tabulations and Logit-Linear multivariate analysis. All computations were done using SPSS-17 statistical software.
Traditional circumcision
In Zambia, traditional circumcision is limited to eight ethnic groups: Chokwe, Kaonde, Luchazi, Lunda, Luvale, Mbunda, Mbowe and Ndembu (Van Binsbergen, Reference Van Binsbergen, Chrétien, Perrot, Prunier and Raison-Jourde1993). These groups accounted for 10.5% of the total population of the country at the 2000 census. They live primarily in the north-western corner of the country, currently the North-West province, and parts of the Western province (see Fig. 1). They originated from areas now located in eastern Angola and southern Congo, and migrated to the Upper Zambezia Valley in the 19th century, bringing with them the practice of circumcision. As recent migrants, they have a lower social status than other well-established groups (such as the Lozi), so that traditional male circumcision is associated with lower social status in Zambia. Although about two-thirds of them still live in their province of origin, some migrated to urban areas, and to other parts of the country, mainly to the Copperbelt and Lusaka provinces, where they often have low-status occupations. It appears that even in these groups, male circumcision was not universal in 2000 (as in some other groups in West Africa), and that, according to the DHS, some 76% of adults were circumcised, with no obvious trend by cohort, meaning that the traditional practice has been stable over the past 50 years. Overall, these groups tend to be less urbanized than average (29.4%), and to live in areas with less HIV infection, except those living in the Copperbelt province.
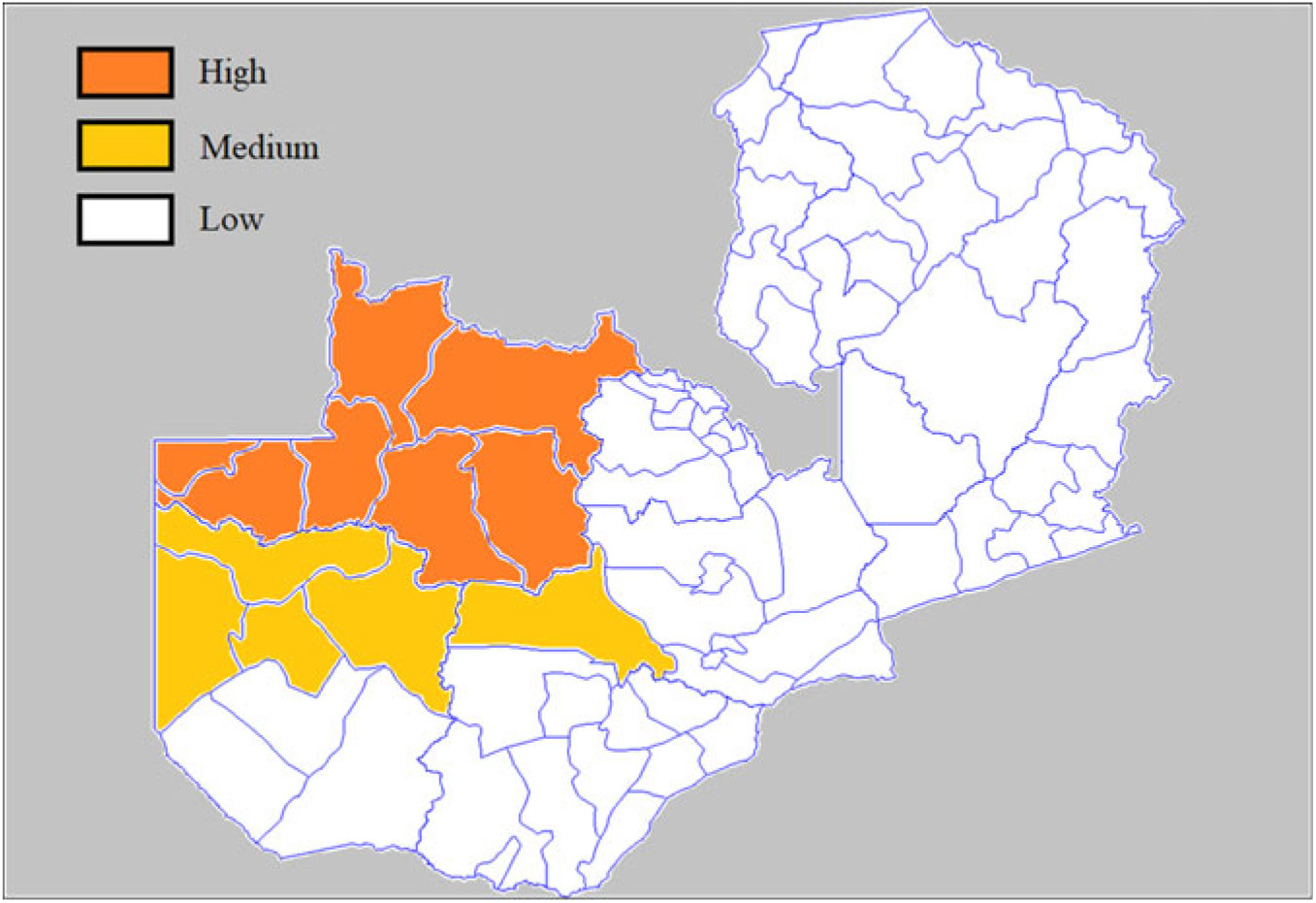
Figure 1. Map of traditional male circumcision, Zambia.
Prevention campaigns
Prevention campaigns in Zambia had started already in the late 1980s with blood safety measures, prevention of mother-to-child transmission (PMTCT), selected workplace programmes (around the mines in particular) and voluntary counselling and testing (VCT). Numerous health education campaigns have been conducted in Zambia since the early 1990s. They focused primarily on changing behaviour, under the slogan ‘ABC’ for Abstinence, Be-faithful, and Condom use. They were directed towards the general population, pregnant women and selected groups such as commercial sex workers (CSWs) and truck drivers. Several programmes were added over the years, such as ‘social and behaviour change communication’ (SBCC), ‘peer education’, ‘mass communication’ and ‘community mobilization’. Over the years, the country has made huge efforts to limit the size of the epidemic (Bloom et al., Reference Bloom, Banda and Songolo2000).
VMMC campaigns
Medical circumcision was offered in health facilities even before 2007. The policy to promote VMMC was adopted in June 2007 in Zambia, and by 2008 VMMC campaigns were well underway, targeting first urban areas, then rural areas. A new push was made in 2011, aiming at circumcising the majority of the adult population. The campaigns focused on the 15–29 age group, but allowed for earlier and later ages as well (Ministry of Health of Zambia, 2012).
Results
The three DHS conducted in 2001, 2007 and 2013 permit documentation of the dynamics of the HIV epidemic and the changing risk factors at national level over the 2001–2013 period, the socio-demographic correlates and the effect of VMMC on individual infection in 2013. The main focus of this analysis was on men aged 15–29, the target of the VMMC campaigns, although other age groups were also considered.
Trends in HIV prevalence and risk factors
According to the DHS, HIV prevalence among adults aged 15–49 declined slowly and regularly over the study period, from 15.6% in 2001, to 14.3% in 2007 and 13.3% in 2013. However, the decline was not identical for men and women, or for all age groups. Firstly, HIV prevalence increased among older adults, primarily because of a cohort effect: for women, the peak of HIV infection was at age 30–34 in 2001, therefore at age 40–44 12 years later and for men at age 35–39 in 2001 and at older ages 12 years later. Secondly, and most important for this study, HIV prevalence declined for women aged 15–29, from 14.2% in 2001 to 9.8% in 2013 (p < 0.001), while it remained steady for men aged 15–29 at around 6.6%. For men, HIV prevalence even increased at age 15–24 (p < 0.002), contrary to expectations (Table 1). This was most surprising because HIV prevalence declined among women of the same age group (most of their partners), and because VMMC has been catching up, inducing an increase in circumcised men from 11.7% in 2007 to 24.2% in 2013.
Table 1. Trends in population characteristics for men aged 15–29 and comparisons with women by HIV prevalence, sexual behaviour and socioeconomic status, Zambia 2001, 2007 and 2013 DHS

All data were from DHS, which provide details on sexual behaviour in the past 12 months, and on the last three sexual partners at time of survey.
CSW = commercial sex worker.
* p < 0.05.
With respect to sexual behaviour, changes were favourable among men aged 15–29 over the 2001–2013 period: age at first sexual intercourse was delayed, so that the proportion of men who had first sex after age 20 increased from 12.9% to 27.4% (p < 0.001); the proportion of men who ever paid for sex declined from 29.0% to 14.2% (p < 0.001); the proportion of men who abstained in the past 12 months increased from 16.8% to 19.9% (p < 0.001); the proportion of men who paid for sex in the past 12 months declined from 17.9% to 6.9% (p < 0.001). For men who had a partner in the past 12 months, the proportion of those who had two or more partners declined from 28.0% to 22.8% (p = 0.064), the proportion who had extramarital sex declined from 32.6% to 9.1% (p < 0.001), the proportion who used condoms increased from 37.5% to 43.0% (p < 0.001) and only the proportion of those who used commercial sex services did not change significantly (p = 0.308), and remained at a relatively low level: 1.7% on average (Table 1).
In contrast, changes in socioeconomic status (SES) were rather conducive to more HIV transmission, since higher SES was associated with higher HIV prevalence. However, changes were small over time and unlikely to have any major effect: increase in urbanization from 40.8% to 48.0% (p < 0.001); increase in the proportion of men living in the Copperbelt and the Lusaka provinces (two provinces with higher HIV prevalence than average) (p = 0.026): and increase in level of education (p < 0.001). According to elasticities (regression coefficients) found in 2013, the increase in urbanization for men aged 15–29 between 2001 and 2013 induced an absolute increase of 0.3% in HIV prevalence (from a baseline of 6.6%), and the increase in level of education had hardly any effect (+0.04%).
Impact of VMMC campaigns
The VMMC campaigns started in early 2008, although medical circumcision by trained health workers existed before in Zambia. The campaigns had a large impact on the number of young men circumcised. They focused on men aged 15–29, but also allowed for earlier and later ages. According to DHS, they did not affect older people, those born before 1968 (age 45+ in 2013) and had the largest effect for the younger men born between 1988 and 1993 (age 20–24 in 2013). By comparing the level of circumcision by cohort between 2007 and 2013, and multiplying the difference by the size of the population (as estimated by the United Nations Population Division, 2015 revision), some 428,000 men were circumcised between 2007 and 2013, of which 362,000 were aged 15–29; that is 17% of the 15–29 population, or about 1 out of 6 men, which is remarkable, and denotes the intensity of the efforts made (Fig. 2). This figure corresponds quite closely to the official figure of VMMC activities: 488,000 men circumcised by the mid-2013, which includes some children and elderly, and which makes Zambia one of the three countries most affected by VMMC campaigns by 2013, together with Botswana and Swaziland. The efforts continued thereafter, and by the end of 2016 some 1.485 million men had been circumcised in Zambia, representing 39% of the male adult population aged 15–49 – the highest value among the fourteen countries targeted by WHO (World Health Organization, 2016)
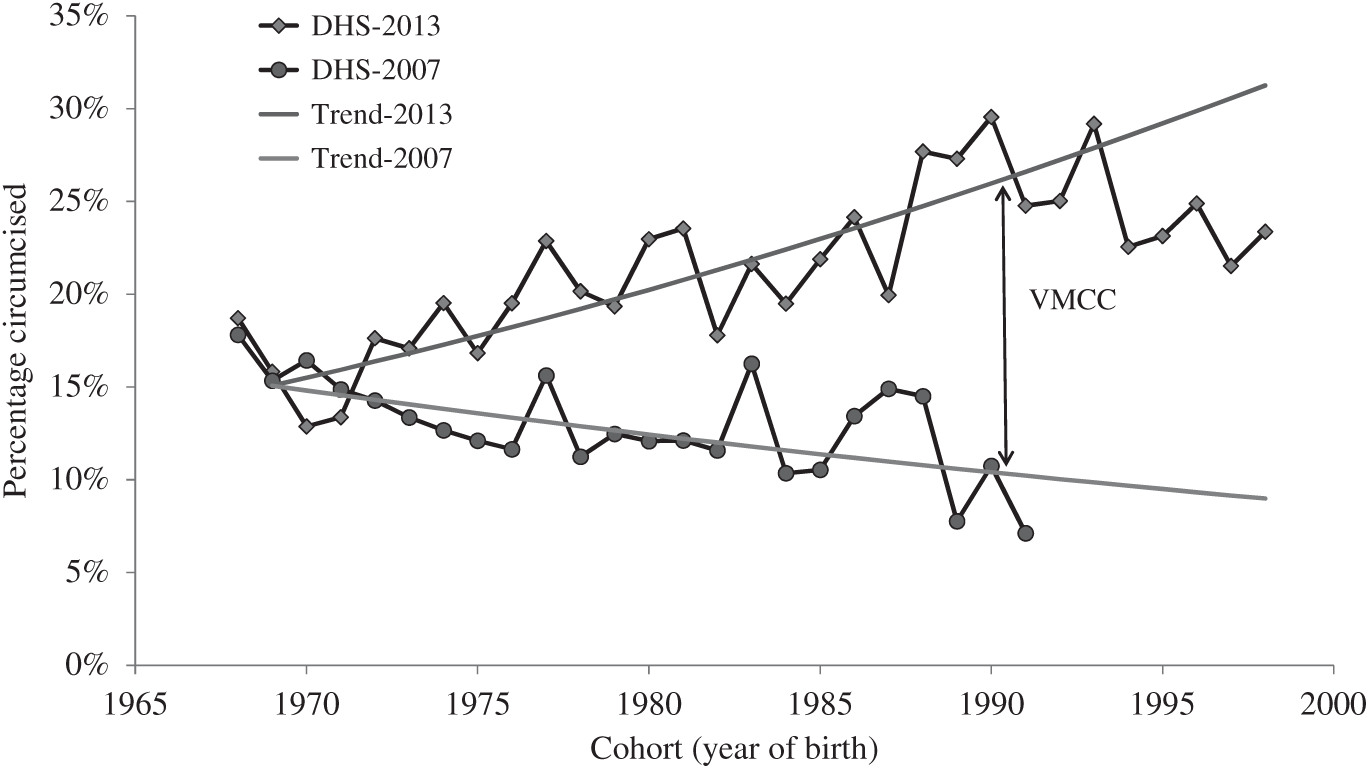
Figure 2. Proportion of men circumcised by cohort, Zambia, 2007 and 2013 DHS.
Relationships between SES, VMMC and HIV in 2013
The relationships of socioeconomic status (SES) with medical circumcision (VMMC) and HIV are complex in Zambia. Based on the 2013 DHS, the correlations between VMMC and HIV prevalence usually appear positive: that is, that more VMMC is associated with more HIV (Table 2). Urban residence was associated with more circumcision and more HIV (ρ = +1.00). Provinces with higher proportions of circumcised men tended to have higher HIV prevalence (ρ = +0.67) – in particular the Copperbelt province (the mining area), whereas provinces with less circumcision tended to have less HIV, such as the Southern and Muchinga provinces; there were, however, exceptions, such as the North-Western province (high circumcision, low HIV) or the Northern province (low circumcision, high HIV). Groups with higher levels of education (ρ = +0.74) or more years of schooling (ρ = +0.54) also had more HIV. The correlation was positive for ethnic groups (ρ = +0.54), with large differences among the 52 groups considered. The only apparent exception was religion (ρ = −0.56), although this was based on tiny Muslim groups who were more circumcised and had less HIV, but accounted only for 0.5% of men aged 15–29. Most of those correlations may be easily understood, since groups with more modern attitudes tend to have more risky sexual behaviour and therefore more HIV, and at the same time tend to be more receptive to VMMC campaigns.
Table 2. Correlations between circumcision and HIV by socioeconomic status variables, Zambia, 2013

ns = not statistically significant.
Multivariate analysis
At the individual level, the net effect of VMMC on HIV infection among men aged 15–29 was investigated with multivariate statistical methods after controlling for a variety of known factors of HIV prevalence, using the 2013 DHS (Table 3). The first control was ‘time since first sex’, measuring the duration of exposure to sexual transmission of HIV, and found to be highly significant: each additional year of exposure had a 1.082 relative risk, which gives a prevalence of 14.1% after 12 years of exposure; that is, at exact age 30 (14.3% found in the DHS). Urban residence was a strong risk factor, with a 2.16 relative risk compared with rural areas, where overall prevalence was much lower, implying less exposure. The number of sexual partners was also significant, with a 1.012 relative risk for each additional partner (the mean number of partners was 3.3 in this population with a wide range). All these values were in the expected direction and of the expected magnitude. In contrast, the coefficient for VMMC was slightly positive and not significant, meaning that the fact of being medically circumcised did not have any effect on the risk of being infected by HIV among men aged 15–29. The effect of ever having paid for sex and that of level of education were also not significant in this analysis.
Table 3. Results of the multivariate analysis of HIV prevalence among men aged 15–29, Zambia, 2013 DHS

ns = not statistically significant; *p < 0.05.
Behaviour of circumcised men in 2013
In order to document the lack of effect of VMMC on HIV infection, the sexual behaviour of circumcised men aged 15–29 was compared with that of uncircumcised men in 2013 (Table 4). Overall, circumcised men tended to have riskier behaviour in the 12 months before the survey: they had more partners (not significant), more extramarital partners (p < 0.05) and they used more commercial sex services (not significant), even though they had the same level of use of condoms in the case of high-risk partners. Even if these differences are rather small, more frequent risky behaviour could well compensate for the small biological effect of male circumcision on HIV transmission from female to male (Table 4).
Table 4. Sexual behaviour of men aged 15–29 in past 12 months by circumcision status, Zambia, 2013 DHS

RR = Relative Risk circumcised/uncircumcised.
CSW = commercial sex worker.
ns = not statistically significant; *p < 0.05.
Discussion
Trends in HIV prevalence among Zambian adults were similar to those in nearby countries of southern and eastern Africa, with a slow decline in prevalence since the early 2000s. However, the fact that prevalence was steady for young men aged 15–29 while it was decreasing for women of the same age was surprising. Among the 20 African countries with at least two DHS, Zambia was the only country where prevalence did not decrease among men while it decreased significantly for women. This situation appears even more surprising considering the fact that large-scale circumcision campaigns have been conducted in Zambia, reaching a significant proportion of the population.
The lack of evidence of any demographic impact of circumcision campaigns in southern and eastern Africa is not unique to Zambia. Five of the fourteen targeted countries have had at least two DHS with HIV assessment since 2012. Among those, three showed no decline in HIV prevalence among men aged 15–29 (Lesotho, Mozambique, Rwanda) and in the other two countries (Malawi and Zimbabwe) HIV prevalence was declining already before the VMCC campaigns, and showed no sign of changing trends (see Table 5 for details).
Table 5. Trends in HIV prevalence among men aged 15–29 in relation to VMMC efforts, selected target countries with at least two DHS/AIS surveys, the last survey being conducted after 2012
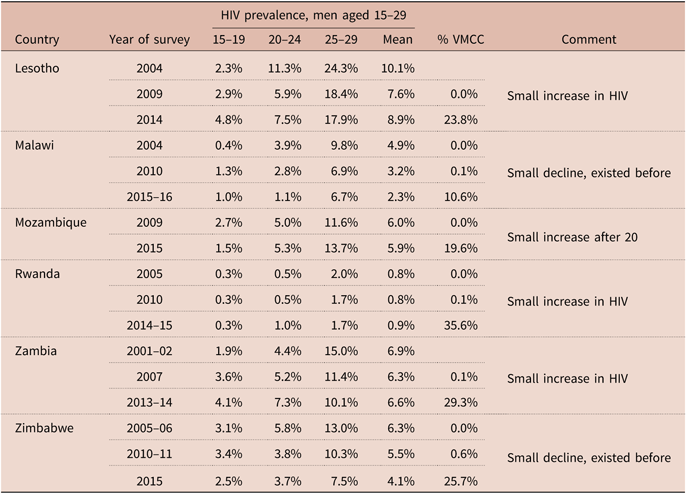
HIV prevalences are as published in the DHS/AIS reports. Circumcision: ratio of men circumcised in VMMC campaigns (source: WHO) to target population of men aged 15–29 (source: United Nations Population Division).
The main argument of this paper is about the net demographic effect on HIV prevalence of VMMC in the population of men aged 15–29. One could argue that the VMMC campaigns are too recent for a proper evaluation. If only official statistics of large campaigns are taken into account, men were circumcised on average 1.42 years before the 2013 DHS survey. This is a rather small interval, although basically the same as that used by the randomized controlled trials. However, many of the men aged 15–29, the target of this study, were circumcised in other circumstances by medically trained personnel. The 2013 DHS provides the age at circumcision and the type of operator. Men aged 15–29 included in this study were circumcised on average 5.8 years before, and a majority (58.2%) were circumcised before their first sexual intercourse. These men were exposed before age 30, at an age when the majority of HIV infections occur. Therefore, the study’s main argument remains valid: that those men who were circumcised before encountering the HIV virus did not benefit from any protection.
The positive association between socioeconomic status and HIV has been documented many times in African countries (Hargreaves & Glynn, Reference Hargreaves and Glynn2002). In Zambia a positive correlation between SES and VMMC was found, leading to a positive correlation between VMMC and HIV prevalence. These associations have been rarely documented and need to be taken into account to understand the dynamics of the changes in African countries. The fact that increasing urbanization and level of education could lead to more HIV is also rarely taken into account. Ironically, the VMMC campaigns changed the relationship between circumcision and HIV: earlier on, areas of traditional circumcision (the North-West province) were associated with less HIV and lower socioeconomic status.
The equivalent levels of HIV prevalence among circumcised and uncircumcised men aged 15–29, even after controlling for a variety of factors, was also surprising. Most likely it was due to the fact that newly circumcised men took more risks in sexual encounters. This could have been anticipated, given the fallacious messages provided at the time of medical circumcision. Arguing that circumcision provides ‘partial protection’, as often said in VMMC campaigns, could well lead to a feeling of being less vulnerable, and therefore conducive to taking more risks. Even though these men were told to continue using condoms to be really protected, they seem to not have listened to this safe advice. Would not it have been better to continue encouraging them to use condoms all the time if uninfected, and to use ARVs if infected, and not to circumcise them?
‘Risk compensation’, that is changing to more risky behaviour when feeling protected by VMMC, has been described earlier in qualitative studies (Abbott et al., Reference Abbott, Haberland, Mulenga and Hewett2013). This study confirms the existence of risk compensation in Zambia for several parameters. This is not necessarily the case elsewhere. For instance, a study in Kenya found no effect of recent circumcision on condom use (Westercamp et al., Reference Westercamp, Agot, Jaoko and Bailey2014). Another study across ten African countries found no effect of circumcision on condom use at last sexual intercourse, or on the number of non-cohabiting sexual partners in past 12 months (Shi et al., Reference Shi, Li and Dushoff2017). So, risk compensation may be context specific, or may vary over time. This issue needs to be further explored.
Some models have presented very optimistic views on the costs and benefits of VMMC in the long run, when 80% of the population is circumcised (Njeuhmeli et al., Reference Njeuhmeli, Forsythe, Reed, Opuni, Bollinger and Heard2011; Kripke et al., Reference Kripke, Njeuhmeli, Samuelson, Schnure, Dalal and Farley2014, Reference Kripke, Opuni, Schnure, Sgaier, Castor and Reed2016). These models are probably correct regarding the financial costs involved, but are based on highly simplified hypotheses on their demographic impact, not substantiated by statistical analysis. In particular, they ignore population heterogeneity with respect to sexual behaviour. Indeed, in a population there is always a large fraction of persons who are at very low risk for HIV (couples in stable relationships), and a fraction of very sexually mobile persons for whom circumcision will have no effect. Furthermore, these models make a direct link between reduced incidence in the short run and long-term effect on prevalence, which are different issues. The Zambia case study is a reminder that these hypotheses are not realistic.
Concerns have been raised about the quality of HIV prevalence data in the 2013 DHS, but this does not seem to affect the results of this analysis. Firstly, all DHS follow very careful procedures for HIV testing. In the case of Zambia, the three DHS (2001, 2007, 2013) followed the same procedure: dried blood spots, two ELISA tests and in case of discrepancy a Western Blot. The ELISA tests were different in the 2001 survey (Wellcozyme HIV 1&2 GACELISA and BIONOR HIV 1&2), but identical in the 2007 and 2013 surveys (Vironostika® HIV Uni-Form II Plus O, Biomerieux and Enzygnost® Anti-HIV 1/2 Plus, Dade Behring). These ELISA tests have high sensitivity and specificity. Secondly, the levels of HIV prevalence for the same cohorts were very consistent between the three surveys. They even permit calculation of cohort incidence after taking mortality into account. If anything, the level of HIV prevalence in 2013 was slightly lower above age 30 than expected from the 2007 survey: 19.6% among men aged 30–53 in 2007, and 19.1% among men aged 36–59 in 2013 (same cohorts born in 1954–1977). What was strikingly different was the high prevalence among men aged 15–24 in the last survey. This cannot be attributed to a failure (false positive) in the testing procedure in 2013. Lastly, declining mortality or ARVs cannot be considered as a cause of high prevalence, since most HIV cases among men aged 15–29 are likely to be recent.
Since this study was based on retrospective data, whether men were circumcised before or after being infected by HIV remains unknown. Therefore, the results may differ from prospective studies when men are circumcised before being infected. Indeed, this analysis reflects accurately the situation in populations, when men are circumcised at various ages and often independently from their HIV serostatus, and is therefore more informative on the population impact of VMMC campaigns. In Zambia, however, HIV testing is recommended before VMMC, and those found with HIV are advised not to get circumcised.
The DHS surveys do not provide information on migration. It is possible that with the sustained economic growth that occurred between 2001 and 2013 (+4.3% a year), men previously living in rural areas migrated to cities and mining areas, where they found an environment with more HIV and new opportunities for circumcision. Indeed, the proportion of men age 15–29 living in urban areas increased from 41% to 49% between the 2001 and 2013 DHS.
The effectiveness of VMMC could therefore be seriously questioned. It seems important to investigate what is going to happen in other countries practising VMMC on a large scale, such as Uganda, Tanzania, Lesotho, Swaziland, Botswana, Zimbabwe, Rwanda, Kenya, Mozambique and South Africa. Only a few years of monitoring are currently available for these countries, whereas the long-term effect is likely to be seen after year 2020. Taking the last DHS available as an end-point, prevalence of HIV among men aged 15–24 tended to rather increase in five countries (Lesotho, Rwanda, Tanzania, Zambia, Uganda), while it tended to decline in only two countries (Malawi and Zimbabwe). It is too early to draw firm conclusions, not counting the fact that confidence intervals are wide in this age range in DHS surveys. However, it seems that medical circumcision had not led so far to any decrease in HIV prevalence. This observation is far from the optimistic view that young men will immediately benefit from VMMC campaigns. Were they useful or harmful? Was the money spent on VMCC efficiently used?
Acknowledgements
This study was supported by the Institut Pasteur and IRD (MG), and the University of Kwazulu Natal (AM), without any external grant. The authors thank the DHS programme and the Zambia Central Statistical Office for providing free access to the survey data.
Funding
This research received no specific grant from any funding agency, commercial entity or not-for-profit organization.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









