INTRODUCTION
The quokka, Setonix brachyurus, is a vulnerable, small macropodid marsupial similar in appearance to a wallaby or kangaroo. It is endemic to Western Australia (WA), but has faced widespread decline since the arrival of the European red fox (Vulpes vulpes) (Hayward et al. Reference Hayward, De Tores and Banks2005). The present distribution of the quokka includes a number of sites on mainland WA, ranging from the Darling Plateau near Perth to the Green Range on the south coast east of Albany and two offshore islands, Bald Island and Rottnest (De Tores et al. Reference De Tores, Hayward, Dillon and Brazell2007). Rottnest Island is located 18 km off the coast of WA and has been an important local holiday destination for over 50 years. Quokkas are the only native marsupial to inhabit Rottnest Island and a close relationship has developed between tourists and quokkas (Hart et al. Reference Hart, Bradshaw and Iveson1985; Sinclair, Reference Sinclair1998). To date there is little available information on the potential impact of pathogenic parasites on the health of native wildlife in Australia. Recent studies on quokkas captured from Two Peoples Bay in Albany and on Bald Island have identified trypanosomes in quokka blood samples, while epimastigote, trypomastigote, sphaeromastigote and promastigote stages were identified from in vitro culture (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009, Reference Austen, Ryan, Friend, Ditcham and Reid2011). This native Australian species of trypanosome recently identified and named Trypanosoma copemani is known to be infective to a variety of Australian marsupials including the critically endangered Gilbert's potoroo, common wombat (Vombatus ursinus), koalas (Phascolarctos cinereus), woylies (Bettongia penicillata), southern brown bandicoot (Isoodon obesulus), tiger quoll (Dasyurus maculatus) and common brushtail possum (Trichosurus vulpecula) (Noyes et al. Reference Noyes, Stevens, Teixeira, Phelan and Holz1999; Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009; McInnes et al. Reference McInnes, Hanger, Simmons, Reid and Ryan2010; Botero et al. Reference Botero, Thompson, Peacock, Clode, Nicholls, Wayne, Lymbery and Thompson2013; Thompson et al. Reference Thompson, Botero, Wayne, Godfrey, Lymbery and Thompson2013). Importantly, as these animals have been shown to be infected with T. copemani, studies by McInnes et al. (Reference McInnes, Gillett, Hanger, Reid and Ryan2011) and Botero et al. (Reference Botero, Thompson, Peacock, Clode, Nicholls, Wayne, Lymbery and Thompson2013) have shown that T. copemani is associated with pathological impacts on both koalas and woylies. Significantly low packed cell volume (PCV) and regenerative anaemia were reported from koalas infected with T. copemani and other trypanosome species, while histopathological changes to cardiac and smooth muscle were reported in woylies, which were speculated to be due to the amastigote life-cycle stage (McInnes et al. Reference McInnes, Gillett, Hanger, Reid and Ryan2011; Botero et al. Reference Botero, Thompson, Peacock, Clode, Nicholls, Wayne, Lymbery and Thompson2013). Mixed trypanosome infections have been reported in woylies and koalas, with woylies co-infected with Trypanosoma vegrandis and T. copemani and koalas with Trypanosoma irwini, Trypanosoma gilletti, T. copemani and T. vegrandis (McInnes et al. Reference McInnes, Hanger, Simmons, Reid and Ryan2010; Botero et al. Reference Botero, Thompson, Peacock, Clode, Nicholls, Wayne, Lymbery and Thompson2013; Barbosa et al. Reference Barbosa, Austen, Gillett, Warren, Paparini, Irwin and Ryan2015). The tick, Ixodes australiensis, has been identified as the vector for T. copemani (Austen et al. Reference Austen, Ryan, Friend, Ditcham and Reid2011), but the vector for other trypanosome species infecting marsupials is unknown. Presently no treatment programs exist for native Australian trypanosomes. Importantly, T. copemani has also been shown to be resistant to normal human serum and therefore may be potentially zoonotic (Austen et al. Reference Austen, Ryan, Ditcham, Friend and Reid2015).
Morphologically T. copemani is highly polymorphic with three main trypomastigote forms (thin, medium and broad) detected in blood smears from quokkas and the Gilbert's potoroo (Potorous gilbertii) (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009). Broad and slender trypomastigote forms of T. copemani have also been observed in blood films from woylies (Thompson et al. Reference Thompson, Botero, Wayne, Godfrey, Lymbery and Thompson2013). In culture, the life-cycle stages previously detected were described and characterized according to parameters by Hoare (Reference Hoare1972) and Mackerras (Reference Mackerras1959) and have been shown to represent epimastigote, sphaeromastigote, promastigote and amastigote stages (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009).
The main aim of the present study was to elucidate the different morphological forms of T. copemani and other native Australian trypanosomes within the circulatory systems of the quokka and the Gilbert's potoroo. Knowledge of the different blood stream and in vitro forms of this parasite is imperative for clinical diagnostics and information gained from this study will help in monitoring and management of infected animals and allow for better treatment programs to be implemented.
MATERIALS AND METHODS
Study site and sample collection
Blood samples examined in the present study were collected from quokkas from Two Peoples Bay (34°58′S, 118°11′E) near Albany, Bald Island (34°55′S, 118°27′E) and Rottnest Island (32°00′S, 115°31′E), all of which are in WA. Quokkas were captured either in traps baited with peanut butter and oat mix or netted by hand. The captured quokkas were anaesthetized with isoflurane and 0·5–1 mL of blood was collected by venepuncture of the lateral caudal vein. The blood was mixed with ethylene diamine tetraacetic acid (EDTA) as an anticoagulant in commercial tubes (Sarstedt, Australia) and stored at 4 °C until required. A total of 35, 34 and 41 blood samples were collected from quokkas captured at Two Peoples Bay, Bald Island and Rottnest Island, respectively. Opportunistic samples from two Gilbert's potoroos (P83 and P94) were also examined. All work was carried out under Murdoch University animal ethics permit W2204/09 and Department of Parks and Wildlife permit number SC000767.
Microscopic detection of blood parasites
Thin blood smears were made from quokka whole blood from 34 quokkas from Two Peoples Bay, 28 quokkas from Bald Island and 40 quokkas from Rottnest Island, on the day of collection were possible, and stained using Modified Wright's stain on an automated slide stainer (Hematek, Bayer). A cover-slip was placed over the stained blood smear and the preparation was examined microscopically at 200- and 400x-magnification for the presence of trypanosomes. Smears containing trypanosomes were then observed at 1000x magnification and images of the parasites recorded using an Olympus DP71 Advance digital camera.
Morphological measurements
Digital images of trypanosomes in blood films were used to measure key morphological features, based on parameters described by Hoare (Reference Hoare1972) and Mackerras (Reference Mackerras1959). These included Total Length (length of the body measured along the mid-line including free flagellum), Breadth (maximum breadth measured at the level of the nucleus, including undulating membrane), Posterior to Kinetoplast (distance between the posterior end and the kinetoplast), Kinetoplast to Nucleus (distance between the kinetoplast and posterior edge of the nucleus), Nucleus to Anterior (distance between the anterior edge of the nucleus and the anterior end of the body) and Free Flagellum, (length of the free flagellum). Measurements were taken using Image J (Schneider et al. Reference Schneider, Rasband and Eliceiri2012).
In vitro cultivation of trypanosomes
Modified Sloppy Evans medium (MSEM)
Fresh blood of 20 µL from each animal was transferred into cryopreservation vials (1·8 mL Nunc cryotubes) containing 1 mL of MSEM (Noyes et al. Reference Noyes, Stevens, Teixeira, Phelan and Holz1999) and incubated in the dark at room temperature. Microscopic examination of wet smear preparations from medium was performed weekly to detect motile trypanosomes at 200- and 400x-magnification. A small volume of 200 µL of medium was removed every 10 days and placed into a new culture vial containing fresh MSEM. Once trypanosomes were detected, air-dried smears were prepared with modified Wright's-Giemsa stain using an Ames Hema-Tek® slide stainer (Bayer, Germany) for further microscopic evaluation.
Cunninghams medium
A 100 µL aliquot from each MSEM culture containing trypanosomes was placed into a culture 6 well culture plate, (Nunclon™) containing 5 mL of Cunningham's liquid medium (CM) (JRH Biosciences, Lenexa, Kansas), supplemented with 10% fetal calf serum and 10 mg mL−1 gentamycin (Sigma-Aldrich, St Louis, Missouri). The cultures were maintained at room temperature and monitored daily using an inverted microscope. Cultures were passaged every week by transferring 1 mL aliquots of culture media to a new culture plate containing fresh CM.
Blood incubation infectivity test (BIIT) medium
The BIIT medium was prepared in the same way as MSEM, however human blood from a healthy volunteer was used as a supplement in the medium instead of horse blood. A 50 µL aliquot of fresh quokka blood was placed into separate tubes containing 250 µL of fresh undiluted human serum. These tubes were then incubated in a water bath at 37 °C for 5 h. The entire contents of each incubated tube was then added to individual tubes containing 1 mL of human MSEM and incubated at room temperature in the dark for 24 h, before examination. Microscopic examination of wet-smear preparations of the medium was performed every day at 200- and 400x-magnification to detect the presence of motile trypanosomes. If trypanosomes were detected, modified Wright's-Giemsa stained thin blood smears were prepared for further microscopic examination. The use of human subjects for this study was approved by the Murdoch University human ethics committee (project number 2010/053).
Scanning electron microscopy (SEM)
A 100 µL aliquot of cultured trypanosomes were placed into 500 µL of 5% glutaraldehyde and incubated overnight at 4 °C. The fixed trypanosome cells were washed in 1× phosphate buffered saline (PBS) and placed onto a round coverslip (G401-10, Pro SciTech, Townville, Queensland, Australia) coated with 10% poly-l-lysine and air-dried. A 1% solution of Dalton's Chrome OsO4 was placed over the cells and incubated in the refrigerator for 1·5 h. The cells were then dehydrated by a series of alcohol washes with 5 min intervals. The cells were first washed several times with 30% alcohol followed by 2 washes of 50%, 2 washes of 70%, 2 washes of 80%, 2 washes of 90%, 2 washes of 95% and then 3 final washes of 100% ethanol for 10 min each. After dehydration, the cells were washed in a 50:50 ratio of 100% ethanol and amyl-acetate for 30 min then washed twice in amyl-acetate over a 60 min time period. The coverslip was placed into a critical point dryer and then dry mounted onto a specimen stub with a carbon disc and coated with gold. The stub was stored in a desiccator until required.
Immunofluorescence
To confirm the existence of novel trypanosome stages, immunofluorescent labelling using an antibody specific to the paraflagellar rod (PFR) was used, which is an organelle only found in free-living Dinoflagellates, Euglenoids and Kinetoplastids (Crithidia, Trypanosomes, Leishmania) (Gull, Reference Gull1999). A 3 mL of Cunningham's culture media containing trypanosomes was centrifuged at 1000 g for 5 min. The majority of the supernatant was removed leaving approximately 200 µL of medium to allow the pellet to be re-suspended. The re-suspended pellet containing trypanosomes was placed on a poly-L-lysine coated slide and air dried at room temperature for 30 min. The cells were fixed by incubating at −20 °C in methanol for 2 h. After fixation cells were re-hydrated in 100 mL of PBS for 5 min. The PBS was removed and the cells re-hydrated with fresh PBS for another 5 min. A primary monoclonal antibody (L8C4) of 200 μL, which binds to PFR was added to the air dried cells and incubated in a moist chamber for 60 min at room temperature. The primary antibody was removed and the cells washed twice with 50 µL PBS. A 20 µL aliquot of fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG (Sigma-Aldrich, USA) was then added to the cells and incubated in a dark moist chamber for 60 min at room temperature. The cells were washed twice with 50 µL of PBS. A 50 µL aliquot of diamidino-2-phenylindole (DAPI) (4′, 6-diamidino-2-phenylindole; 1 µg mL−1), a fluorescent DNA intercalating dye, was added to the same cells and incubated for 4 min. The DAPI was then removed and the cells were washed at 30 sec intervals twice with 50 µL of 100 mm hydroxyethyl-piperazineethane-sulfonic acid buffer. A drop of equilibrium buffer (Slowfade, Molecular probes S-7461, Life Technologies, Victoria, Australia) was added to the cells and incubated at room temperature for 4 min. The buffer was removed and a drop of component (A) (Slowfade, Molecular probes S-7461) was added for fluorescence stability. Finally a cover slip was applied, the air expelled and the edges sealed with nail varnish.
Fluorescence in situ hybridization (FISH)
To confirm the presence of novel trypanosome-like life-cycle stages that lacked a free flagellum, FISH was performed on both blood and culture life-cycle stages using a commercially synthesized probe specific to the trypanosome 18S rRNA gene (TRYall1F 5′ ACCGTTTCGGCTTTTGTTGG 3′) manufactured with a 5′ 6-Carboxyfluorescein (FAM) fluorescent tag at the 5′ end (GeneWorks, Adelaide, South Australia) as previously described (Thompson et al. Reference Thompson, Botero, Wayne, Godfrey, Lymbery and Thompson2013), on MSEM culture and whole blood smears. The hybridized slides were examined with a BX51 microscope (Olympus, Japan) using ultraviolet light (330–385 nm) through an emission filter (420 nm) producing green fluorescence. The slides were scanned at 400- and 1000x-magnification and digital images captured using an Olympus DP71 Advance digital camera.
Molecular characterization
Whole genomic DNA was extracted from fresh blood samples using a MasterPure™ DNA Purification Kit (Epicentre® Biotechnologies, Madison, Wisconsin, USA) following the manufacturer's instructions and the DNA stored at −20 °C until required. To confirm the presence of trypanosomes, universal primers were used to amplify and sequence a 1439 bp fragment of the 18S ribosomal RNA (rRNA) gene as previously described (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009). In addition, the samples were also screened with T. vegrandis specific primers (Botero et al. Reference Botero, Thompson, Peacock, Clode, Nicholls, Wayne, Lymbery and Thompson2013) using a modified protocol (Barbosa et al. Reference Barbosa, Austen, Gillett, Warren, Paparini, Irwin and Ryan2015), as this species has previously been detected in marsupial blood from woylies, a Western grey kangaroo (Macropus fuliginosus), a quenda (I. obesulus), koalas, and a tammar wallaby (Macropus eugenii) (Botero et al. Reference Botero, Thompson, Peacock, Clode, Nicholls, Wayne, Lymbery and Thompson2013; Thompson et al. Reference Thompson, Botero, Wayne, Godfrey, Lymbery and Thompson2013; Barbosa et al. Reference Barbosa, Austen, Gillett, Warren, Paparini, Irwin and Ryan2015). All samples were also screened for piroplasms (Theileria and Babesia), using 18S nested primers as previously described (Jefferies et al. Reference Jefferies, Ryan and Irwin2007).
RESULTS
Molecular confirmation of trypanosomes and piroplasms
To confirm the presence of trypanosomes, all blood samples collected from quokkas and Gilbert's potoroos were screened by polymerase chain reaction (PCR) and confirmed as T. copemani by sequence analysis of the 18S rRNA gene. To determine the potential of trypanosome co-infections, all marsupials were also screened for T. vegrandis using a species-specific PCR. All samples tested were negative, with the exception of one quokka (Q1340), from Two Peoples Bay, which was positive for both T. vegrandis and T. copemani. This is the first report of T. vegrandis in quokkas. The Rottnest Island quokkas were all negative for piroplasms, whereas all the quokkas from both Two Peoples Bay and Bald Island were positive for piroplasms by PCR.
Trypanosome morphology
Using light microscopy, the morphology of trypanosomes detected in blood smears from quokkas from both Two Peoples Bay and Bald Island was consistent with the different morphological forms of T. copemani from the Gilbert's potoroo, representing slender, medium and broad forms as previously described (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009). Trypanosomes representing typical trypomastigote blood cycle stages were not detected in any of the Rottnest Island quokka blood smears.
In addition to the three morphologically different trypomastigote forms, various potential trypanosome life-cycle stages were detected directly from blood samples from quokkas for the first time using light microscopy, in vitro cultivation, SEM, immunofluorescence and FISH. Trypanosome forms representing promastigote (Fig. 1) and sphaeromastigote (Fig. 2) stages, as previously described (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009) were identified. In addition, an amastigote stage (Fig. 3) and three novel trypanosome life-cycle stages representing an oval stage (Fig. 4), an extremely thin stage (Fig. 5) and an adherent stage (Fig. 6) were detected directly within blood films and in vitro, while two novel culture stages representing a tiny stage (Fig. 7) and a circular stage (Fig. 8) were only detected in vitro.
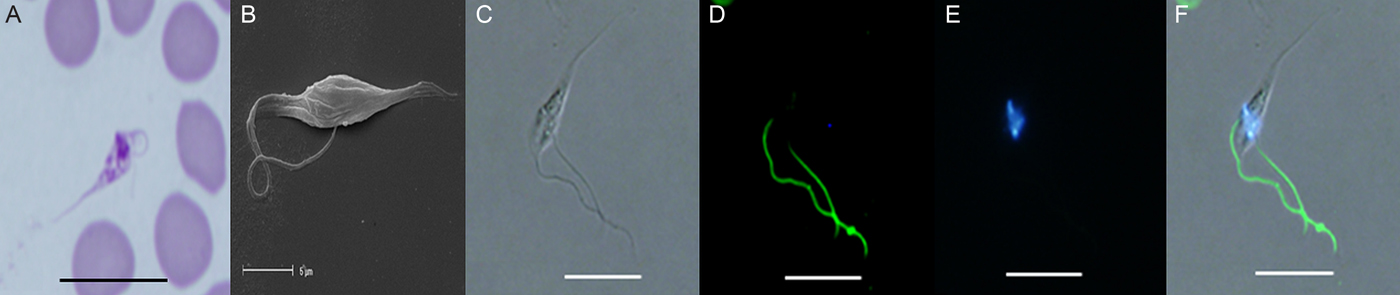
Fig. 1. (A) Light photomicrograph of the promastigote stage in a modified Wright's-Giemsa stained blood film from quokka Q1340 from Two Peoples Bay. (B) Scanning electron photomicrograph of the promastigote stage from in vitro culture originally isolated from Gilbert's potoroo P83. (C) Unstained trypanosome using DIC from Gilbert's potoroo P83. (D) Immunofluorescent staining of the PFR of a trypanosome isolated from Gilbert's potoroo P83 using monoclonal antibody (L8C4). (E) DAPI staining of nuclear DNA from Gilbert's potoroo P83. (F) Combined images of C, D and E. Abbreviations: DIC, differential interference contrast; DAPI, diamidino-2-phenylindole; PFR, paraflagellar rod. Scale bars represent 10 µ m.

Fig. 2. Light photomicrographs of sphaeromastigote stage (indicated by arrow) in a modified Wright's-Giemsa stained blood film. (A) Sphaeromastigote stage from quokka Q1342-1338 from Two Peoples Bay. (B) Sphaeromastigote stage from in vitro culture originally isolated from Gilbert's potoroo P94. (C) Immunofluorescent staining using monoclonal antibody (L8C4) of the sphaeromastigote stage in vitro isolated from a quokka (Q1) from Bald Island. Scale bars represent 10 µ m.
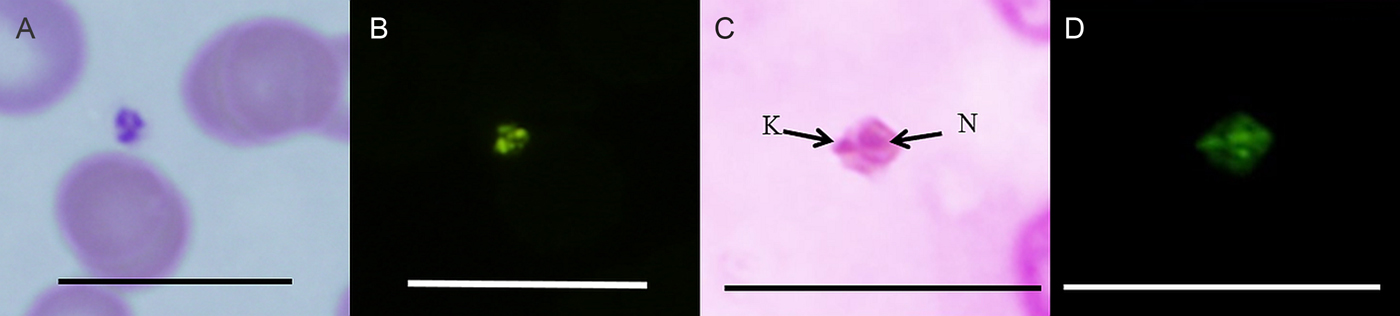
Fig. 3. (A) Light photomicrograph of the amastigote stage in vivo from quokka QRS3. (B) FISH analysis of the amastigote stage in vivo from quokka Q4908-4846. (C) Light photomicrograph of the amastigote stage in vitro stained with Giemsa from quokka Q1342-1338 N = nucleus, K = kinetoplast. (D) FISH analysis of the same amastigote stage in in vitro culture. Abbreviations: FISH, fluorescence in situ hybridization. Scale bar represents 20 µ m.

Fig. 4. (A) Light photomicrograph of the oval stage from quokka Q1051 from Two Peoples Bay. (B) Oval shape stage from in vitro culture originally isolated from Gilbert's potoroo P94. (C) Oval shape stage from in vitro culture from quokka Q2088-2050 stained with modified Wright's-Giemsa stain. Scale represents 10 µ m.

Fig. 5. (A) Extremely thin stage (indicated by arrow) in a modified Wright's-Giemsa stained blood film from quokka Q3343-3304 from Bald Island. (B) Light photomicrograph of the extremely thin stage in BIIT in vitro culture, originally isolated from Q2088-2050. Abbreviations: BIIT, blood incubation infectivity test. Scale bars represent 10 µ m

Fig. 6. Light photomicrographs of (A) Initial adherent stage on the surface of the erythrocyte (indicated by arrow) from quokka Q2088-2050. (B) A more developed adherent stage on an erythrocyte in MSEM in vitro culture originally isolated from Q3336-3325 from Bald Island. (C) Immunofluorescent staining using L8C4 monoclonal antibody of the adherent stage showing green fluorescence of the flagellum and co-staining of nuclei (blue fluorescence) using DAPI from quokka Q1.(D) FISH analysis of two initial adherent stages showing green fluorescence (white arrows) adhered to the surface of an erythrocyte in vitro (Q1). (E) FISH analysis of the more advanced adherent stage (white arrow) on the surface of an erythrocyte in vivo isolated from Q4633-4613 from Two Peoples Bay. Abbreviations: MSEM, modified Sloppy Evans medium; DAPI, diamidino-2-phenylindole; FISH, fluorescence in situ hybridization. Scale bars represent 10 µm.

Fig. 7. (A) The tiny stage from in vitro culture originally isolated from Gilbert's potoroo P83. Scale bars represent 10 µ m. (B) SEM of the tiny round flagellum stage (indicated by arrow) from in vitro culture originally isolated from Gilbert's potoroo P83.. Abbreviations: SEM, scanning electron microscopy. Scale bars represent 2·5 µ m

Fig. 8. (A) Immunofluorescent staining (L8C4) of the circular stage in vitro showing green fluorescence (white arrows) of the flagellum around the circumference of the parasite (Q1). (B) The same stage showing the nuclear region within the centre stained with DAPI. Abbreviations: DAPI, diamidino-2-phenylindole. Scale bar represents 10 µ m.
The detection rate of trypanosomes in direct blood films was very low and often required several smears from the same individual to be made in order to detect a single trypomastigote. In comparison, trypanosome-positive blood cultured in vitro, yielded more abundant trypanosome life-cycle stages that were detected as early as 5 days post inoculation. The most prevalent life-cycle stage in vitro were epimastigotes, followed by thin trypomastigotes, sphaeromastigotes, amastigotes and promastigotes, respectively. With regards to the potential novel trypanosome stages, the most prevalent form in culture and in blood films was the adherent stage which is in contrast to the extremely thin stage that was only detected once in both in vivo and in vitro.
During screening of blood smears, several erythrocyte abnormalities were observed in the blood of infected quokkas. These included the appearance of acanthocytes (star shaped erythrocytes), echinocytes (erythrocytes with many small, evenly spaced thorny projections on the cell membrane), schistocytosis (irregular shaped erythrocytes), dacrocytes (tear drop erythrocytes), microspherocytes (small round dense erythrocytes less than 4 µ m with no central pallor) and burst erythrocytes (data not shown), which are all cell types associated with haemolytic anaemia (Rodak et al. Reference Rodak, Fritsma and Keohane2012).
Promastigote stage
Only one promastigote stage (represented by an elongated form and an antenuclear kinetoplast with a flagellum arising near it and emerging from the anterior end of the body), was detected in a single blood film for the first time (Fig. 1A), from quokka Q1340, (which was co-infected with T. vegrandis and T. copemani) captured from Two Peoples Bay and from in vitro culture. Morphologically this stage was granular in appearance and was observed as having a long, pointed posterior, no undulating membrane, a rounded anterior containing the nucleus, an ante-nuclear kinetoplast and a free flagellum. The measurable morphological dimensions of this novel trypanosome-like stage from in vivo were; 17·0 µ m in total length, 8·9 µ m from posterior to kinetoplast and 1·3 µ m from nucleus to anterior.
The promastigote stage was more abundant in in vitro and was detected using SEM (Fig. 1B) and immunofluorescence. Immunofluorescent staining using a monoclonal antibody (L8C4) (specific for the PFR for trypanosomes) and DAPI showed bright green fluorescence along the length of the free flagellum and bright blue nuclear staining of both the nucleus and kinetoplast confirming the promastigote stage (Fig. 1C–F).
Sphaeromastigote stage
Only one sphaeromastigote trypanosome stage was observed in a blood smear from one quokka (Q1342-1338) captured from Two Peoples Bay (Fig. 2A) and a few from a quokka captured from Rottnest Island (QBS5). This is in contrast to the in vitro sphaeromastigote stages observed as the third most abundant life-cycle stage with epimastigotes and thin trypomastigotes the most prevalent forms. The in vitro sphaeromastigote forms originating from the blood of a Gilbert's potoroo (P94) (Fig. 2B) and a quokka from Bald Island (Q1) were detected using light microscopy and confirmed as a trypanosome stage with the use of the immunofluorescence monoclonal antibody L8C4 and DAPI (Fig. 2C). Morphologically the sphaeromastigote stage was small and rounded with a free flagellum. The morphological dimensions of the sphaeromastigote stage from in vivo were; 6·28 µ m in total length, 2·3 µ m in breath and 3·3 µ m in free flagellum.
Amastigote stage
The amastigote stage was observed in blood smears from quokka QRS3 from Rottnest Island (Fig. 3A) and Q4908-4846 (Fig. 3B) and in vitro (Fig. 3C) from quokka Q1342-1338 and confirmed using FISH (Fig. 3B). Morphologically this stage was small and round to oval, with a nuclear region and a peripheral kinetoplast with either a small or absent free flagellum. This stage measured 1·79 µ m in length and 1·02 µ m in width. The amastigote stages were observed in culture generally clumped together, however single independent motile stages with short beating flagellum were also observed, generally swimming in a circular motion.
Oval stage
Two singular oval trypanosome stages were observed in a blood smear (Fig. 4A) from one quokka (Q1051) captured from Two Peoples Bay and also in vitro from a Gilbert's potoroo P94 (Fig. 4B) and quokka Q2088-2050 (Fig. 4C). Morphologically this oval stage was granular in appearance with structures that stained with modified Wright's-Giemsa stain, had a short pointed posterior, oval body and free flagellum. The presence of the stained structures made it difficult to accurately determine the nucleus and kinetoplast. The morphological dimensions of this novel trypanosome stage from in vivo were; 9·4 µ m in total length and 4·4 µ m in free flagellum.
Extremely thin stage
An extremely thin trypanosome-like stage was observed once in a blood smear (Fig. 5A) from a quokka (Q3343-3304) captured from Bald Island and only detected once within in vitro culture (Fig. 5B) from quokka Q2088-2050. Morphologically this thin novel stage was observed as having a kinetoplast, a nucleus, a long pointed posterior end.
Adherent stage
Adherent trypanosome stages were represented by a small rounded independently rapid moving forms and forms observed adherent to erythrocytes (Fig. 6A, Q2088-2050). Initially the adherent stage was observed as a tiny rounded circle, clear to greenish brown in appearance. It then appeared to develop into a small dark circle once adhered to the erythrocyte. The adherent region developed further and could be seen with a cell body radiating out on either side of the nucleus when attached to the surface of an erythrocyte (Fig. 6B, Q2088-2050). Immunofluorescent staining using the L8C4 monoclonal antibody, showed binding of the antibody (observed as green fluorescence) to the novel adherent flagellum and co-staining with DAPI stained the nuclei (Fig. 6C, Q1). FISH analysis using a specific Trypanosome probe further confirmed these stages, with binding of the DNA probe (observed as green fluorescence) to both the initial (Fig. 6D, Q4633-4613) and more advanced adherent stages (Fig. 6E, Q4633-4613) on the surface of the erythrocyte.
Tiny round stage
A very tiny round trypanosome-like stage was detected in cultures by light microscopy (Fig. 7A) and SEM (Fig. 7B) originally from the blood of a Gilbert's potoroo, P83. This stage had a prominent pointed posterior end, a rounded anterior end and free flagellum and was often observed independently motile and rapid in motion.
Circular stage
A circular trypanosome stage was detected in vitro (Fig. 8, Q1) using immunofluorescent staining with the L8C4 monoclonal antibody. Green fluorescence of the flagellum could be seen around the circumference of the parasite (Fig. 8A) and DAPI co-staining of the nuclear region in the centre (Fig. 8B).
DISCUSSION
The present study is the first report of trypanosomes (confirmed as T. copemani by DNA analysis) in quokkas on Rottnest Island. The previous lack of detection of trypanosomes on Rottnest Island is likely due to the absence of the typical diagnostic trypomastigote stages in previously examined blood films (Austen et al. Reference Austen, Ryan, Friend, Ditcham and Reid2011). Morphologically the trypanosomes from the Rottnest quokkas represented sphaeromastigote and amastigote stages.
Trypanosoma copemani has a polymorphic life-cycle with three trypomastigote forms: a thin, medium and broad form, described from the Gilbert's potoroo and a medium form described from the quokka (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009). The thin and broad trypomastigote forms of T. copemani have also been described from woylies (Thompson et al. Reference Thompson, Botero, Wayne, Godfrey, Lymbery and Thompson2013). Previous studies have only detected trypomastigote life-cycle stages in the peripheral blood of Australian native marsupials. In the present study, we have detected the promastigote, sphaeromastigote and amastigote trypanosome forms (Hoare, Reference Hoare1972), directly in blood films and have preliminary confirmation that these three stages are trypanosomes with the use of trypanosome specific FISH and immunofluorescent probes.
The promastigote, sphaeromastigote and amastigote trypanosome-like forms appeared quite granular in appearance with structures stained by Modified Wright's-Giemsa, making it difficult to clearly distinguish the nucleus and kinetoplast regions, thus limiting our morphometric analysis. The small size and the lack of distinguishing features may be the cause of these life-cycles being previously unidentified, as well as the low level of parasitemia observed in Australian marsupials (Noyes et al. Reference Noyes, Stevens, Teixeira, Phelan and Holz1999). The promastigote stage however had a more defined nuclear region and was shown to be more prevalent in culture compared with in vivo with only one promastigote stage identified directly from within the host.
The sphaeromastigote stage has previously been identified in vitro, from quokkas and Gilbert's potoroos (Austen et al. Reference Austen, Jefferies, Friend, Ryan, Adams and Reid2009). This however is the first time that it has been detected directly in quokka blood and confirmed in vitro using immunofluorescent staining. The presence of the sphaeromastigote stage in blood supports the free circulation of amastigotes in the blood stream, since sphaeromastigotes represent transitional stages between amastigotes and epimastigotes in trypanosome stercorarian life-cycles (Hoare, Reference Hoare1972).
The amastigote stage was identified directly within blood films from quokkas for the first time and confirmed using FISH. Amastigote stages are generally considered intra-cellular, with multiplication occurring exclusively within cells. Studies however have shown that they can be present within the circulatory system (Ley et al. Reference Ley, Andrews, Robbins and Nussenzweig1988; Tyler and Engman, Reference Tyler and Engman2001). Pyogranulomatous myocarditis, endocarditis, muscle degeneration and necrosis with structures present suggestive of amastigotes have been reported in histopathological studies of heart sections from woylies naturally infected with T. copemani (Botero et al. Reference Botero, Thompson, Peacock, Clode, Nicholls, Wayne, Lymbery and Thompson2013). Further research however is needed to confirm these likely stages as truly amastigote forms.
Four novel potential trypanosome life-cycle stages were detected in the present study representing an oval stage, an extremely thin stage, an adherent stage and a tiny stage. These stages were identified both in vitro and in quokka blood films. A circular stage was also identified only in vitro and may represent a transitional epimastigote stage rounding up and forming into an amastigote stage. The novel oval stage may represent a transitional stage between a sphaeromastigote stage and an epimastigote stage, as no clear nucleus or kinetoplast was distinguishable due to granular staining. This oval stage appears to be morphologically similar to a cultured trypanosome isolate (ABF), previously isolated from a wallaby, which also lacked a distinctive nucleus and kinetoplast region (Hamilton et al. Reference Hamilton, Stevens, Gidley, Holz and Gibson2005). The extremely thin stage may represent a transitory life-cycle form or an early intracellular trypomastigote form, just released from a ruptured host cell. The latter form is known to migrate in the circulation system to re-infect new cells (Tanowitz et al. Reference Tanowitz, Kirchhoff, Simon, Morris, Weiss and Wittner1992). The tiny stage appears to morphologically resemble a metacylic trypomastigote stage, with the exception of a shorter free flagellum, as described previously by Tyler and Engman (Reference Tyler and Engman2001). To fully validate these novel stages as trypanosomes, further analysis is needed such as binding of trypanosome specific probes to these potential life forms and the use of transmission electron microscopy (TEM) for ultra-structural identification.
The adherent form was detected on the surface of erythrocytes in the blood smears of quokkas and Gilbert's potoroos naturally infected with trypanosomes, and in vitro from all three geographical locations. It is possible that this is a piroplasm life-cycle stage as it has similar morphological characteristics to a marginal stage of two piroplasm species (Babesia tachyglossi and Theileria tachyglossi) identified in the blood of an echidna (Clark et al. Reference Clark, Adlard, Spratt and Clark2004). However, Babesia and Theileria were absent in all the Rottnest Island quokkas by both microscopy and PCR screening at the 18S locus. The binding of the L8C4 monoclonal antibody and DNA probe also supports the hypothesis that these novel forms are potential trypanosome stages because both these probes are specific for Trypanosoma.
Generally the adherent stage was observed in a more advanced stage in vitro, containing a kinetoplast with a cell body radiating out from either side and attached to the surface of the erythrocyte. Whether the adherent stage is actually attached to the surface of the erythrocyte is unknown. However, trypanosomes attaching to erythrocytes, have previously been demonstrated in Trypanosoma gambiense, Trypanosoma rhodesiense, T rypanosoma brucei brucei, Trypanosoma congolense and Trypanosoma lewisi (Silva et al. Reference Silva, Herrera, Domingos, Ximenes and Darvila1995). Light and electron microscopy studies by Anosa and Kaneko (Reference Anosa and Kaneko1983) have reported the adhesion of T. b. brucei to erythrocytes in deer mice (Peromyscus maniculatus), while Silva et al. (Reference Silva, Herrera, Domingos, Ximenes and Darvila1995) reported Trypanosoma evansi adhered to erythrocytes from dogs and horses. These stages were also detected in the BIIT medium supporting a recent study, which reported the resistance of T. copemani to human serum lysis (Austen et al. Reference Austen, Ryan, Ditcham, Friend and Reid2015). Before the adherent stage can be confidently validated, TEM analysis, and the use of nuclear staining, such as DAPI, is vital to show that these forms are freely independent and living trypanosome stages.
The adherent forms may have the potential to cause erythrocyte destruction inducing immune antigen:antibody complexes and consequently leading to both erythrophagocytosis and haemolytic anaemia, a common feature of trypanosomiasis, with the exact cause unknown (Woodruff, Reference Woodruff1973; Woo and Kobayashi, Reference Woo and Kobayashi1975; Amole et al. Reference Amole, Clarkson and Shear1982).
Consistent with our findings of erythrocyte abnormalities, abnormalities including vacuolation, torocytes, acanthocytes, microspherocytes, schistocytes and dacrocytes have previously been reported in trypanosome-infected blood (Anosa and Kaneko, Reference Anosa and Kaneko1983; Silva et al. Reference Silva, Herrera, Domingos, Ximenes and Darvila1995), with microspherocytes and schistocytes typically associated with haemolytic anaemias (Mallah et al. Reference Mallah, Brown, Rossi and Block2010; Rodak et al. Reference Rodak, Fritsma and Keohane2012). Examination of erythrocytes from a quokka blood sample negative for trypanosomes was consistent with eosinophilic discocyte erythrocytes that showed a moderate central pallor (Clark et al. Reference Clark, Adlard, Spratt and Clark2004) with the exception of a few dacrocytes detected. However more in depth research is needed to determine if Australian native trypanosomes can cause trypanosomiasis and the effects they may have on the health of marsupial erythrocytes.
The present study demonstrates our limited understanding of the complex life-cycles of Australian native trypanosomes. Given that all the quokkas and both Gilbert's potoroo isolates were positive for T. copemani by sequence analysis at the 18S rRNA locus and negative for T. vegrandis, with the exception of one quokka (Q1340), the life-cycle forms identified in this study are highly suggestive of T. copemani stages. The promastigote stage identified in Q1340, which was co-infected with both T. copemani and T. vegrandis by molecular characterization, is unlikely to represent a T. vegrandis life-cycle stage, as T. vegrandis is the smallest trypanosome identified to date (Thompson et al. Reference Thompson, Botero, Wayne, Godfrey, Lymbery and Thompson2013). Measurements for T. vegrandis promastigotes are not available but trypomastigote stages measure 8·30 ± 0·28 (6·92–10·50) µ m in length (Thompson et al. Reference Thompson, Botero, Wayne, Godfrey, Lymbery and Thompson2013). In contrast, the promastigote stage described from Q1340 in the present study was 17·0 µ m in length, which is too large to be T. vegrandis. However to conclusively identify the trypanosome species represented by each life-cycle stage, species-specific probes need to be applied. The importance of identifying and understanding all the stages of native Australian trypanosome life-cycles is important when considering clinical diagnosis, false negatives in epidemiological studies, management of native wild life and translocation studies and preventing disease outbreaks as no treatment to date exists.
ACKNOWLEDGEMENTS
The authors wish to thank the Rottnest Island Authority, Shane Kearney the environmental coordinator from Rottnest Island and Professor Keith Gull for supplying the PFR antibody. The assistance of Gordon Thompson, Peter Fallon, Amanda Barbosa and fieldwork support by Dr Linda Davies and Dr Tegan McNab is also acknowledged.
FINANCIAL SUPPORT
The Rottnest Island Authority funded activities on Rottnest Island (JA, no grant number).











