Introduction
Intrauterine growth restriction (IUGR) refers to a pathological condition of pregnancy in which the developing fetus does not reach its growth potential for any given gestational age.Reference Rizzo and Arduini 1 IUGR constitutes one of the most common pregnancy complications worldwide affecting over 10% of all pregnancies.Reference Ananth and Vintzileos 2 Using an animal model, we have previously demonstrated that adult offspring born from pregnancies complicated with IUGR secondary to a prenatal hypoxic insult, exhibit an increase in left-ventricular mass, early in vivo signs of heart failureReference Rueda-Clausen, Morton and Davidge 3 and impaired tolerance to myocardial ischemia.Reference Xu, Williams, O'Brien and Davidge 4 Moreover, we have also shown that adult male offspring born from pregnancies complicated with IUGR exhibit myocardial structural changes characterized by increased deposition of type I and III collagen, an increase in the β/α myosin heavy change ratio and decreased myocardial activity of matrix metalloproteinases.Reference Xu, Williams, O'Brien and Davidge 4 Together, these findings suggest the presence of an active cardiac fibrosis/remodeling process in offspring born IUGR. One factor that could be involved in most of the phenotypical characteristics observed in these adult animals born IUGR is increased myocardial oxidative stress.
Oxidative stress occurs when there is an excess of free radicals or reactive oxygen species (ROS) relative to the amount of antioxidants; leading to damage of DNA, proteins and lipid membranes.Reference Droge 5 Cells have developed several mechanisms to remove excess ROS such as thiol reducing elements (glutathione and thioredoxion) and enzymes such as superoxide dismutase, catalase and glutathione peroxidase.Reference Murphy and Oudit 6
Among the several mechanisms that could induce oxidative stress and cardiac remodeling, we decided to study the potential role of iron metabolism and myocardial iron accumulation for a number of reasons: (i) iron is an essential element with a high reduction–oxidation potential, involved in many metabolic process and characterized for its potential as a source of oxidative stress,Reference Murphy and Oudit 6 , Reference Oudit, Trivieri, Khaper, Liu and Backx 7 (ii) the uptake, transport and storage of this element are closely regulated by the organism through a number of mechanisms, in fact, hypoxia is one of the major regulators of iron absorption and metabolism.Reference Murphy and Oudit 6 , Reference Anderson, Frazer and McLaren 8 , Reference Oudit, Sun and Trivieri 9 (iii) pathological conditions such as hemochromatosis, in which tissue and plasma iron levels are substantially increased, are characterized by the deposition of iron in multiple organs including the heartReference Murphy and Oudit 6 and (iv) we have shown that murine models of iron overloadReference Murphy and Oudit 6 exhibit a cardiac phenotype that is very similar to that which we have described in adult rats born IUGR.Reference Rueda-Clausen, Morton and Davidge 3 Moreover, these cardiovascular features are compatible with the most common manifestations of chronic iron toxicity in humans.Reference Lee and Beutler 10 , Reference Shander, Cappellini and Goodnough 11 Interestingly, it has been previously demonstrated that IUGR newborn babies exhibit an impaired iron handling characterized by decreased serum ferritin concentrations and increased transferrin levels.Reference Siimes and Siimes 12 , Reference Chockalingam, Murphy, Ophoven, Weisdorf and Georgieff 13 However, little is known regarding the long-term effects of prenatal hypoxia insults on the mechanisms that regulate iron homeostasis.
Given all these considerations, we hypothesize that offspring resulting from hypoxia-induced IUGR will have long-term changes in their ability to regulate iron metabolism leading to myocardial iron deposition, induction of myocardial oxidative stress, cardiac remodeling and cardiac dysfunction later in life.
Methods
Animals
Female Sprague Dawley rats were obtained at 3 months of age (Charles River, Quebec, Canada), acclimatized and then mated within the animal facility. A vaginal smear obtained the following morning was examined for the presence of sperm, which we designated as day 0 of pregnancy (term ≈21 days). Throughout pregnancy, rats were housed in standard rat cages with ad libitum access to water and food (standard lab rat chow) and weighed daily. On day 15 of pregnancy, rats were randomized to control (n = 8) or maternal hypoxia (n = 8) and treated as described previously in detail.Reference Rueda-Clausen, Morton and Davidge 3 Briefly, rats assigned to the maternal hypoxia group were placed inside a Plexiglas chamber continuously infused with nitrogen to maintain an oxygen concentration of 11.5% during the last 6 days of pregnancy. The level and duration of hypoxia used to induced IUGR in this model was chosen based on a number of considerations: (i) the effect of this protocol on offspring growth and development has been extensively described and has consistently shown to produce long-term effects on cardiovascular structure and function,Reference Rueda-Clausen, Morton and Davidge 3 , Reference Xu, Williams, O'Brien and Davidge 4 , Reference Rueda-Clausen, Morton, Lopaschuk and Davidge 14 (ii) time of exposure to hypoxia (starting on gestational day 15) was chosen to prevent interference with the implantation of embryos and to match the period of rapid fetal growth in this species and (iii) the level of hypoxia was chosen based on our previous experience showing that a more severe hypoxic insult can compromise the viability of the offspring and may not be optimal when studying the programing. Just before birth, dams were returned to normal housing conditions (21% oxygen). At the time of birth, litters were reduced to eight pups (four male and four female) in order to control the postnatal environment. Pups and dams were then weighed bi-weekly until weaning at week 3 and then again on the experimental day at 4 or 12 months of age when they were considered to be young adults or aged adults, respectively. All animals were housed in standard rat cages with 60% humidity, a 12 h:12 h light:dark cycle and ad libitum access to water and food (standard lab rat chowLabDiet 5001* with an iron content of 270 ppm) in the animal facilities of the University of Alberta. All procedures in this study were approved by the University of Alberta Animal Welfare Committee and are in accordance with the guidelines of the Canadian Council on Animal Care and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Blood withdrawal and processing
On the experimental day, blood samples were obtained from anesthetized animals by aortic transection. Blood samples were collected in tubes containing ethylenediaminetetraacetic acid, heparin or no anticoagulant and placed on ice for 10 min and then centrifuged (10 min at 4000 rpm, 4°C). Serum and plasma were then separated into aliquots and stored at −80°C. Lipemic or severely hemolytic samples were discarded.
Cardiac samples
Hearts were excised from anesthetized rats and perfused for 10 min in retrograde Langendorff mode; with Krebs–Henseleit prepared as previously reported.Reference Xu, Williams, O'Brien and Davidge 4 Hearts were switched to anterograde working perfusion mode and perfused in a closed recirculating system at 37°C with 120 ml of modified Krebs–Henseleit solution prepared as described previously and exposed to an ischemia reperfusion (IR) protocol 4. After perfusion, hearts were placed on dry gauze to remove excess water and snap frozen in liquid nitrogen.
Myocardial oxidative stress
Levels of total, oxidized and reduced glutathione were determined in myocardial samples (80–100 mg) obtained from the free wall of the left ventricle (LV) of frozen myocardial specimens obtained as mentioned before. A 10% w/v homogenate was created by adding ice-cold 5% metaphosphoric acid (0.9 ml/100 mg tissue) and homogenizing. The homogenates were then centrifuged at 12,000 rpm for 15 min at 4°C and supernatants were collected. Myocardial levels of glutathione in its reduced (GSH) and oxidized (GSSG) states were determined using a commercially available kit [OxiSelect™ Total Glutathione (GSSG/GSH) Assay Kit Catalog no. STA-312, Cell Biolabs Inc., San Diego, CA, USA] and following the manufacturer specifications. Levels of malondialdehyde (MDA) were determined in myocardial samples (100–150 mg) from the same specimens as mentioned before and using a commercially available kit (Bioxytech MDA-586™, assay kit catalog no. 21044, OxisResearch, Percipio Biosciences Inc., Los Angeles, CA, USA) and following the manufacturer specifications.
Myocardial histology
In a different set of animals, perfused hearts were collected after IR protocol and were preserved in 10% formalin for 48 h and then transferred to 10% ethanol for dehydration. Following dehydration in methanol, axial myocardial samples, including left and right ventricle, were sent to the Alberta Diabetes Institute Histology Core (Edmonton, Canada) for histopathological preparation (by paraffin embedding) followed by slicing and staining (hematoxylin/eosin and Masson's trichrome). Prussian blue staining for the detection of iron deposits in the myocardium was performed using the Gomori's methodReference Gomori 15 on 5 μm slices of myocardial tissue previously fixed in 10% formalin and embedded in paraffin. All procedures were performed according to standardized protocols.
Myocardial iron content
Myocardial samples (100–200 mg) were obtained from the free wall of the LV of frozen myocardial specimens. Samples underwent nitric acid digestion followed by dilution and injection into a High Resolution Sector Field Inductively Coupled Plasma Mass Spectrometer (ICP-MS; Element 2®, Thermo Scientific; Trace Elements Laboratory at London, ON, Canada). The reported coefficient of variation of this technique is 3–14% and the minimal detectable concentration of iron is 0.5 μg/g.
Statistical analysis
Data are presented as mean ± standard error of the mean (se), except where stated otherwise. Due to the marked phenotypical differences between sexes, data obtained from male and female offspring were analyzed separately. Differences in the measurements performed among the groups were tested using two-way analysis of variance (ANOVA) with age and prenatal exposure to hypoxia as sources of variation. A Bonferroni post-hoc test was then used to compare replicate means by groups. A P-value < 0.05 was considered statistically significant.
Results
Characteristics of newborn and adult offspring that resulted from this model have been previously published.Reference Rueda-Clausen, Morton, Lopaschuk and Davidge 14 , Reference Rueda-Clausen, Dolinsky and Morton 16 , Reference Morton, Rueda-Clausen and Davidge 17 Briefly, the hypoxic insult used in this study caused a sex-independent reduction in both body weight (∼17%) and absolute placental weight (∼13%) and an increase in relative cardiac weight both in male and female fetuses (∼23%). This kind of prenatal insult, however, had no significant effect on perinatal parameters such as litter size, proportion of stillborn offspring or sex distribution. After birth, offspring exposed to hypoxia in utero rapidly caught up with controls in terms of body weight. Within four days after birth, there were no differences in body weight between sex- and age-matched experimental groups.Reference Rueda-Clausen, Morton, Lopaschuk and Davidge 14 , Reference Rueda-Clausen, Dolinsky and Morton 16 , Reference Morton, Rueda-Clausen and Davidge 17
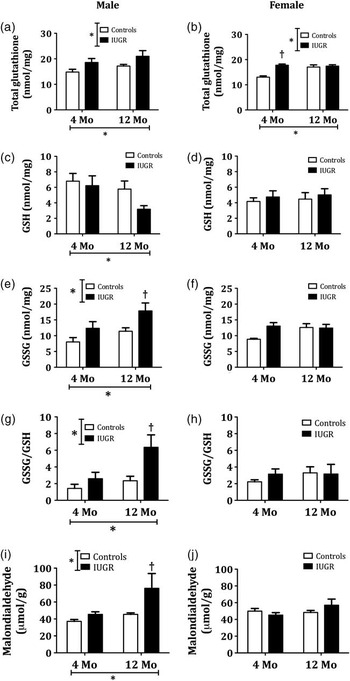
Overall, in both male and female offspring, IUGR and aging were associated with an increase in the myocardial levels of total glutathione (GSH + GSSG; Fig. 1a and 1b). The myocardial concentration of GSH, however, presented a dimorphic behavior depending on the sex of the offspring. In male, but not in female offspring, aging was associated with a decrease in the reduced fraction of glutathione in the myocardium (Fig. 1c and 1d). By presenting these results as a ratio between the oxidized and reduced fractions of glutathione (GSSG/GSH), it is evident that in male, but not in female offspring, both aging and IUGR were associated with an increased presence of myocardial oxidative stress (Fig. 1g and 1h). Glutathione determinations were consistent with the measurements of lipid peroxidation (MDA) performed in the same tissues and showing similar results (Fig. 1i and 1j).

Fig. 1 Long-term effects of intrauterine growth restriction (IUGR) on the myocardial levels of glutathione. Measurements performed in myocardial samples from Control and IUGR offspring of both sexes at 4 and 12 months (Mo) of age. (a and b) Total glutathione levels (GSH + GSSG), (c and d) levels of reduced glutathione (GSH), (e and f) oxidized glutathione (GSSG), (g and h) ratio of oxidized to reduced glutathione (GSSG/GSH), (i and j) myocardial levels of malondialdehyde. *Represents values of P < 0.05 for the respective sources of variation (IUGR or age) using two-way analysis of variance. † P < 0.05 v. controls after a Bonferroni post-hoc test comparing IUGR and control offspring of the same age (n = 6 per group).
To better characterize the increase in collagen deposition that has been described in the myocardium from offspring born IUGR,Reference Xu, Williams, O'Brien and Davidge 4 we performed qualitative histological studies with Masson's trichrome staining. Neither in young offspring nor aged female offspring was IUGR associated with changes in cardiac structure or collagen deposition that could be detected in the histological preparations. Close to 80% of the samples obtained from aged male offspring born IUGR, exhibited myocardial remodeling characterized by myocyte loss dispersed throughout the ventricular wall (Fig. 2).

Fig. 2 Myocardial deposition of collagen in offspring born IUGR at 12 months of age. Representative images of myocardial samples stained with Masson's trichrome in which muscle fibers are stained red, collagen is green, cytoplasm is pink and cell nuclei are dark brown. Images obtained from the inter-ventricular septum of male offspring at 12 months of age using (a) low (b) mid or (c) high magnification. Arrows indicate intra-myocardial areas of myocyte loss and connective fiber deposition that were observed only in aged, male offspring born IUGR. Calibration bars represent 50 μm.
From a systemic perspective, neither IUGR nor aging were associated with changes in any of the plasma markers of iron metabolism evaluated in this project (Fig. 3). Interestingly, it was notable that in female offspring both plasma concentrations of free iron and total iron binding capacity were substantially greater (∼50%; P < 0.01) than those in male rats regardless of which experimental group they belonged to (Fig. 3a, 3b, 3e and 3f). Both ferritin levels and iron saturation indices showed a trend towards an increase in male offspring born IUGR, however, these differences did not reach statistical significance. In order to evaluate the presence of localized myocardial iron deposition, we measured iron levels in myocardial samples from male offspring in all experimental groups using the gold standard technique (mass spectrometry). As summarized in Figure 4, aging was associated with an increase in the total iron content; however, contrary to our hypothesis, IUGR did not affect total myocardial iron content.

Fig. 3 Long-term effect of intrauterine growth restriction (IUGR) on plasma levels of iron homeostasis markers. Measurements performed in serum samples from Control and IUGR offspring of both sexes at 4 and 12 months (Mo) of age (n = 6 per group). (a and b) blood free iron levels, (c and d) total iron binding capacity (TIBC), (e and f) iron saturation index, (g and h) serum ferritin levels.

Fig. 4 Long-term effect of intrauterine growth restriction (IUGR) on myocardial accumulation of iron in male offspring. Measurements performed in left ventricle tissue from Control and IUGR offspring at 4 or 12 months (Mo) of age. *Represents values of P < 0.05 for the respective sources of variation (IUGR or Age) using two-way analysis of variance (n = 6 per group). Determinations performed by the Trace Elements Laboratory in London, ON using inductively coupled plasma mass spectroscopy.
Finally, we performed histological preparations with Prussian blue in samples from aged, male offspring which would allow us to identify small iron depositions that could not be detected by mass spectrometry; however, no iron deposits were identified in any of the analyzed samples. For positive controls we performed the same histological technique in samples of myocardium obtained from a murine model of iron overload, which demonstrated that our technique was appropriate (Fig. 5).

Fig. 5 Representative images of myocardial samples from control and intrauterine growth restriction male offspring stained for iron detection. Representative images of myocardial slides stained with Prussian blue to detect iron accumulation (blue). No evidence of cardiac iron deposits was identified in any of the experimental groups. Positive control tissues were obtained from a murine model of iron nutritional overload. Calibration bars represent 100 μm.
Discussion
Our study represents experimental evidence showing that hypoxia-induced IUGR was associated with increased levels of oxidative stress in the myocardium. Previous clinical studies have reported that babies born from pregnancies complicated with IUGR, as a result of maternal undernourishment, exhibit increased levels of oxidative stress at birth.Reference Gupta, Narang, Banerjee and Basu 18 Moreover, weReference Xu, Williams, O'Brien and Davidge 4 and othersReference Elmes, Gardner and Langley-Evans 19 have demonstrated that IUGR resulting from maternal hypoxia or nutritional restriction, is associated with increased susceptibility to cardiac I/R injury during adulthood. Interestingly, it has also been suggested that inducing antioxidant mechanisms in the offspring could reverse susceptibility to ischemia in some of these models.Reference Elmes, McMullen, Gardner and Langley-Evans 20 Together, these results suggest that programed increases in myocardial oxidative stress could play a role in the phenotypical characteristics observed in offspring born IUGR.
One additional programing factor that needs to be considered is the potential effect of postnatal maternal stress caused by litter reduction. Previous studies have shown that these kind of postnatal insults can have long-term consequences on offspring behavior and health.Reference Barnes and Ozanne 21 We anticipate, however, that the effect of this particular factor on the results will be minimal in our study given that litters from both experimental groups were reduced after birth.
Glutathione is a key intracellular tripeptide thiol composed of glutamic acid, cysteine and glycine.Reference Henderson and Tyagi 22 Glutathione helps protect cells from free radical damage by acting as an antioxidant. Within cells, glutathione exists in reduced (GSH) and oxidized (GSSG) states.Reference Parodi, De Maria and Roubina 23 Reduced glutathione's thiol group provides reducing equivalents to other unstable ROS, and in turn then becomes unstable itself. This unstable GSH readily reacts with another unstable GSH to form a stable GSSG molecule.Reference Parodi, De Maria and Roubina 23
In our study we found that, in both male and female offspring, IUGR was associated with a consistent and significant increase in the total levels of glutathione, which suggests that these tissues exhibit an up-regulation of the expression of this tripeptide, probably as a compensatory mechanism in response to the enhanced oxidative stress.Reference Sharma, Dewald and Adrogue 24 , Reference Yeh, Ching and Yen 25
In healthy myocardial cells, more than 90% of the total glutathione pool is normally found in the reduced form due to activity of the enzyme glutathione reductase.Reference Fineschi, Baroldi and Centini 26 This enzyme is constitutively active and, in addition, can be induced by oxidative stress.Reference Tsutsui, Kinugawa and Matsushima 27 An increased GSSG/GSH ratio is considered a marker of oxidative stress.Reference Tsutsui, Kinugawa and Matsushima 27 Interestingly, our results showed that close to 50% of all the glutathione in the myocardium of all our experimental groups was in the oxidized form. This finding could be a consequence of the characteristics of the samples used to perform these measurements since the tissues used for these determinations were collected after I/R, a condition in which oxidative stress is known to be increased.Reference Hariharan, Zhai and Sadoshima 28 , Reference Zhao and Zhao 29 In addition, it is possible that the increased susceptibility to ischemic injuries described in these animals may be due to increased levels of oxidative stress in the pre-ischemic heart, or that increased levels of oxidative stress in the myocardium are the result of the decreased recovery after ischemia that we have described previously in these same animals.Reference Rueda-Clausen, Morton, Lopaschuk and Davidge 14 The results from female aged offspring, however, provide an interesting finding given that, in this particular group of animals, IUGR was associated with a decreased recovery after a 10 min ischemic challengeReference Rueda-Clausen, Morton, Lopaschuk and Davidge 14 but was not associated with changes in intra-myocardial markers of oxidative stress. These results suggest that the observed differences in the myocardial GSSG/GSH ratio between control and IUGR offspring is not secondary to the observed differences in response to the I/R challenge. These results were further supported by an alternative method to determine myocardial lipid peroxidation, which is a well-recognized effect of increased oxidative stress.
Epidemiological studies have shown that premenopausal women exhibit a certain degree of cardiovascular risk protection due to the action of ovarian derived hormones. This sex-related cardioprotection, therefore, gradually disappears after menopause. As their cardiovascular risk rises, post-menopausal women also exhibit an increase in systemic markers of oxidative stress,Reference Sanchez-Rodriguez, Zacarias-Flores, Arronte-Rosales, Correa-Munoz and Mendoza-Nunez 30 , Reference Abdul-Rasheed, Al-Shamma and Zillo 31 which suggest that sex hormones could also be involved in the aging-induced increase in oxidative stress.Reference Sanchez-Rodriguez, Zacarias-Flores, Arronte-Rosales, Correa-Munoz and Mendoza-Nunez 30 Relative to humans, female rats do not undergo menopause as they age, instead they undergo ovarian senescence.Reference Hunter, Kostyak, Novotny, Simpson and Korzick 32 At this stage, female rats are infertile but their estrogen levels remain constant compared with the decrease seen with human menopause. The animal aging model used in this study, therefore, does not mimic a post-menopausal but a premenopausal aging state in female offspring. The sustained blood levels of estrogen could explain why the marked increase in oxidative stress observed in aged male rats born IUGR was absent in their female counterparts exposed to a similar prenatal insult.
The results obtained using Masson's trichrome histological preparations demonstrated that, in male offspring, IUGR and aging interact to produce a particular cardiac phenotype characterized by multiple isolated areas of intra-ventricular myocyte death and connective tissue deposition. These results are consistent with our previous observations demonstrating that the myocardium of male adult offspring born IUGR exhibits an increase in collagen deposition.Reference Xu, Williams, O'Brien and Davidge 4 In addition to these histological results, the strong and close association between hypoxia and iron metabolism detailed in the literature supported the hypothesis that hypoxic prenatal insults could produce long-term effects on iron metabolism leading to cardiac siderosis, inflammation and fibrosis.Reference Murphy and Oudit 6 , Reference Anderson, Frazer and McLaren 8 , Reference Ben-Yosef, Miller, Shapiro and Lahat 33 Contrary to our hypothesis, however, we did not identify differences in either systemic parameters associated with iron overload or levels of iron in the myocardium of IUGR offspring as measured using high resolution ICP-MS; the gold standard technique for this kind of measurement. It is plausible that the samples of myocardium used for these determinations (100 mg per sample) were not representative of the total myocardial tissue and failed to include areas of iron deposition. However, all samples were collected from approximately the same anatomical location in the LV free wall where focal loss of cardiomyocytes and disrupted extracellular matrix were observed. Moreover, histological preparations with Prussian blue offered an alternative evaluation of cardiac structure and iron deposition that confirmed our initial findings.
As no differences were observed in myocardial iron accumulation or systemic iron balance markers, it is unlikely that the observed changes in cardiac oxidative stress of male offspring born IUGR could be explained by myocardial iron accumulation. The interesting sex differences in this particular finding suggest that sex hormones may contribute to the modulation of pathophysiological mechanisms leading to this increase in myocardial oxidative stress, however, the specific pathways still need to be determined.
Conclusions
The present study suggests that hypoxic insults leading to IUGR produce long-term effects on the levels of oxidative stress in the post-ischemic myocardium of male but not female offspring. We also demonstrated that in only male offspring, IUGR and aging interact to produce an interesting cardiac phenotype characterized by isolated intra-myocardial clusters of myocyte loss and connective fiber deposition. Contrary to our hypothesis, no changes in systemic markers of iron overload or total iron accumulation in the myocardium were observed among groups. The potential effects of IUGR on myocardial oxidative stress warrant further investigation. Moreover, further studies are required to understand the specific mechanisms leading to the observed changes in the cardiac structure of offspring born IUGR.
Acknowledgments
This work was supported by a research grant from the Canadian Institutes of Health Research (CIHR) and a Personnel Award from the Heart and Stroke Foundation of Canada (H&S). C.R.-C. and J.M. are supported by H&S; Alberta Innovates Health Solutions (AI-HS). G.Y. Oudit is supported by HSFC, CIHR and AI-HS; Z.K. is an AI-HS Scholar and is supported by CIHR and HSFC, S.T. Davidge is funded by AI-HS as an Alberta Heritage Foundation for Medical Research Scientist and is a Canada Research Chair (CRC) in Women's Cardiovascular Health.