The commercially available Echium oil is extracted from the seeds of Echium plantagineum, an herbaceous plant of the Boraginaceae family. E. plantagineum oil (EPO) is characterised by a distinctive fatty acid (FA) profile, being rich in α-linolenic (18:3 n-3, all cis-9,12,15; ALA), stearidonic (18:4 n-3, all cis-6,9,12,15; SDA) and γ-linolenic (18:3 n-6, all cis-6,9,12; GLA) acids (about 30, 13 and 10% of total FA, respectively) (Kitessa et al. Reference Kitessa, Nichols, Abeywardena, Preedy, Watson and Patel2011).
SDA and GLA are intermediate products of the metabolic pathways of n-3 and n-6 polyunsaturated fatty acids (PUFA), respectively. Both SDA and GLA are directly and respectively formed from ALA and linoleic acid (18:2 n-6, all cis-9,12; LA), the precursors of the n-3 and n-6 PUFA series. This first step of both metabolic pathways involves the enzyme Δ6-desaturase (EC 1·14·19·3) and it is known to be rate-limiting (Patterson et al. Reference Patterson, Wall, Fitzgerald, Ross and Stanton2012). For this reason, it lowers the overall efficiency of ALA and LA conversion to longer chain PUFA [such as eicosapentaenoic (20:5 n-3, all cis-5,8,11,14,17; EPA), docosahexaenoic (22:6 n-3, all cis-4,7,10,13,16,19; DHA) and dihomo-γ-linolenic (20:3 n-6, all cis-8,11,14; DGLA) acids] which have been associated with numerous health benefits in humans (Swanson et al. Reference Swanson, Block and Mousa2012; Wang et al. Reference Wang, Lin and Gu2012).
Few published trials have attempted to include EPO in livestock rations mainly as a way to increase the content of very long-chain (VLC) n-3 PUFA in animal-derived food products, trying to meet levels recommended for optimal human health (Kris-Etherton et al. Reference Kris-Etherton, Grieger and Etherton2009; Walker et al. Reference Walker, Jebb, Calder and Phil2013). Regarding ruminants, promising results in raising EPA in cow milk fat can be obtained protecting EPO against ruminal biohydrogenation (Kitessa & Young, Reference Kitessa and Young2011). When ruminally unprotected, unsaturated fatty acids (UFA) in EPO are expected to be largely biohydrogenated, and other different associated health benefits can be awaited. In vitro investigations of SDA metabolism by bovine rumen microbial populations showed, for example, the possibility to increase sharply the concentration of the biohydrogenation intermediate vaccenic acid (18:1 trans-11, VA) (Maia et al. Reference Maia, Correia, Alves, Fonseca and Cabrita2012) which is known to be largely converted to the beneficial (Lock et al. Reference Lock, Kraft, Rice, Bauman, Destaillats, Sébédio, Dionisi and Chardigny2012) rumenic acid (18:2 cis-9trans-11, RA) within the ruminant mammary gland (Bernard et al. Reference Bernard, Leroux, Chilliard and Ntambi2013) and human tissues (Niwińska, Reference Niwińska2010).
EPO effects on lipid metabolism have been investigated only using dairy cow as the model (Kitessa & Young, Reference Kitessa and Young2011; Bainbridge et al. Reference Bainbridge, Lock and Kraft2015). However, remarkable inter-species differences are known to exist in milk production and milk composition responses to dietary lipid supplementations (Bernard et al. Reference Bernard, Leroux, Chilliard and Bosze2008; Toral et al. Reference Toral, Chilliard, Rouel, Leskinen, Shingfield and Bernard2015). Moreover, despite the abundant published information on including vegetable and marine lipids in dairy goat diets, no information is available on in vivo ruminal biohydrogenation of dietary SDA and GLA and on their effects on goat milk production and composition. The current study aimed therefore to investigate the effects of ruminally unprotected EPO on performance and milk FA composition in mid-lactation goats. Particular emphasis was purposely given on biohydrogenation intermediates and specific FA known for their putative favourable or adverse effects on human health.
Materials and methods
Animals and dietary treatments
The experiment was carried out in a farm located in None (Torino province, Piedmont, NW Italy) from May 5 to June 17, 2014. Twenty-four terziparous Camosciata goats in mid-lactation were selected from a flock of 80 lactating goats and allocated to two balanced groups of 12 animals each, according to their stage of lactation (89 ± 5·8 d in milk), milk yield (2·2 ± 0·22 kg/head per day), milk gross composition (fat, protein, and lactose contents), FA profile of milk fat, and mammary Δ9-desaturase (EC 1·14·19·1) activity (estimated by the computation of different desaturase indexes: DI14 = 14:1 cis-9/14:0; DI16 = 16:1 cis-9/16:0; DI18 = 18:1 cis-9/18:0). The groups were then randomly assigned to a control or an experimental diet. The control group (CON) was fed mixed hay ad libitum and 0·800 kg/head per day of a commercial concentrate containing corn, partially dehulled sunflower meal, roasted dehulled soybean meal, wheat bran, barley, soybean hulls, roasted soybean, dehydrated alfalfa, cane molasses, calcium carbonate, precipitated dicalcium phosphate dihydrate, sodium bicarbonate, sodium chloride, magnesium oxide, and sulphur. The concentrate was administered in equal amounts during milkings, at 7·00 and 19·00. The other group (EPO group, Echium) received the same diet but, during the morning milking and immediately before feeding, the concentrate was supplemented with 40 ml/head per day of human food grade EPO, unprotected against ruminal biohydrogenation (Elixarome Botanicals, Tonbridge, Kent, UK).
All selected goats were housed indoors in individual pens and had free access to water.
Feed intake, sampling and analysis
Orts were weighed daily to estimate feed intake. Representative samples of hay and concentrate were collected at 11, 22, 33, and 44 d after the beginning of EPO supplementation and ground with a cutting mill to pass a 1-mm screen sieve (Pulverisette 15 – Fritsch GmbH, Idar-Oberstein, Germany). AOAC (2000) procedures were used to determine dry matter (DM, method no. 930·15), ash (method no. 942·05), crude protein (CP, method no. 984·13), ether extract (EE, method no. 920·39), acid detergent fibre and acid detergent lignin (ADF and ADL, method no. 973·18). Neutral detergent fibre (NDF) was analysed according to Van Soest et al. (Reference Van Soest, Robertson and Lewis1991); α-amylase (Sigma Aldrich, Saint Louis, MO, USA), but no sodium sulphite, was added, and results were corrected for residual ash content.
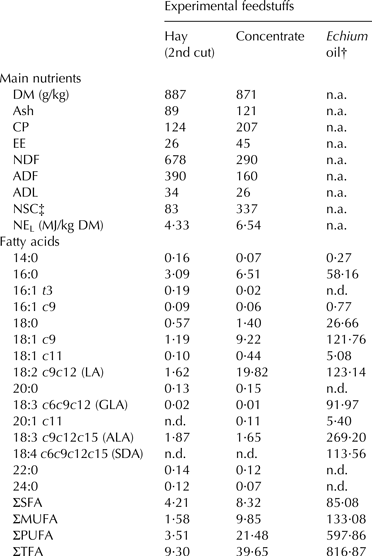
The FA composition of hay, concentrate and EPO was assessed using a combined direct transesterification and solid-phase extraction method as described by Alves et al. (Reference Alves, Cabrita, Fonseca and Bessa2008). Separation, identification and quantification of fatty acid methyl esters (FAME) were performed as described by Renna et al. (Reference Renna, Gasmi-Boubaker, Lussiana, Battaglini, Belfayez and Fortina2014). All analyses were performed in duplicate. The proximate composition of hay and concentrate and the FA profile of the feedstuffs are presented in Table 1.
Table 1. Proximate composition (g/kg DM, unless otherwise stated) and fatty acid profile (g/kg DM) of the experimental feedstuffs

DM, dry matter; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; NSC, nonstructural carbohydrates; NEL, net energy for lactation; n.a., not analysed; n.d., not detected; LA, linoleic acid; GLA, γ-linolenic acid; ALA, α-linolenic acid; SDA, stearidonic acid; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; TFA, total fatty acids
† Fatty acids expressed as g/kg sample
‡ Calculated as 1000 − (NDF + CP + EE + ash)
Milk sampling and analysis
Individual milk yield was recorded and individual composite samples (n = 96) of morning and afternoon (1:1) milkings were collected following the same schedule as described for feed samples collection. Each milk sample was divided into two aliquots, immediately stored at 4 °C in a portable refrigerator and transported to the laboratory. The first aliquot (50 ml) was analysed for fat, protein, lactose and urea (MilkoScan FT 6000, Foss Electric, Hillerød, Denmark). The second aliquot (150 ml) was frozen at −80 °C and successively analysed for the FA composition. Lipid extraction, and FAME separation, identification and quantification were performed as detailed in Renna et al. (Reference Renna, Cornale, Lussiana, Malfatto, Fortina, Mimosi and Battaglini2012). All analyses were performed in duplicate.
Statistical analysis
The changes in dry matter intake (DMI), milk yield, and milk composition were analysed using the MIXED procedure of the Statistical Analysis System (SAS, 2008) for repeated measures over time. The goat was considered as the experimental unit. Compound symmetry, first order autoregressive or unstructured covariance structure, according to the smallest Schwarz Bayesian information criterion, was applied (Littell et al. Reference Littell, Henry and Ammerman1998). The following model was used:
where Y ijk = mean of response variable, μ = population mean, DTi = fixed effect of dietary treatment, G (i)j = random effect of goat within the treatments, STk = fixed effect of sampling time, (DT × ST)ik = fixed effect of interaction between dietary treatment and sampling time, and εijk = experimental error.
Significance was declared at P ⩽ 0·05. Results of statistical analysis are reported as estimate least-squares means.
Results
Milk yield and gross composition
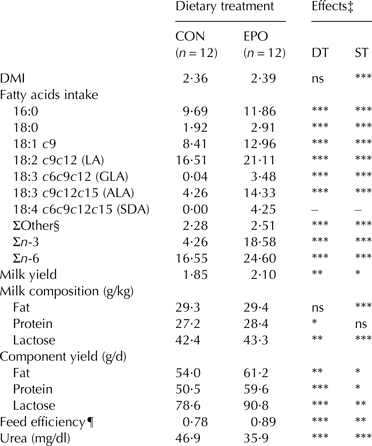
The dietary treatment had no significant effect on DMI. Intakes of EPO-derived FA were exclusive for EPO goats (SDA, 4·25 g/d) or significantly higher in EPO than CON goats (ALA: 14·32 vs. 4·25 g/d; GLA: 3·48 vs. 0·04 g/d; P ⩽ 0·001; Table 2). The EPO supplementation enhanced milk yield (+14%, P ⩽ 0·01). Protein and lactose contents were also significantly higher in EPO than CON milk (+1·2 g/kg, P ⩽ 0·05 and +0·9 g/kg, P ⩽ 0·01, respectively), while milk fat content remained unaffected by treatment. The supplementation increased milk fat (+13%, P ⩽ 0·01), protein (+18%, P ⩽ 0·001) and lactose (+16%, P ⩽ 0·001) yields and decreased milk urea (−23%, P ⩽ 0·001) (Table 2).
Table 2. Mean values of dry matter intake (kg/d), fatty acids intake (g/d), milk yield (kg/d) and milk main constituents of goats fed the control (CON) and Echium oil (EPO) supplemented diets†

n, number of samples; DT, dietary treatment; ST, sampling time; DMI, dry matter intake; LA, linoleic acid; GLA, γ-linolenic acid; ALA, α-linolenic acid; SDA, stearidonic acid
† Total number of samples analysed for each group equal to 48 (12 goats × 4 sampling days)
‡ *** P ⩽ 0·001; ** P ⩽ 0·01; * P ⩽ 0·05; ns, not significant (P > 0·05). The effect of interaction between dietary treatment and sampling time (DT × ST) was not significant; therefore significance is only presented for main effects
§ 14:0, 16:1 t3, 16:1 c9, 18:1 c11, 20:0, 20:1 c11, 22:0, 24:0
¶ Calculated as: milk yield (kg)/dry matter intake (kg)
Milk, fat and protein yields significantly decreased over time in both experimental groups (P ⩽ 0·05; data not shown). The DT × ST interaction was not significant for the considered parameters.
Milk fatty acid composition
The EPO supplementation significantly affected the FA composition of goat milk fat (Tables 3–5).
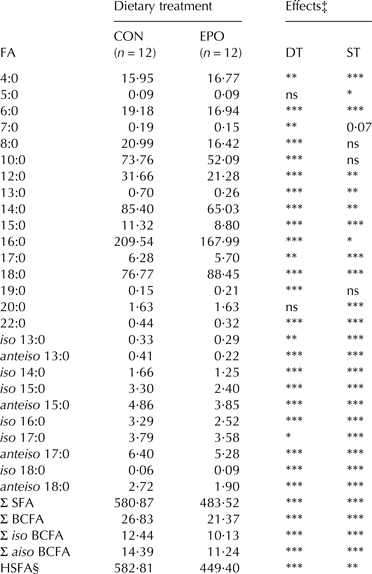
Table 3. Mean contents (g/kg fat) of saturated (SFA) and branched-chain fatty acids (BCFA) in milk fat of goats fed the control (CON) and Echium oil (EPO) supplemented diets†

n, number of samples; DT, dietary treatment; ST, sampling time; HSFA, hypercholesterolaemic saturated fatty acids
† Total number of samples for each group equal to 48 (12 goats × 4 sampling days)
‡ P ⩽ 0·001; **P ⩽ 0·01; *P ⩽ 0·05; ns, not significant (P > 0·05). The effect of interaction between dietary treatment and sampling time (DT × ST) was not significant; therefore significance is only presented for the main effects
§ Calculated as: 12:0 + 4 × 14:0 + 16:0
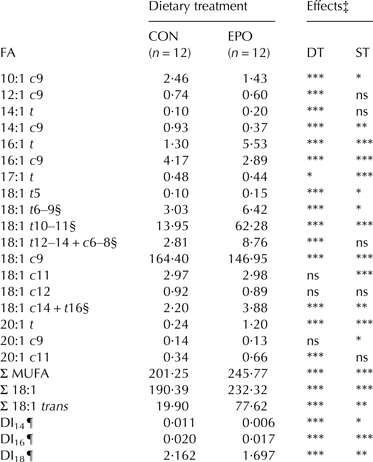
Table 4. Mean contents (g/kg fat) of monounsaturated fatty acids (MUFA) in milk fat of goats fed the control (CON) and Echium oil (EPO) supplemented diets†

n, number of samples; DT, dietary treatment; ST, sampling time; c, cis; t, trans
† Total number of samples for each group equal to 48 (12 goats × 4 sampling days)
‡ P ⩽ 0·001; **P ⩽ 0·01; *P ⩽ 0·05; ns, not significant (P > 0·05). The effect of interaction between dietary treatment and sampling time (DT × ST) was not significant; therefore significance is only presented for the main effects
§ Co-eluted in the applied chromatographic conditions
¶ DI14: 14:1 cis-9/14:0; DI16: 16:1 cis-9/16:0; DI18: 18:1 cis-9/18:0
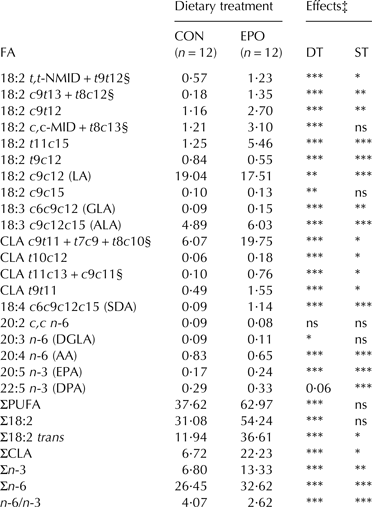
Table 5. Mean contents (g/kg fat) of individual polyunsaturated fatty acids (PUFA) in milk fat of goats fed the control (CON) and Echium oil (EPO) supplemented diets†

n, number of samples; DT, dietary treatment; ST, sampling time; c, cis; t, trans; NMID, non-methylene interrupted diene; MID, methylene interrupted diene; LA, linoleic acid; GLA, γ-linolenic acid; ALA, α-linolenic acid; CLA, conjugated linoleic acid; SDA, stearidonic acid; DGLA, dihomo-γ-linolenic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid
† Total number of samples for each group equal to 48 (12 goats × 4 sampling days)
‡ ***P ⩽ 0·001; **P ⩽ 0·01; *P ⩽ 0·05; ns, not significant (P > 0·05). The effect of interaction between dietary treatment and sampling time (DT × ST) was not significant; therefore significance is only presented for the main effects
§ Co-eluted in the applied chromatographic conditions
Total saturated fatty acids (SFA) and all individual SFA from 6 to 17 carbon atoms were significantly reduced (SFA: −17%, P ⩽ 0·001), while milk concentration of stearic acid (18:0) was increased (+15%, P ⩽ 0·001) as a consequence of EPO supplementation. The hypercholesterolaemic saturated fatty acids (HSFA) index (calculated as: 12:0 + 4 × 14:0 + 16:0) was significantly lower (−23%, P ⩽ 0·001) in EPO if compared to CON milk. Odd- and branched-chain fatty acids (OBCFA) generally showed diminished responses to EPO supplementation. Total iso and total anteiso BCFA were reduced by 19% and 22%, respectively (P ⩽ 0·001).
All detected trans-octadecenoic isomers showed remarkable increases in EPO milk if compared to CON milk (P ⩽ 0·001), with the greatest modifications observed for the sum of 18:1 trans-10 and trans-11 isomers (+346%). The raise in total trans-octadecenoic isomers was equal to 290% (P ⩽ 0·001). On the contrary, all monounsaturated fatty acids (MUFA) with a cis-9 double bond (with the exception of C20:1 cis-9) significantly decreased as a consequence of EPO inclusion in the diet (P ⩽ 0·001). Mammary Δ9-desaturase activity, estimated by desaturase indexes (DI14, DI16 and DI18), was inhibited by EPO. On a whole, the concentration of total MUFA was significantly higher (+22%, P ⩽ 0·001) in EPO milk.
Total PUFA was also enhanced by the lipid supplementation (+67%, P ⩽ 0·001). The concentrations of conjugated linoleic acid (CLA) isomers and non-conjugated trans-octadecadienoic acids [both methylene interrupted (MID) and non-methylene interrupted (NMID) dienes)] were significantly higher (from +116 to +660%) in EPO than CON milk (P ⩽ 0·001). Regarding n-6 PUFA, milk concentration of LA was significantly lower (−8%, P ⩽ 0·01), while both GLA (+67%, P ⩽ 0·001) and DGLA (+22%, P ⩽ 0·05) were significantly higher in EPO than CON milk. The concentration of total n-3 FA was raised by about 96% in EPO milk if compared to CON milk (P ⩽ 0·001). A remarkable increase was observed for SDA (more than +1000%, P ⩽ 0·001). ALA and EPA increased by about 23% and 41%, respectively (P ⩽ 0·001). DPA showed a tendency towards higher concentrations in EPO if compared to CON milk (+14%, P = 0·06), while DHA was undetectable in all analysed milk samples. The EPO supplementation significantly decreased the n-6/n-3 FA ratio of goat milk fat (−36%; P ⩽ 0·001).
In the EPO group, the apparent transfer rate of EPO-derived FA (GLA, ALA and SDA) from feed into milk fat [calculated as the ratio between the FA secreted in milk and its level provided by the diet (%); Bernard et al. Reference Bernard, Rouel, Leroux, Ferlay, Faulconnier, Legrand and Chilliard2005] was very low: 0·3% for GLA, 2·7% for ALA, and 1·7% for SDA.
Discussion
Milk yield and gross composition
Dairy goat response to lipid supplementation is modulated by type of fat, inclusion level and basal diet composition (e.g., forage type, forage to concentrate ratio). Goat milk yield is reported to increase at low dietary inclusion of extra fat and then to decrease from higher (about 5% DM) inclusion levels onwards (Martínez Marín et al. Reference Martínez Marín, Pérez Hernández, Pérez Alba, Carrión Pardo, Garzón Sigler and Gómez Castro2013). In the present trial, the level of lipid supplementation was relatively low (1·6% diet DM) and the observed increase in milk yield in the EPO supplemented goats was most likely the consequence of the higher energy concentration of the diet.
The inclusion of plant and marine oils in goat diets shows contradictory results regarding the milk fat response. Usually, unprotected plant lipids enhance milk fat concentration and secretion (Martínez Marín et al. Reference Martínez Marín, Pérez Hernández, Pérez Alba, Carrión Pardo, Garzón Sigler and Gómez Castro2013). Conversely, unprotected marine oils (used alone or in combination with plant lipids) are reported to exert negative (Kitessa et al. Reference Kitessa, Gulati, Ashes, Fleck, Scott and Nichols2001; Bernard et al. Reference Bernard, Leroux, Rouel, Delavaud, Shingfield and Chilliard2014) or non-significant (Tsiplakou & Zervas, Reference Tsiplakou and Zervas2013; Toral et al. Reference Toral, Rouel, Bernard and Chilliard2014) effects on milk fat content and/or yield in this species. The results obtained in the present trial showed that EPO supplementation did not alter milk fat content, but its daily yield was increased as consequence of the enhanced milk yield. Moreover, it is known that a significant availability and uptake of stearic acid is an important factor for milk fat secretion in goats (Bernard et al. Reference Bernard, Leroux, Chilliard and Bosze2008) and the observed positive effect of EPO supplementation on fat yield could be partly related to the increased availability of 18:0 from dietary UFA biohydrogenation within the rumen.
Oilseeds, plant oils and fish oils are reported to exert variable effects on milk protein response in goats, with the majority of observations showing unchanged or increasing effects (Martínez Marín et al. Reference Martínez Marín, Pérez Hernández, Pérez Alba, Carrión Pardo, Garzón Sigler and Gómez Castro2013; Tsiplakou & Zervas, Reference Tsiplakou and Zervas2013; Bernard et al. Reference Bernard, Leroux, Rouel, Delavaud, Shingfield and Chilliard2014; Toral et al. Reference Toral, Rouel, Bernard and Chilliard2014). In this trial, the increase in milk protein in EPO goats could be ascribed to small differences in energy balance between groups and/or to the defaunating effect of PUFA provided by EPO on rumen protozoa, which may have determined an increase of microbial protein availability in the intestine (Nudda et al. Reference Nudda, Battacone, Atzori, Dimauro, Rassu, Nicolussi, Bonelli and Pulina2013; Cozma et al. Reference Cozma, Andrei, Pintea, Miere, Filip, Loghin and Ferlay2015).
The comparison of our results with those from dairy cows trials (Kitessa & Young, Reference Kitessa and Young2011; Bainbridge et al. Reference Bainbridge, Lock and Kraft2015) highlighted, as expected, remarkable differences between the two species, with cows showing unchanged or diminished response of milk yield and main constituents to dietary supplementation of ruminally protected EPO (Kitessa & Young, Reference Kitessa and Young2011; Bainbridge et al. Reference Bainbridge, Lock and Kraft2015).
Milk fatty acid composition
This trial provided new information on both the effects of vegetable lipid supplementation on goat milk FA and in vivo biohydrogenation of SDA and GLA.
The observed significant reduction of the concentration of medium-chain SFA in EPO milk, due to inhibitory effects of dietary PUFA on FA synthesis de novo, is favourable from a human health perspective (Siri-Tarino et al. Reference Siri-Tarino, Sun, Hu and Krauss2010) and in agreement with the majority of previously published trials evaluating the effects of dietary lipid supplementation in goats (Chilliard et al. Reference Chilliard, Glasser, Ferlay, Bernard, Rouel and Doreau2007).
PUFA are also known to exert inhibitory effects on the rumen microbiota. As odd- and branched-chain FA derive from microbial synthesis (Fievez et al. Reference Fievez, Colman, Castro-Montoya, Stefanov and Vlaeminck2012), dietary lipid supplementations are usually expected to decrease OBCFA concentration in ruminant milk fat. Studying SDA metabolism by mixed ruminal microorganisms in vitro, Maia et al. (Reference Maia, Correia, Alves, Fonseca and Cabrita2012) showed a quadratic decrease of OCFA and a linear decrease of BCFA in fermentation contents associated with increasing levels of SDA supplementation. Such findings are also reflected in our trial, as the milk of goats supplemented with EPO showed significantly lower concentrations of OBCFA if compared to the CON group.
In vitro and in vivo studies also suggest that the main EPO-derived FA (ALA, SDA and GLA) are largely biohydrogenated by rumen microbes (Lee & Jenkins, Reference Lee and Jenkins2011; Alves et al. Reference Alves, Maia, Bessa, Fonseca and Cabrita2012; Maia et al. Reference Maia, Correia, Alves, Fonseca and Cabrita2012). Consistent with the rumen biohydrogenation pathways of these FA (Kemp & Lander, Reference Kemp and Lander1983; Lee & Jenkins, Reference Lee and Jenkins2011; Alves et al. Reference Alves, Maia, Bessa, Fonseca and Cabrita2012), we observed significant increases in the concentrations of both trans-octadecadienoic and trans-octadecenoic isomers in EPO milk if compared to CON milk. Particularly, vaccenic acid is one of the major FA formed during ALA, SDA and GLA biohydrogenation, which is confirmed by the large increase occurred for 18:1 trans-10 and trans-11 isomers (their sum reaching about 8% of total detected FA in milk fat) in our trial. According to known ruminant species specificities (larger increases in trans-FA in lipid-supplemented goats than cows; Bernard et al. Reference Bernard, Leroux, Chilliard and Ntambi2013), such response by goats was higher if compared to that obtained in dairy cows fed lipid encapsulated EPO (Bainbridge et al. Reference Bainbridge, Lock and Kraft2015) or ruminally infused with SDA-enriched soybean oil (Bernal-Santos et al. Reference Bernal-Santos, O'Donnell, Vicini, Hartnell and Bauman2010). From a human health point of view, this is favourable as vaccenic acid is known to exert both direct and indirect (via conversion to rumenic acid) benefits (Field et al. Reference Field, Blewett, Proctor and Vine2009; Lim et al. Reference Lim, Oh, Wang, Lee, Kim, Kim and Lee2014).
The observed inhibitory effects of EPO supplementation on the estimated Δ9-desaturase activity is consistent with the hypothesised negative transcriptional or post-transcriptional regulation of Stearoyl-CoA Desaturase gene expression by dietary PUFA and/or their ruminal biohydrogenation intermediate products (e.g., 18:2 trans-9trans-11) in dairy goats (Bernard et al. Reference Bernard, Leroux, Chilliard and Ntambi2013; Toral et al. Reference Toral, Chilliard, Rouel, Leskinen, Shingfield and Bernard2015). Dietary PUFA biohydrogenation and conversion of 18:1 trans-7 and 18:1 trans-11 to 18:2 trans-7cis-9 and 18:2 cis-9trans-11 (respectively) within the mammary gland led to a marked increase in the sum of these CLA isomers in EPO milk if compared to CON milk. On a whole, total CLA approached 2·8% of total FA in EPO milk (corresponding to about 65 mg per 100 g of milk).
Despite the highly significant increase in milk concentration of ALA, SDA and GLA in EPO if compared to CON goats, the apparent transfer rate of these FA from feed to goat milk fat in the EPO group was very low, confirming data previously obtained with dairy cows (Bernal-Santos et al. Reference Bernal-Santos, O'Donnell, Vicini, Hartnell and Bauman2010; Bainbridge et al. Reference Bainbridge, Lock and Kraft2015). Differently from EPO-fed dairy cows, in the current trial GLA showed a lower transfer rate than ALA and SDA, which may indicate differences among PUFA of the n-3 and n-6 series in the caprine species; such finding needs additional investigations to obtain further confirmation.
Overall, the observed low transfer rates indicates as well that only low amounts of EPO-derived PUFA were available for carbon chain elongation. Goat milk concentrations of beneficial VLC PUFA (such as DGLA, EPA and DPA) significantly or tended to increase with EPO treatment, but their sum remained very low (about 2 mg/100 g of milk) due to the extensive ruminal biohydrogenation. The observed lack of DHA response is consistent with previous findings in the bovine species (regardless of lipid protection) and monogastrics (Kitessa & Young, Reference Kitessa and Young2009, Reference Kitessa and Young2011; Bernal-Santos et al. Reference Bernal-Santos, O'Donnell, Vicini, Hartnell and Bauman2010; Bainbridge et al. Reference Bainbridge, Lock and Kraft2015) and may be ascribed to a competition between ALA and 24:5 n-3 for Δ6-desaturase (Patterson et al. Reference Patterson, Wall, Fitzgerald, Ross and Stanton2012; Bainbridge et al. Reference Bainbridge, Lock and Kraft2015).
The sampling time significantly affected many of the detected FA. The majority of these changes were of small entity and occurred during the experimental period (weeks 14–21 into lactation) without any clear increasing or decreasing trend (data not shown).
Conclusion
Supplementing a high forage diet with EPO at 1·6% DM is useful to increase goat milk yield and milk protein content, as well as to improve the overall goat milk FA profile. Significant reductions of the hypercholesterolemic medium-chain SFA and of the n-6/n-3 FA ratio, significant increases of beneficial VLC PUFA and several-fold enrichment of rumenic and vaccenic acids were observed. However, without proper ruminal protection against biohydrogenation, apparent transfer rates of EPO-derived FA to goat milk fat remain very low (<3%). Consequently, ruminally unprotected EPO cannot be considered a valuable strategy to develop goat functional dairy products enriched with VLC n-3 PUFA for human consumption.
This research was supported by a ‘‘University of Torino (ex 60%)’’ grant (Es. fin. 2013–2014). The authors gratefully acknowledge Mrs. Vanda Malfatto and Dr Eleonora Caro for technical support and the farmers for assistance and care of animals.