Published online by Cambridge University Press: 01 November 2004
Objectives: The frequency and consequences of major bleeding associated with anticoagulant prophylaxis for prevention of venous thromboembolism is examined.
Methods: We conducted a systematic review and meta-analysis of controlled trials that reported rates of major bleeding after pharmaceutical thromboprophylaxis in patients undergoing major orthopedic surgery. Thromboprophylactic agents were divided into four groups:warfarin/other coumarin derivatives (WARF), unfractionated heparin (UFH), low molecular weight heparin (LMWH), and pentasaccharide (PS). Meta-analysis was conducted comparing LMWH with each of WARF, UFH, and PS. The frequency of re-operation due to major bleeding was reviewed and combined with published costs to estimate the mean cost of managing major bleeding events in these patients.
Results: Twenty-one studies including 20,523 patients met inclusion criteria for the meta-analysis. No evidence of significant between-trial heterogeneity in risk ratios was found. Combined (fixed effects) relative risks (RR) of major bleeding compared with LMWH were WARF – RR 0.59 (95 percent confidence interval [CI], 0.44–0.80); UFH – RR 1.52 (95 percent CI, 1.04–2.23); PS – RR 1.52 (95 percent CI, 1.11–2.09). Seventy-one studies including 32,433 patients were included in the review of consequences of major bleeding. We estimated that the average cost of major bleeding is $113 per patient receiving thromboprophylaxis.
Conclusions: LMWH results in fewer major bleeding episodes than UFH and PS but more than WARF. These events are costly and clinically important.
Venous thromboembolism (VTE) is a common complication of major orthopedic surgery. Patients undergoing total hip replacement (THR), total knee replacement (TKR), and surgery for correction of fracture of the upper femur (hip fracture) are at very high risk of VTE (35). In the absence of prophylaxis, between 40 percent and 80 percent of these patients will experience venographically demonstrable deep vein thrombosis, 4–10 percent will develop clinical pulmonary embolism (PE), and 0.2–5 percent of patients will die from PE (35).
The American College of Chest Physicians recommends that thromboprophylaxis should be routinely used in patients at highest risk of VTE (35). Prophylaxis against VTE with low dose unfractionated heparin, low molecular weight heparins, warfarin, and mechanical methods has been shown to be effective in reducing the incidence of VTE compared with placebo (2;3;9;53;75;79). More recently, pentasaccharide has been introduced into the United States and other countries as a new agent indicated for use in thromboprophylaxis in orthopedic surgery (5;20;57;99). Many orthopedic surgeons now use thromboprophylaxis for at least some of their patients undergoing major procedures (4;35;38).
However, use of anticoagulant drugs for thromboprophylaxis is associated with increased risk over placebo of clinically significant bleeding (64). Bleeding is particularly troublesome in joint replacement surgery, as hemorrhage at the surgical site may require re-intervention and an infected hematoma may threaten the viability of the joint graft. Perceived risk of bleeding may influence the choice of whether to give prophylaxis or influence choice between different prophylactic agents.
Several well-conducted clinical studies have evaluated the effectiveness and safety of thromboprophylactic agents. However, clinically significant bleeding and symptomatic VTE are uncommon, and even large studies may not have sufficient power to detect statistically significant differences between agents in these important events. Meta-analysis combines data from different studies to generate additional statistical power (18) and is an appropriate tool to research the frequency of uncommon events when the consequences of these events are serious. The purpose of our study was to compare rates of major, total, and fatal bleeding associated with different agents, and to examine the consequences and costs of major bleeding.
We conducted a systematic review and meta-analysis of rates of major bleeding (74). We conducted secondary analyses to identify the consequences and estimate the costs of managing major bleeding episodes.
All inclusion criteria for study selection were predefined. To be included in the meta-analysis studies had to: (i) describe patients undergoing major orthopedic surgery, defined as one or more of THR, TKR or hip fracture surgery; (ii) be controlled trials of thromboprophylactic agents; (iii) report rates of major bleeding; (iv) report currently licensed doses and recommended administration regimens of thromboprophylactic agents; (v) include comparisons between two or more of the following agents: low-molecular weight heparins (LMWH), warfarin or another coumarin derivative (WARF), unfractionated heparins (UFH), or pentasaccharide (PS); and, (vi) report rates of major bleeding during the period of acute hospitalization after surgery with up to 14 days of follow-up (i.e., studies reporting out of hospital prophylaxis only were excluded).
Studies were identified by a search conducted in September 2001. Databases searched included Medline; Pubmed; Embase; The Cochrane Library; Oldmedline; National Research Register; Medical Research Council trials; Science Citation Index; Health Technology Assessment database; Current Controlled trials; National Health Service Economic Evaluation Database (NHSEED); Econlit; and Database of Abstracts of Reviews of Effectiveness (DARE). Full details of the search strategy and protocol are available on request from the authors.
Data extraction and quality assessment were carried out by one reviewer and checked by a second reviewer. Any differences were resolved by consensus.
From each study, we extracted data on study setting; description of participants; number of patients; bleeding episodes; and thromboprophylaxis interventions used. We classified interventions into one of five groups: UFH (unfractionated heparin at a dose of 10,000–15,000 IU per day), LMWH (any low molecular weight heparin at a dose licensed in orthopedic surgery), WARF (adjusted dose warfarin or other coumarin derivative), PS (pentasaccharide at a licensed dose), OTH (other).
We abstracted data for major bleeding according to the criteria defined by Hull and colleagues (46): “Bleeding was classified as major if it was overt and associated with a fall in hemoglobin level of 2 grams per deciliter or more, if it led to transfusion of two or more units of blood, if it was retroperitoneal, if it occurred in a major prosthetic joint, or if it was intracranial.” When this definition was not used, we attempted to derive this measure from reported information. If this was not possible, the authors' definition of major bleeding was accepted. In particular, we accepted a Bleeding Index (99) of >2 as constituting a major bleeding event.
We assessed components of methodological quality separately (72;88), including details of randomization (generation of allocation sequence and allocation concealment), blinding, and recording of study withdrawals.
Major bleeding was the primary end point, and total and fatal bleeding were secondary end points. We combined results from individual studies on the risk ratio scale, using a fixed-effects model (Mantel–Haenszel method), and performed tests of heterogeneity (17). We also performed random effects analyses for comparison (DerSimonian and Laird method [17]). We examined publication bias and related biases in funnel plots and carried out tests of funnel plot asymmetry (6;19). Sensitivity analyses examined the importance of the methodological quality of studies, publication status (abstract or full publication), study size (trials with 300 or more patients), and the funding source (i.e., if funded by industry). L'Abbé plots and influence analyses were used to examine heterogeneity and to investigate whether the results are robust to exclusion of individual studies (92). All analyses were performed in Stata version 7.0 (Stata Corporation, College Station, TX).
To explore the consequences and cost implications of major bleeding, we abstracted data on the site of major bleeding (wound site, gastrointestinal [GI], other) and action taken (surgery or medical management) in response to major bleeding events. Analysis of the cost of management of major bleeding included studies which reported: original estimates of cost or resource use, findings for the United States, costs in patients undergoing major orthopedic or other surgery and in which the definition of major bleeding was clearly reported. Data were inflated to 2002 US dollars using the Consumer Price Index to facilitate comparisons between studies.
We multiplied the pooled frequency of surgical and medical management by estimated cost to derive the mean cost of management of a major bleeding event. This value was multiplied by the estimated frequency of major bleeding events, pooled across all groups, to estimate the cost of major bleeding per patient given thromboprophylaxis.
Twenty-one studies including 20,523 patients satisfied the inclusion criteria for the meta-analyses. Three studies were identified as abstracts only: two of which subsequently have been published (57;99) with the other in press (39). Eight published studies reported direct comparisons between WARF and LMWH (11;25;30;42;48;51;52;61). Nine published and one unpublished study reported direct comparisons between UFH and LMWH (12;13;24;31;39;55;59;63;84;96), and four published studies reported direct comparisons between PS and LMWH (5;20;57;99).
Most studies reported major bleeding using the Hull et al. (46) or similar criteria, although not all studies reported rates of minor or total bleeding, and definitions of minor bleeding used were heterogeneous. Fatal bleeding was reported rarely (3/20,523 = 0.01 percent of patients given prophylaxis). All twenty-one studies were included in the meta-analysis.
Compared with LMWH, we estimate the relative risk of major bleeding with WARF to be 0.59 (95 percent CI, 0.44–0.80), with UFH to be 1.52 (1.04–2.23), and with PS to be 1.52 (1.11–2.09). Forest plots corresponding to these analyses (fixed effects models) are shown in Figures 1–3. There was little evidence of between-study heterogeneity (p>.10). Relative risks of total bleeding (all compared with LMWH) were estimated to be 0.77 (0.68–0.88) with WARF, 1.17 (0.92–1.48) with UFH, and 1.27 (1.04–1.55) with PS, again with little evidence of heterogeneity (p>.10). L'Abbé plots did not reveal clear outliers and funnel plots were symmetrical, with statistical tests of funnel plot asymmetry nonsignificant (p>.10). Influence analysis showed little impact of excluding studies one-by-one.

Warfarin/other coumarin derivatives versus low molecular weight heparin, major bleeding. Heterogeneity chi-squared=4.55 (d.f.=6), p=.602.

Unfractionated heparin versus low molecular weight heparin, major bleeding. Test of heterogeneity chi-squared=4.02 (d.f.=8), p=.856.

Pentasaccharide versus low molecular weight heparin, major bleeding. Heterogeneity chi-squared=5.88 (d.f.=3), p=.118.
Hull et al. (51) was identified as a possible outlier in the WARF vs LMWH analysis, as this study demonstrated higher rates of bleeding. Closer examination found that the definition of major bleeding used was consistent with the author's earlier work (46) and no other discrepancies were identified. Removing this study from the analysis resulted in an estimate RR for WARF vs LMWH of 0.60 (0.40–0.90) compared with 0.77 in the main analysis. The random effects results were similar to those from the fixed effects analyses, although confidence limits tended to be wider. Relative risks of major bleeding compared with LMWH were 0.59 for WARF (0.44–0.80), 1.55 for UFH (1.05–2.29), and 1.53 for PS (0.92–2.55). There was little evidence for differences in results according to methodological quality of studies, type of publication, study size, and source of funding. Full results of the sensitivity analyses are available on request from the authors.
Seventy-one studies (32,433 patients), including the twenty-one studies in the meta-analysis, reported rates of major bleeding for at least one of the four agents (1;7;8;10;14–16;21–23;26–29;33;34;36;37;40;41;43;45;47;48;50;54;58;60;62;65;66;68;69;71;73;76;78;80–83;85–87;89;90;93–95;97;98;101). A total of 632 episodes of major bleeding were reported (632/32,433 = 1.95 percent of patients receiving thromboprophylaxis). Only five cases of fatal bleeding were identified.
The location of bleeding was reported for 60 percent (378/632) of major bleeding episodes, of which 71 percent (267/378) were at the wound site, 7 percent (28/378) in the GI tract, and the remainder at other sites. Action taken in response to bleeding was reported for only 19 percent (118/632) of major bleeding episodes, of which 20 percent (24/118) required re-operation at the wound site, 1 percent (1/118) other surgical intervention, and the remainder were managed medically.
We identified fifty-two studies describing costs of major bleeding: thirty-seven were secondary references (referring back to costs from other publications); seven did not report U.S. costs (six European, one South African); eight reported original estimates of the cost of bleeding in the United States. A summary of the studies is given in Table 1.
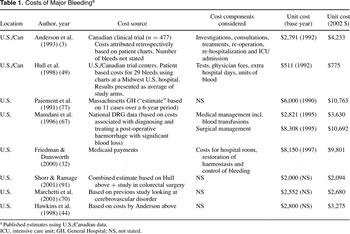
Combining the estimated frequency of surgery and medical management with the estimated cost per event from Mamdani et al. (67) resulted in an estimated cost of management of an episode of major bleeding of $5,801 (2002 prices), equal to approximately $113 per patient receiving prophylaxis.
This systematic review and meta-analysis found significant differences in rates of bleeding after thromboprophylaxis in major orthopedic surgery. Compared with LMWH, WARF resulted in significantly fewer major and total bleeding episodes, UFH resulted in significantly more major bleeding episodes, and PS resulted in significantly more episodes of major and total bleeding.
Limitations of the study include the following: this study considered only the safety end points of major, total, and fatal bleeding and not the efficacy and effectiveness of agents in preventing VTE. Previous, good quality meta-analyses have reported that LMWH significantly reduced VTE compared with WARF (2;79) or UFH (2;3;53;75;79), although one study (56) reported a trend in favor of LMWH versus UFH and WARF, but this finding did not reach statistical significance. Since our analysis was performed, Turpie and colleagues (100) have published a meta-analysis of the four PS studies. The authors found a significant reduction in VTE with PS compared with LMWH and that major bleeding occurred significantly more frequently with PS (5;57).
We were unable to search non-English language sources and, therefore, may not have identified all published studies. This finding will introduce bias only if the results of trials published in other languages differ systematically from trials published in English. We are not aware of significant trials that have not been published in English, but the risk remains that we have overlooked material information.
Our study did not distinguish between different preparations of LMWH, and there may be differences in risk of bleeding, although current evidence is inconclusive (80;82;97). Similarly we did not distinguish between dosing regimens that initiate LMWH before and after surgery or between different dosing schedules for UFH, and we included studies reporting both twice and three times daily dosing. However, older studies reporting the use of adjusted dose UFH were excluded, as adjusted dosing is no longer recommended in prophylaxis (35). Finally, we pooled results for warfarin with those for other coumarin drugs, as we are not aware of any evidence that different coumarin derivatives have substantively different characteristics. We believe these simplifications are justified as they allow us to generate sufficient statistical power to show differences between therapies.
We did not include bleeding rates reported more than 2 weeks after surgery. This means that we have not considered the potential impact on bleeding rates of extending prophylaxis beyond the duration of hospitalization. This strategy is a particular limitation in the interpretation of the warfarin findings. Warfarin prophylaxis in practice often continues for more than 14 days (38), potentially extending the time for which patients are at elevated risk of bleeding. Extended warfarin regimens were not reported in the literature we reviewed, and insofar as extended prophylaxis is used in practice, the relevance of our finding may be ques-tioned.
Bleeding rates used in this analysis were those observed in the clinical trial setting. We included all controlled studies that reported major bleeding, in an attempt to include studies with pragmatic designs that may have been excluded from other analyses. However, clinical trials typically report data from selected patient populations, who are experiencing close monitoring in close to ideal circumstances. In ordinary practice bleeding rates may be increased by practical problems with administration and monitoring of prophylaxis and with food or drug interactions.
Finally, the estimate of the cost of major bleeding relied on published cost data from a group of patients not included in the main analysis and should be viewed as an order of magnitude rather than a definitive cost finding. As major bleeding events are infrequent in these patients, primary analysis of cost would have been a substantial undertaking, beyond the scope of this project. We believed that the included analysis was justified as it provides an indication of the scale of the economic consequences of major bleeding in these patients.
This analysis has shown that rates of major and total bleeding vary between classes of agents. These data and the costs of managing bleeding events should be taken into account by health-care decision-makers when selecting the appropriate method of thromboprophylaxis to be used.
This analysis considers in detail the risk and consequences of bleeding after prophylaxis against VTE. However, the economic implications of VTE prophylaxis also include expenditure on drugs and related administration and monitoring. Choice of prophylactic regimen should also consider impact on the occurrence and costs of management of VTE events and their sequelae. A full systematic review of all these factors was beyond the scope of this review. It is our hope that the findings of this review will be of use to other authors in subsequent economic analyses of VTE prophylaxis.
We thank Liz Payne of the Wessex Institute for Health Research & Development, University of Southampton, United Kingdom, for information support. We thank Professor Sylvia Haas, Institute for Experimental Oncology and Therapeutic Research, Technical University Munich, Germany, for provision of data in press. This analysis was funded by a grant from Aventis Pharma, Inc. The funding source had no role in study design, data collection, data analysis, data interpretation, or in the writing of the report.

Warfarin/other coumarin derivatives versus low molecular weight heparin, major bleeding. Heterogeneity chi-squared=4.55 (d.f.=6), p=.602.

Unfractionated heparin versus low molecular weight heparin, major bleeding. Test of heterogeneity chi-squared=4.02 (d.f.=8), p=.856.

Pentasaccharide versus low molecular weight heparin, major bleeding. Heterogeneity chi-squared=5.88 (d.f.=3), p=.118.

Costs of Major Bleedinga