Introduction
Identifying individuals at high risk of developing dementia before the onset of their functional impairment is important for early clinical intervention and for prospective research. Mild cognitive impairment (MCI) was proposed as a concept to fit this need by Reisberg and colleagues in Reference Reisberg, Ferris, de Leon, Franssen, Kluger, Mir and Cohen1998 and has since become the topic of intense research interest. MCI patients are reported to convert to dementia at a rate of approximately 5–10% per year, whereas the conversion rate of healthy controls is 1–2% (Petersen, Reference Petersen2011). Importantly, MCI is not a homogeneous entity, neither in its presentation nor prognosis. Individuals with MCI can be impaired due to medications, depression, or incipient neurological diseases such as Alzheimer's disease (AD), frontotemporal dementia (FTD), or vascular brain disease.
Current conceptualizations of MCI recognize multiple subtypes centered on the presence or absence of memory impairment, namely amnestic (aMCI) and non-amnestic (naMCI). These, in turn, have single or multiple cognitive domain sub-classifications and may represent the prodromes of different dementia types. For example, a minority of MCI individuals present with single domain naMCI, which can progress to non-AD dementing conditions such as FTD, whereas multiple domain naMCI can progress to Lewy body dementia or vascular dementia (Jak, Bangen, et al., Reference Jak, Bangen, Wierenga, Delano-Wood, Corey-Bloom and Bondi2009; Petersen et al., Reference Petersen, Roberts, Knopman, Boeve, Geda, Ivnik and Jack2009). In contrast, most MCI individuals present with predominantly memory complaints and 90% of these aMCI patients who progress to dementia demonstrate clinical signs of AD (Petersen et al., Reference Petersen, Doody, Kurz, Mohs, Morris, Rabins and Winblad2001). These aMCI individuals may or may not show impairments in other cognitive domains in addition to memory and thus can be characterized as multiple or single domain aMCI (the former tending to progress to AD or vascular dementia). However, not all individuals with MCI will progress to dementia according to these classifications or, indeed, progress to dementia at all (Petersen et al., Reference Petersen, Roberts, Knopman, Boeve, Geda, Ivnik and Jack2009). Recently published diagnostic criteria for “MCI due to AD” (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox and Phelps2011) describe these patients as non-demented individuals with an expressed concern for change in cognition, objective impairment in one or more cognitive domain, and preserved independence in functional abilities. The current consensus is that episodic memory is the cognitive domain most commonly impaired in MCI patients who go on to develop AD, although other cognitive domains, including executive function, may also be impaired (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox and Phelps2011).
AD neuropathology progresses in a predictable sequence, with neurofibrillary pathology and neuronal atrophy beginning in medial temporal areas and progressing to temporal, parietal, and posterior cingulate cortices before affecting the anterior cingulate and frontal lobes. Amyloid deposition is initially seen in the basal portions of frontal, temporal, and occipital lobes, followed by cortical association areas and the hippocampus, finally affecting extensive cortical and subcortical areas (Braak & Braak, Reference Braak and Braak1997; Thompson et al., Reference Thompson, Hayashi, Dutton, Chiang, Leow, Sowell and Toga2007). Most aMCI patients show neuropathological features intermediate between normal aging and very early AD, including neurofibrillary pathology in the medial temporal lobes and diffuse amyloid in the neocortex (Petersen et al., Reference Petersen, Doody, Kurz, Mohs, Morris, Rabins and Winblad2001, Reference Petersen, Parisi, Dickson, Johnson, Knopman, Boeve and Kokmen2006). Structural imaging shows neuronal loss in the medial and inferior temporal lobes in both single- and multiple-domain aMCI, with the posterior temporal lobe, parietal association cortex and posterior cingulate also affected in multiple domain aMCI (e.g., Whitwell, Petersen, et al., Reference Whitwell, Petersen, Negash, Weigand, Kantarci, Ivnik and Jack2007).
The traditional description of AD as a disease that begins with episodic memory impairment gradually progressing to global decline in cognitive functioning is consistent with the neuropathological progression (Becker, Huff, Nebes, Holland, & Boller, Reference Becker, Huff, Nebes, Holland and Boller1988; Collie & Maruff, Reference Collie and Maruff2000). However, evidence suggests that non-memory cognitive deficits may be present even in the earliest stages. While the presence of global cognitive deficits, including difficulties in executive functioning, is commonly reported in moderate AD (see Duke & Kaszniak, Reference Duke and Kaszniak2000; Perry & Hodges, Reference Perry and Hodges1999), some suggest that executive dysfunction may occur in early AD (e.g., Baddeley, Baddeley, Bucks, & Wilcock, Reference Baddeley, Baddeley, Bucks and Wilcock2001; Baudic et al., Reference Baudic, Barba, Thibaudet, Smagghe, Remy and Traykov2006), possibly even in the preclinical phase (e.g., Albert, Blacker, Moss, Tanzi, & McArdle, Reference Albert, Blacker, Moss, Tanzi and McArdle2007; Chen et al., Reference Chen, Ratcliff, Belle, Cauley, DeKosky and Ganguli2001; Perri, Serra, Carlesimo, & Caltagirone, Reference Perri, Serra, Carlesimo and Caltagirone2007). Although atrophy of the frontal lobes is not typically observed in MCI, recent studies point to a possible disconnection between brain areas (e.g., Brambati et al., Reference Brambati, Belleville, Kergoat, Chayer, Gauthier and Joubert2009; Chao et al., Reference Chao, Pa, Duarte, Schuff, Weiner, Kramer and Johnson2009; Chen et al., Reference Chen, Chen, Cheng, Hua, Liu and Chiu2009; Delano-Wood et al., Reference Delano-Wood, Bondi, Sacco, Abeles, Jak, Libon and Bozoki2009), which could underlie this early executive impairment. Thus, the traditional view that AD primarily involves episodic memory deficits during the early phases may not be entirely accurate. For this reason, it is important to fully describe the profile of executive function in early and preclinical AD to more accurately define the early stages of the illness, and to identify people at risk of developing the disease. Early identification is essential for implementing strategies for the prevention and/or slowing of neuronal damage (see Lopez & Belle, Reference Lopez and Belle2004). In addition, early and precise diagnosis is necessary for case management and for selecting appropriate subjects for pharmaceutical trials and other research.
The present study was designed to provide a comprehensive description of the severity and frequency of executive function impairment in MCI. Previous studies have often not explored multiple sub-domains of executive functioning; therefore, we tested MCI patients on measures of divided attention, working memory, inhibitory control, verbal fluency, and planning to determine whether certain sub-domains are more severely or frequently impaired than others. The pervasiveness of deficits in executive functioning is the focus of the current study, in which we examine patients with MCI, and also of a companion paper, which examines FTD and Lewy body dementia (Johns et al., Reference Johns, Phillips, Belleville, Goupil, Babins, Kelner and Chertkow2009).
Executive Functions
Despite a large literature on executive functions, a consensus on a precise definition of the construct has yet to be reached. Nevertheless, executive functions are commonly conceptualized as higher-order cognitive capacities that are necessary to support independent, purposive, goal-directed behavior or high-level control over lower level cognitive functions (Perry & Hodges, Reference Perry and Hodges1999; Royall et al., Reference Royall, Lauterbach, Cummings, Reeve, Rummans, Kaufer and Coffey2002; Stuss & Levine, Reference Stuss and Levine2002). Although the subcomponents of executive functioning are not agreed upon, they generally include planning, initiation, organization, self-monitoring, cognitive flexibility, set shifting, inhibitory control, generative behavior or fluency, abstraction, working memory, and divided attention (Alvarez & Emory, Reference Alvarez and Emory2006; Royall et al., Reference Royall, Lauterbach, Cummings, Reeve, Rummans, Kaufer and Coffey2002; Stuss & Levine, Reference Stuss and Levine2002). These subcomponents can be further reduced by examining tests that tap into five overarching sub-domains: divided attention, working memory, inhibitory control, verbal fluency, and planning. Successful performance on neuropsychological tests in these sub-domains often requires the use of other aspects of executive functioning as well; however, we have chosen tests thought to involve one of these five sub-domains as its primary cognitive process to succinctly cover several aspects of executive functioning.
Executive Function in Mild Cognitive Impairment
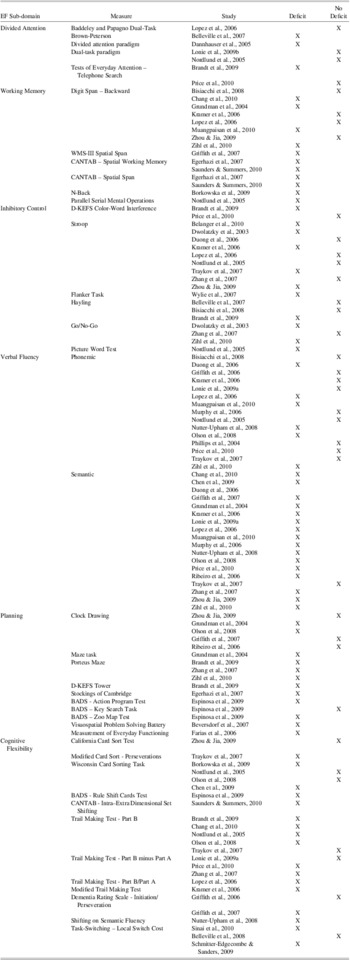
Recent studies suggest that impairment in multiple cognitive domains is common in MCI (e.g., Kramer et al., Reference Kramer, Nelson, Johnson, Yaffe, Glenn, Rosen and Miller2006; Libon et al., Reference Libon, Xie, Eppig, Wicas, Lamar, Lippa and Wambach2010; Loewenstein et al., Reference Loewenstein, Acevedo, Agron, Issacson, Strauman, Crocco and Duara2006), and progression to dementia is much more common in such cases (Bozoki, Giordani, Heidebrink, Berent, & Foster, Reference Bozoki, Giordani, Heidebrink, Berent and Foster2001). Furthermore, there are marked preclinical deficits in executive functioning in individuals who later go on to develop AD (Backman, Jones, Berger, Laukka, & Small, Reference Backman, Jones, Berger, Laukka and Small2005). In one study, a worsening of executive functioning deficits, but not a worsening of memory, was associated with conversion to AD over a 1-year follow-up period (Rozzini et al., Reference Rozzini, Chilovi, Conti, Bertoletti, Delrio, Trabucchi and Padovani2007). Emerging evidence increasingly supports the notion that executive functioning is commonly impaired in aMCI. Although a complete review of all studies that have examined executive function performance in aMCI is outside the purview of this introduction, the interested reader can find a summary of the studies in the Appendix. This table shows that there is indeed evidence for statistically significant poorer performance of aMCI patients on several tests of executive function. However, there are clearly studies that fail to find such evidence and not all measures yield consistent findings. Moreover, on the whole, studies that have reported poorer performance did not examine the frequency or severity of such deficits.
Previous studies have examined some aspects of the severity and frequency of executive deficits observed in MCI. Although there were significant group differences between MCI patients and controls on measures of working memory, inhibition, verbal fluency, and planning, the average degree of impairment exceeded one standard deviation (SD) only for inhibitory control (Grundman et al., Reference Grundman, Petersen, Ferris, Thomas, Aisen, Bennett and Thal2004; Nordlund et al., Reference Nordlund, Rolstad, Hellstrom, Sjogren, Hansen and Wallin2005; Ribeiro, de Mendonca, & Guerreiro, Reference Ribeiro, de Mendonca and Guerreiro2006). Others have examined frequency of impairment and found impairment (> −1.0 SD) in 75% of MCI patients on dual task performance, and 30–50% on a measure of inhibition (Belanger & Belleville, Reference Belanger and Belleville2009; Belleville, Chertkow, & Gauthier, Reference Belleville, Chertkow and Gauthier2007). In studies examining impairment in multiple sub-domains, 50–90% of MCI patients were mild to moderately impaired on one or more measures (Belleville et al., Reference Belleville, Chertkow and Gauthier2007; Nordlund et al., Reference Nordlund, Rolstad, Hellstrom, Sjogren, Hansen and Wallin2005). While these studies suggest clinically significant deficits on some measures, most did not evaluate a broad range of executive measures and the frequency of impairment was compared against other non-executive cognitive domains. Thus, it is not known if only certain sub-domains of executive functioning are affected. Furthermore, the relationship between the degree and frequency of impairment on different executive tests has yet to be examined.
The Present Study
Given the demonstration of executive function deficits in MCI, we set out to determine (1) the severity of the deficits in various sub-domains of executive function and (2) the frequency of impairment in each sub-domain. Assessing individuals with a clinical diagnosis of amnestic MCI, we first demonstrate that there are statistically reliable group differences between MCI patients and controls on all tests of executive functioning. In light of the previous literature, we then predicted that MCI patients would exhibit a mild-to-moderate impairment on the measures of inhibitory control and smaller but reliable deficits in other sub-domains. Second, based on the few previous studies of prevalence, we predicted that impairment would be common in one or more sub-domains.
Method
Participants
The Consortium on Cognition and Aging of the Quebec Research Network on Aging recruited 40 MCI patients who met the criteria for testing from seven memory clinics across Quebec. Thirty-two normal elderly controls (NECs) were recruited as controls matched on age, education, and sex. MCI patients were referred by one of the participating physicians as part of their normal clinical work. Informed consent was obtained from all participants and ethical approval for the study was obtained from all institutions involved.
A standardized diagnostic checklist was used at all sites, with a standardized clinical and neuropsychological assessment in French or English. Physicians performed a mental status assessment and a physical evaluation for each patient and confirmed the diagnosis of MCI using agreed-upon criteria (Petersen et al., Reference Petersen, Roberts, Knopman, Boeve, Geda, Ivnik and Jack2009; Winblad et al., Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund and Petersen2004). Patients were not selected a priori to have executive function deficits. In fact, in our MCI sample, 100% were judged by the referring physician based on his/her clinical evaluation to have amnestic MCI, with a primary memory impairment at the time of diagnosis. In 15 patients, memory was judged to be the only domain impaired, and the remaining 25 patients were judged to have deficits in multiple cognitive domains, including memory. Notably, only 6 of the 40 MCI patients (15%) were judged to have executive function or judgment difficulties, based on standard clinical assessment using functional questions, verbal fluency, and problem solving. Thus, our sample was a mixture of single- and multiple-domain aMCI patients, suggesting that many will go on to develop AD. Once a patient was diagnosed with aMCI, they were referred for an extensive cognitive evaluation using the Consortium's neuropsychological battery. To ensure that there was no objective functional impairment, patients were administered the Barthel Index (Mahoney & Barthel, Reference Mahoney and Barthel1965) and the Functional Activities Questionnaire (Pfeffer, Kurosaki, Harrah, Chance, & Filos, Reference Pfeffer, Kurosaki, Harrah, Chance and Filos1982).
NECs were excluded if they demonstrated an abnormal score on either the Mini-Mental State Examination (MMSE < 25; Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975) or the Montreal Cognitive Assessment (MoCA < 26; Nasreddine et al., Reference Nasreddine, Phillips, Bedirian, Charbonneau, Whitehead, Collin and Chertkow2005). The MoCA is a cognitive screening test specifically designed to detect MCI and was included as a screen for control participants because it is more sensitive to detecting MCI than the MMSE (sensitivity = 90%; Nasreddine et al., Reference Nasreddine, Phillips, Bedirian, Charbonneau, Whitehead, Collin and Chertkow2005); thus, we can be confident that the NEC group was cognitively intact. Lastly, NECs were excluded if subjective memory complaints were present (Subjective Memory Complaints Scale >9; Schmand, Jonker, Hooijer, & Lindeboom, Reference Schmand, Jonker, Hooijer and Lindeboom1996).
Exclusion criteria for all participants included evidence of serious health problems, other brain disease such as tumor, Parkinson's disease, or large vessel stroke (evidenced clinically or on computed tomography [CT] scan; magnetic resonance imaging [MRI] assessment was not routinely carried out to assess small vessel disease), medical disorders which might impair cognition (e.g., metabolic dysfunction such as uncontrolled diabetes, thyroid dysfunction, B12/folic acid deficiency, or alcohol abuse), or a chronic psychiatric disorder (other than mild depressive symptoms). For MCI patients, this information was obtained through the physician's physical examination, and for control participants, through self-report.
Table 1 shows group characteristics for demographic and clinical screening variables. As Quebec is a bilingual province and participants were tested in their primary language, the language distribution of the two groups was examined. The MCI group had a significantly higher Francophone-to-Anglophone ratio. Correlations between language and executive measures revealed significant correlations with some Tower of London (TOL) and all Hayling scores, p < .05 (two-tailed). However, when grouped according to language, there were no significant differences between Francophones and Anglophones on the TOL; therefore, language was co-varied in the Hayling test only. To control for the possible effects of mild depression, we used the Geriatric Depression Scale score as a covariate for the tests with which it correlated.
Table 1 Demographic and clinical variables in mild cognitive impairment (MCI) and normal elderly controls (NEC)

Note. MMSE = Mini-Mental State Examination; GDS = Geriatric Depression Scale; SMCS = Subjective Memory Complaints Scale.
Materials and Procedure
MCI patients were tested at the individual clinics; the controls were tested at Concordia University and the Institut Universitaire de Gériatrie de Montréal. To ensure equivalent standards in data collection at the memory clinics, common evaluation tools and standardized procedures were provided. The neuropsychologists, nurses, and graduate students engaged in data collection were trained on test administration and provided with a testing manual detailing the procedures. During data collection, a study coordinator visited the sites to verify that the uniform testing protocol was used.
Participants completed six tests of executive functioning as part of a larger battery administered in a standardized order, which included tests of learning and memory, language, visual-spatial function, attention, and motor praxis. The six measures of executive functioning were: the Brown-Peterson TaskFootnote 1 (BPT; adapted from Belleville, Chatelois, Fontaine, & Peretz, Reference Belleville, Chatelois, Fontaine and Peretz2003), the Letter-Number Sequencing subtest of the Wechsler Adult Intelligence Scale-Third Edition (LNS; Wechsler, Reference Wechsler1997a), the Hayling testFootnote 2 (Burgess & Shallice, Reference Burgess and Shallice1997), the Stroop test (Victoria version; Spreen & Strauss, Reference Spreen and Strauss1998), phonemic and semantic verbal fluency (F, A, S & animals; Spreen & Strauss, Reference Spreen and Strauss1998), and the Tower of LondonFootnote 3 (TOL; Shallice, Reference Shallice1982).
Results
Group Comparison
We treated each neuropsychological test as a separate family of statistical tests and used the appropriate Bonferroni correction for follow-up comparisons. The Huynh-Feldt correction was used for violations of sphericity. Not all participants completed all tests; missing data were primarily due to difficulties performing the task or discontinuation due to fatigue. Statistical comparisons between groups are presented in Table 2. As predicted, MCI patients exhibited reduced performance compared to controls on all measures of executive function administered.
Table 2 Performance of patients with mild cognitive impairment (MCI) and normal elderly controls (NEC) on cognitive measures
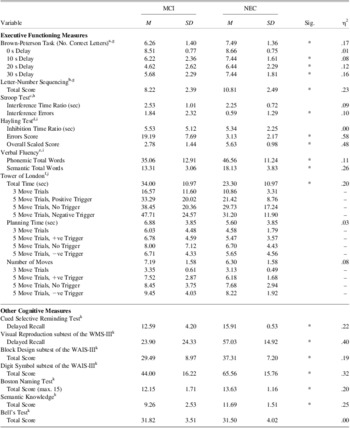
Note. aMixed ANOVA with group (MCI, NEC) as the between-subjects factor and delay (0 s, 10 s, 20 s, 30 s) as the within-subjects factor, significant main effect of group, main effect for delay, and Group × Delay interaction. bUnivariate ANOVA. cInterference time ratio = time for colour (interference) condition divided by time for dot condition. Interference errors = errors for colour (interference) condition minus errors for dot condition. MANOVA, λ(2, 67) = .899, p=.028, η2 = .101. dInhibition time ratio = mean response latency of Section 2 divided by mean response latency of Section 1. Weighted error score was obtained by weighting connected errors by 3 and somewhat connected errors by 1 and summing. Overall scaled score was calculated according to the procedure outlined in Burgess and Shallice (Reference Burgess and Shallice1997). MANOVA with GDS score and language as covariates, λ(3, 63) = .408, p < .001, η2 = .592. eMANOVA with GDS score as a covariate, λ(2, 64) = .706, p < .001, η2 = .294. fTotal time, planning time, and number of moves were analyzed with separate mixed ANOVAs, with group (MCI, NEC) as a between-subjects factor and trial type (N3, N5, T+, T−) as the within-subjects factor. Significant main effect of group for total time and main effects of trial type for all three measures. gMissing data for 3 MCI patients. hMissing data for 2 MCI patients. iMissing data for 4 MCI patients. jMissing data for 8 MCI patients. kMissing data for 1 MCI patient.
* p < .05.
Profile of Executive Functioning
Having demonstrated reliable group differences, it was important to determine the magnitude of the deficits. Therefore, we calculated standardized scores for each MCI patient based on the mean and SD of the control group (Figure 1). Mean standardized scores between −1.0 and −1.5 SDs were considered to reflect mild impairment, scores between −1.5 and −2.0 SDs a moderate impairment, and scores greater than −2.0 SDs a severe impairment.
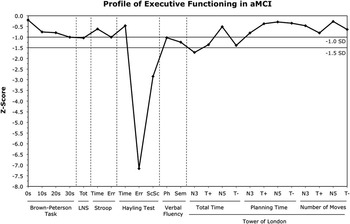
Fig. 1 Average degree of impairment across tests of executive functioning in amnestic mild cognitive impairment (aMCI) in comparison to our sample of normal elderly controls. Tot = total score; LNS = Letter-Number Sequencing; Err = errors scaled score; ScSc = Overall Scaled Score; Ph = phonemic; Sem = semantic; N3 = 3-move trial; T+ = 5-move trial, positive trigger; N5 = 5-move trial, no trigger; T− = 5-move trial, negative trigger.
As shown in Figure 1, in the sub-domain of divided attention, MCI patients were not impaired on the BPT, with the exception of mild impairment in the 30 s delay condition. For working memory, patients exhibited a mild impairment on the LNS. For inhibitory control, MCI patients were not impaired on Stroop time but showed a mild impairment on errors. The greatest magnitude of deficits was observed on the Hayling test, on which severe impairment was found for errors and the overall scaled score. Scores were not impaired for inhibition time. Mild impairment was observed for both phonemic and semantic fluency, with slightly greater impairment on semantic fluency. Finally, patients demonstrated a mild-to-moderate impairment on total time for three TOL trial types (N3, T+, T−). Other TOL variables were not impaired.
Frequency of Impairment
Having identified the mean severity of executive function deficits, it was then important to determine the prevalence of impairment on each test and in each sub-domain. As shown in Figure 2, of those who completed all tests in a given sub-domain, at least half of MCI patients were impaired within each of the sub-domains (>1.0 SD below the mean), with impairment in inhibitory control being the most frequent, followed by fluency, planning, divided attention, and working memory. The majority were also impaired at the −1.5 SD level in all sub-domains. Of the patients who completed all of the tests in this study, 100% were impaired in at least one sub-domain, 96.4% were impaired in two sub-domains or more, 96.4% were impaired in three or more sub-domains, 67.9% were impaired in four or more sub-domains, and 42.9% were impaired in all five sub-domains.
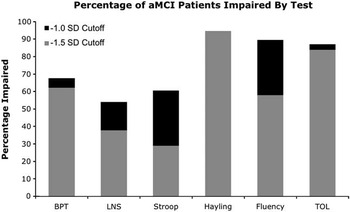
Fig. 2 Frequency of executive impairment in amnestic mild cognitive impairment (aMCI) on each test of executive functioning. BPT = Brown-Peterson Task; LNS = Letter-Number Sequencing; TOL = Tower of London.
Executive Function and Other Cognitive Domains
We computed Pearson product-moment correlations between the executive function tests to determine whether they were indeed measuring distinct aspects of executive function, controlling for Type I error (p < .001). No correlations were significant. It was then important to rule out the possibility that impaired executive function was driven by impairment in other cognitive domains. Thus, we examined its relationship with overall disease severity (measured by the MMSE), memory performance (delayed recall on the Cued Selective Reminding Test; Grober & Buschke, Reference Grober and Buschke1987; and the Wechsler Memory Scale-III Visual Reproduction subtest; Wechsler, Reference Wechsler1997b), and language performance (Boston Naming Test; Mack, Freed, Williams, & Henderson, Reference Mack, Freed, Williams and Henderson1992; Semantic Knowledge Test; Saumier & Chertkow, Reference Saumier and Chertkow2002). We computed the mean Z-scores for tests of executive function, memory, and language, respectively. First, the mean Z-score of delayed recall (M = −4.2; SD = 4.2) was significantly lower than that of the executive function tests (M = −1.94; SD = .89; t(38) = −3.3; p = .002) but these did not correlate (r = −.13; p = .42). Moreover, the mean Z-score of the executive function tests did not correlate with the MMSE (r = −.08; p = .61) or with the mean Z-score of language tests (r = .08; p = .63). Taken together, this suggests that the impairment in executive function was not merely tracking along with impairment in memory or overall disease severity. It is also interesting to note that the mean Z-score for Hayling errors (−7.2) was actually larger than the mean Z-score for delayed recall on the Selective Reminding Test (−6.3), indicating that patients showed deficits at least as severe as on a test of memory. Hayling test performance did not correlate with language function (r = −.04; p = .80), or working memory (LNS; r = .09; p = .61).
Discussion
Our goals were to determine whether amnestic MCI patients (with or without other deficits) exhibited impairment in various sub-domains of executive functioning as well as the severity and frequency of any such impairment. There were reliable and significant group differences on all tests of executive control administered. Strikingly, impairments greater than 1.0 SD below the mean of normal controls were found in all five executive sub-domains. MCI patients showed mild impairment on the most demanding condition involving divided attention (BPT), were mildly impaired on working memory (LNS), verbal fluency, and planning (TOL), and severely impaired on one measure of inhibitory control (Hayling test) and mildly impaired on the other (Stroop). Finally, impairment was frequent on each of the tests of executive functioning, ranging from 54% (LNS) to 95% (Hayling test). Moreover, we found that overall performance on tests of executive functioning was not related to overall disease severity, memory performance, language performance, or demographic variables. Furthermore, impairment on errors on the Hayling test was as severe as on a measure of verbal delayed recall.
Thus, amnestic MCI individuals who were not judged in the clinic to have particular impairments in executive functioning based on functional testing and simple mental status evaluation, in fact performed lower than controls on a variety of executive functioning tests. This finding is consistent with the emerging literature in which executive functioning deficits are increasingly reported in MCI (see the Appendix). Our results question the view that aMCI and early AD predominantly involve deficits in episodic memory, and suggest that executive dysfunction may be an important and underappreciated area of impairment in these disorders. Previous studies have typically not examined multiple sub-domains of executive functioning, and doing so in the present study allowed us to determine that MCI patients perform lower than controls in several areas. The examination of multiple sub-domains also allowed us to compare the sub-domains in terms of severity and frequency of impairment.
We consistently observed a particular deficit in inhibitory control. Specifically, the Hayling test produced the largest effect sizes in the group comparison, the greatest degree of impairment, and the highest frequency of impairment. The Stroop test (our other inhibition test) had substantially smaller effect sizes and a smaller degree and frequency of impairment. This is consistent with findings in AD (Belleville, Rouleau, & Van der Linden, Reference Belleville, Rouleau and Van der Linden2006). The present results indicate that the Hayling test is sensitive to impairment in inhibitory control and could have substantial clinical utility in both the diagnosis of MCI and monitoring treatment efficacy.
Although the two inhibitory tests differed in their sensitivity to deficits in our MCI patients, this could be due to their task demands. The Stroop test explicitly presents both competing response sets to the examinee (i.e., the to-be-ignored word and the target color of the ink). In contrast, on the Hayling test, the examinee is given a strong context that leads to an expected response that must be inhibited and a novel, unrelated response must be generated. The absence of the alternative response may make the inhibition of the habitual response more difficult. We do not believe that the deficit on the Hayling test was caused by difficulty finding an alternative word, as MCI patients did not take longer to produce their responses nor did the deficit correlate with their language performance; they simply did not inhibit the prepotent response (reflected by the high error rate). We also believe that the sensitivity of the Hayling is due to the very low base rate of errors in control participants, thus making the patients’ errors striking.
The very high prevalence of executive dysfunction in our sample of individuals judged clinically to be amnestic MCI patients is striking. Every MCI patient in our cohort was impaired in at least one sub-domain of executive functioning, 96% were impaired in at least two sub-domains, and 43% were impaired in all five sub-domains. This is notable given recent reports (e.g., Jak, Bondi, et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009) showing that requiring impairment on two or more tests within a cognitive domain improved the stability of diagnosis for single and multiple domain aMCI. There is a striking discrepancy between the high prevalence of executive dysfunction we demonstrated with neuropsychological testing and the physicians’ clinical observation at the time of diagnosis, which was only 15%. These results underline the difficulty in testing executive function ability in a clinical setting or “at the bedside,” largely relying on reports of functional impairment and “loss of judgment,” and demonstrate the importance of a thorough neuropsychological evaluation in revealing these problems. Although the current diagnostic guidelines for MCI (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox and Phelps2011) recognize the importance of neuropsychological testing, they acknowledge that cognitive testing may not always be feasible and that bedside testing may not be sensitive to the subtle deficits in MCI or accurately assess cognitive domains other than memory. Furthermore, it is not universally agreed that neuropsychological testing is routinely needed for diagnosis (Petersen, Reference Petersen2011). The results of the present study suggest that deficits in executive functioning may be frequently missed if neuropsychological testing assessing multiple sub-domains of executive function is not conducted.
A thorough understanding of a patient's cognitive functioning is essential for treatment planning and patient care. If executive impairment is frequently present in patients with MCI but unrecognized without adequate assessment, then the opportunity to appropriately council patients and their caregivers will be missed. Several studies have found that executive functioning is related to functional abilities, such as financial and medical decision making, in community-dwelling older adults (Royall et al., Reference Royall, Lauterbach, Cummings, Reeve, Rummans, Kaufer and Coffey2002). Therefore, careful assessment of possible executive dysfunction should be undertaken, and these cognitive abilities could be used to inform patient care with regards to a patient's ability to function in areas of daily life requiring goal-directed behavior, complex decision-making, and judgment. Furthermore, the presence of executive dysfunction is related to decreased abilities to perform activities of daily living in AD (Pereira, Yassuda, Oliveira, & Forlenza, Reference Pereira, Yassuda, Oliveira and Forlenza2008). Thus, the early identification of executive impairments may help to predict long-term functional outcome.
Another potential implication of the high frequency and severity of executive impairment in MCI is the possibility of using tests of executive function to predict conversion to dementia. MCI patients with impairment in another domain in addition to memory have a greater likelihood of progressing to AD (Bozoki et al., Reference Bozoki, Giordani, Heidebrink, Berent and Foster2001) and are more likely to be functionally impaired (Aretouli & Brandt, Reference Aretouli and Brandt2010). The Hayling test in particular may be useful in demonstrating the presence of a non-memory impairment in MCI patients, thus improving prognostic accuracy. Future research should be directed at examining the predictive utility of the Hayling test and other tests of executive functioning for conversion of MCI to dementia.
Although executive dysfunction has been reported in the earliest stages of AD and in MCI, and the present study found deficits in several sub-domains of executive functioning in MCI, the neural substrates of these deficits remain unclear. Although frontal lobe atrophy is not characteristic of MCI or very early AD (Whitwell, Przybelski, et al., Reference Whitwell, Przybelski, Weigand, Knopman, Boeve, Petersen and Jack2007), recent PET studies using PiB uptake as an in vivo amyloid marker have found uptake in frontal cortex (among other areas), with MCI patients showing a bimodal pattern (the majority being similar to AD with a similar distribution, and a minority showing low binding, or binding intermediate between controls and AD; Rabinovici & Jagust, Reference Rabinovici and Jagust2009; Villemagne et al., Reference Villemagne, Fodero-Tavoletti, Pike, Cappai, Masters and Rowe2008). Although some studies have found an association between PiB uptake and episodic memory performance (see Quigley, Colloby, & O'Brien, Reference Quigley, Colloby and O'Brien2011), the relationship between PiB uptake and cognition varies widely within and across groups (Rabinovici & Jagust, Reference Rabinovici and Jagust2009). Thus, the possibility that amyloid pathology in frontal brain areas may relate to our findings is intriguing but speculative at this point.
One possible explanation for executive dysfunction in early AD and MCI is that AD can be characterized as a disconnection syndrome. This is evidenced by neuronal damage in cortico-cortical connections and a loss of coherence in brain activity between anterior and posterior regions and between hemispheres (for reviews, see Bokde, Ewers, & Hampel, Reference Bokde, Ewers and Hampel2009; De Lacoste & White, Reference De Lacoste and White1993; Delbeuck, Van der Linden, & Collette, Reference Delbeuck, Van der Linden and Collette2003; Salmon & Bondi, Reference Salmon and Bondi2009). Therefore, the executive dysfunction seen in AD and MCI may be due to multiple neuropathological and metabolic changes in anterior and posterior regions and/or the classic limbic system which involves both medial temporal and anterior cingulate regions. This loss of anatomical and functional connectivity could explain deficits in cognitive areas that rely on distributed networks connecting different regions, such as executive functions (Delbeuck et al., Reference Delbeuck, Van der Linden and Collette2003; Morris, Reference Morris2004). It is interesting to note that there is some evidence of impairment in AD and MCI on tasks requiring information integration (Festa et al., Reference Festa, Insler, Salmon, Paxton, Hamilton and Heindel2005; Foster, Behrmann, & Stuss, Reference Foster, Behrmann and Stuss1999; Golob, Miranda, Johnson, & Starr, Reference Golob, Miranda, Johnson and Starr2001). Some groups, including ours, are beginning to investigate cortical connectivity and its relation to cognitive functioning in MCI (e.g., Jiang & Zheng, Reference Jiang and Zheng2006; Johns, Davies, & Phillips, Reference Johns, Davies and Phillips2011; Pijnenburg et al., Reference Pijnenburg, v d Made, van Cappellen van Walsum, Knol, Scheltens and Stam2004), but more work is needed in this area.
Two potential limitations of the present study warrant mention. First, we were unable to statistically control for the presence of mild depression in the MCI patients in our analysis of severity and frequency of impairment; thus, it is unknown to what extent mild depression may influence degree or frequency of impairment on the Hayling test or verbal fluency (i.e., the two tests with which GDS scores were correlated). Nevertheless, we believe our approach was clinically valid, as it is common that MCI patients will present with symptoms of mild depression. Second, it would have been desirable to have had a larger sample size, which would have allowed us to undertake a factor analysis of the multiple measures of executive function to better explore the inter-relationships between the sub-domains.
In summary, we demonstrated that impairment is common in amnestic MCI across multiple sub-domains of executive functioning. Significant group differences were found between MCI patients and normal controls on all executive tests administered in this study. Furthermore, impairment was observed in all five sub-domains, and more than half of MCI patients were impaired within each of the sub-domains, with a particularly severe and frequent impairment in inhibitory control (Hayling test). These results indicate that, in addition to impairments in episodic memory, executive impairment is an important aspect of MCI. As noted above, there is a risk that such deficits might be missed in the course of a clinic or bedside assessment and this points to the importance of having a thorough neuropsychological evaluation when resources exist. Importantly, tests of executive functioning, and particularly inhibitory control, should be used in any neuropsychological test battery used to detect and characterize MCI. Our study contributes to the growing discussion of the importance of assessing multiple measures in multiple domains of cognition and the consequent impact on the diagnosis of MCI (Ganguli et al., Reference Ganguli, Snitz, Saxton, Chang, Lee, Vander Bilt and Petersen2011; Schinka et al., Reference Schinka, Loewenstein, Raj, Schoenberg, Banko, Potter and Duara2010). This will help with early and accurate diagnosis, improve case management, and potentially contribute to the identification of persons with a particularly high risk of developing dementia.
Acknowledgments
This research was supported by the Axe Cognition of the Réseau Québecois de Recherche sur le Viellissement funded by the Fonds de la Recherche en Santé du Québec, and by scholarships from the Center for Research in Human Development, Concordia University, and the Canadian Institutes of Health Research awarded to Erin Johns. We thank Diane Goupil for her central role in the organization of this project. We also thank those who kindly volunteered to participate in this study. A portion of these data was previously presented at 36th annual meeting of the International Neuropsychological Society, Waikoloa, Hawaii, February 2008. This research was completed by the first author in partial fulfillment of the requirements for the degree of Master of Arts (Psychology). Dr. Howard Chertkow has been on the Adjudication board or Advisory Board, or has been a grant recipient from Pfizer Canada, Lundbeck Canada, and Bristol Myers Squib. The other authors declare no conflict of interest.
Appendix
Summary of studies for which the primary goal was to characterize neuropsychological function in aMCI patients and which examined performance on at least one test of executive function.