Introduction
Traumatic brain injury (TBI) is the most common cause of brain damage (Kurtzke, Reference Kurtzke1984). It is highly prevalent in industrialized countries (Kraus, Reference Kraus1993), estimated at approximately 2% of the general population (The National Center for Injury Prevention and Control, 1999), and often leads to long-term disability (Moscato, Trevisan, & Willer, Reference Moscato, Trevisan and Willer1994). Despite the relative pervasiveness of physical, cognitive, emotional, and behavioral difficulties among TBI patients, especially in moderate-to-severe TBI (Hellawell, Taylor, & Pentland, Reference Hellawell, Taylor and Pentland1999; Langlois, Rutland-Brown, & Wald, Reference Langlois, Rutland-Brown and Wald2006), substantial individual differences in severity and duration of clinical impairment are typically seen across patients (Kesler, Adams, Blasey, & Bigler, Reference Kesler, Adams, Blasey and Bigler2003; Vakil, Reference Vakil2005).
The reserve hypothesis (Satz, Reference Satz1993; Stern, Reference Stern2002) has been proposed to explain this mismatch between brain pathology and its clinical expression. According to this hypothesis, individual differences in variables such as intellectual ability and brain capacity provide differential reserve against age-related changes or brain pathology. A key distinction typically made in the literature is between “brain reserve” (BR) (Satz, Reference Satz1993) and “cognitive reserve” (CR) (Stern, Reference Stern2002). BR refers to the brain's ability to cope with increasing damage and is commonly indexed by anatomical measures (such as total intracranial volume, head circumference, and ventricle-to-brain ratio) (Bigler, Reference Bigler2006; Stern, Reference Stern2006). On the other hand, CR reflects differences in processing the relevant task and indexed with cognitive and lifetime experience variables. Numerous epidemiological and neuropsychological studies have examined the concept of reserve, mostly in normal and pathological aging. These studies reported a positive association between various indices of BR (e.g., head circumference, intracranial volume) and CR (e.g., IQ, education, occupation, engaging in leisure activities) with slower cognitive decline in normal aging, as well as reduced risk of dementia (e.g., Manly, Schupf, Tang, & Stern, Reference Manly, Schupf, Tang and Stern2005; Mortimer, Snowdon, & Markesbery, Reference Mortimer, Snowdon and Markesbery2003; Scarmeas, Levy, Tang, Manly, & Stern, Reference Scarmeas, Levy, Tang, Manly and Stern2001).
To date, only a few studies have attempted to examine several indices of “reserve” in the context of TBI. For example, Ropacki and Elias (Reference Ropacki and Elias2003) found that individuals with a history of neurologic insult, psychiatric problems, alcoholism, or drug abuse (i.e., indices of “diminished reserve”) displayed worse cognitive deficits following TBI in comparison to those with no prior neurological history. In another study, Salmond, Menson, Chatfield, Pickard, and Sahakian (Reference Salmond, Menson, Chatfield, Pickard and Sahakian2006) found that higher premorbid intelligence was associated with lower rates of depression following TBI. Kesler et al. (Reference Kesler, Adams, Blasey and Bigler2003) examined the relationships between total intracranial volume, ventricle-to-brain ratio, education level, and standardized testing obtained before injury with post-injury cognitive outcome. Participants with lower post-injury IQ scores had significantly lower total intracranial volume values, irrespective of injury severity, and experienced significantly greater change in IQ. Finally, various demographic and clinical variables that could be regarded as proxies of reserve, including psychiatric history (MacMillan, Hart, Martelli, & Zasler, Reference MacMillan, Hart, Martelli and Zasler2002), history of drug or alcohol abuse (MacMillan et al., Reference MacMillan, Hart, Martelli and Zasler2002; Novack, Bush, Meythaler, & Canupp, Reference Novack, Bush, Meythaler and Canupp2001; Sherer, Bergloff, High, & Nick, Reference Sherer, Bergloff, High and Nick1999), marital status (Kreutzer et al., Reference Kreutzer, Marwitz, Walker, Sander, Sherer, Bonger and Bushnik2003), socioeconomic status (SES) (Hoofien, Vakil, Gilboa, Donovick, & Barak, Reference Hoofien, Vakil, Gilboa, Donovick and Barak2002), occupational status (Gollahar et al., Reference Gollahar, High, Sherer, Bergloff, Boake, Young and Ivanhoe1998; Sherer et al., Reference Sherer, Sander, Nick, High, Malec and Rosenthal2002), and education (Gollahar et al., Reference Gollahar, High, Sherer, Bergloff, Boake, Young and Ivanhoe1998; Novack et al., Reference Novack, Bush, Meythaler and Canupp2001; Sherer et al., Reference Sherer, Sander, Nick, High, Malec and Rosenthal2002) were found to predict various indices of post-TBI functional and occupational outcome.
Despite the widespread support for the reserve hypothesis, much of the relevant scholarship has intuitively addressed the reserve concept by defining it on the basis of a single or a limited number of indicators, without referring to specific constructs and to the nature of their relationships (Satz, Cole, Hardy, & Rassovsky, Reference Satz, Cole, Hardy and Rassovsky2011). Several recent studies have underscored the need for a systematic evaluation of reserve. For example, Siedlecki et al. (Reference Siedlecki, Stern, Reuben, Sacco, Elkind and Wright2009) evaluated three variables of CR (years of education, Wide Range Achievement Test, and Picture vocabulary) across three different samples. The three measures highly correlated with each other across the three samples, and in two samples an overlap was found with between this CR construct and a construct of executive functions. Additionally, using path analysis Richards and Sacker (Reference Richards and Sacker2003) found that three CR measures, childhood IQ, educational attainment, and adult occupation, each provided unique contribution to cognitive function in middle age. Thus, a coherent framework for understanding and evaluating the construct of reserve requires a systematic evaluation of its underlying structure.
Given the large number of indices that could potentially represent the constructs of reserve, along with sample size limitations, our effort in the present study focused on the investigation of the underlying structure of CR concept, after characterizing its components a priori on the basis of variables described in prior studies. The variables that have typically been associated with CR can be divided into three main domains: premorbid Intelligence (e.g., Ropacki, Bert, Ropacki, Rogers, & Stern, Reference Ropacki, Bert, Ropacki, Rogers and Stern2007; Scarmeas et al., Reference Scarmeas, Zarahn, Anderson, Habeck, Hilton, Flynn and Stern2003; Sumowski, Chiaravalloti, Wylie, & Deluca, Reference Sumowski, Chiaravalloti, Wylie and DeLuca2009); SES variables such as years of education (e.g., Kesler et al., Reference Kesler, Adams, Blasey and Bigler2003; Snowdon, Ostwald, & Kane, Reference Snowdon, Ostwald and Kane1989), occupation level (e.g., Legendre, Stern, Solomon, Furman, & Smith, Reference Legendre, Stern, Solomon, Furman and Smith2003; Stern, Albert, Tang, & Tsai, Reference Stern, Albert, Tang and Tsai1999), and other SES indices (e.g., Bickel & Cooper, Reference Bickel and Cooper1994); and Leisure activity, indexed by cognitive (e.g., Wilson et al., Reference Wilson, Mendes de Leon, Barnes, Schneider, Bienias, Evans and Bennet2002), physical (e.g., Dik, Deeg, Visser, & Jonker, Reference Dik, Deeg, Visser and Jonker2003), and social (e.g., Fratiglioni, Paillard-Borg, & Winblad, Reference Fratiglioni, Paillard-Borg and Winblad2004) leisure activities. We examined the CR construct in TBI as little information exists regarding its structure in this clinical population and also because TBI, unlike pathological aging, reflects a sudden and acute injury that produces immediate damage to regions of the brain and decrease in overall brain volume (Bigler, Reference Bigler2001, Reference Bigler2006). Therefore, it involves an identifiable event that may decrease reserve, by potentially setting the process of the aging brain off its normal course. Consistent with this view, several studies have found that TBI was a risk factor for future dementia and neuropsychiatric disorders (Bigler, Reference Bigler2006; Fann et al., Reference Fann, Burington, Leonetti, Jaffe, Katon and Thompson2004; Lye & Shores, Reference Lye and Shores2000; Murrey, Starzinski, & LeBlanc, Reference Murrey, Starzinski and LeBlanc2004). Hence, TBI offers a unique theoretical and practical contribution, as it has the potential to provide insight into the reserve construct, with implications for both TBI and general aging processes.
We indexed premorbid CR variables in a cross-sectional research design in individuals who sustained moderate-to-severe TBI. Based on the aforementioned studies, three CR domains were identified. These included estimated premorbid intelligence, engagement in leisure activities, and SES. Structural equation modeling (SEM) approach was used to compare several hypothesized models to test whether the three CR components reflect a single unitary construct or rather represent separable domains. The identification of the underlying structure of CR is a necessary step for future investigations of its construct validity.
Method
Participants
The study included 89 individuals (80 males, age range, 19–73 years), with moderate-to-severe TBI. We focused on the moderate-to-severe TBI group because the differential diagnosis is much clearer than in cases of mild TBI. The characterization of TBI severity was based on three measures: Glasgow Coma Scale (GCS), loss of consciousness (LOC), and post-traumatic amnesia (PTA). Moderate TBI was defined as GCS 9–12, LOC 20 min—36 hr, and PTA 1–7 days; severe TBI was defined as GCS 3–8, LOC more than 36 hr, and PTA more than 7 days (Williamson, Scott, & Adams, Reference Williamson, Scott and Adams1996). Participants were recruited from the Day Treatment Rehabilitation unit and the outpatient clinics of the Rehabilitation Hospital at the Chaim Sheba Medical Center, Ramat-Gan, Israel (n = 62) and from the Rehabilitation Center for Veterans after TBI, Jaffa, Israel (n = 27). We only included participants who were at least 18 years old at the time of injury to avoid potential confounds related to neural plasticity in children. Additionally, we included only participants that were at least a year after injury, to ensure certain stability in their neuropsychological condition. Participants’ demographic and injury severity data are presented in Table 1. All participants gave written informed consent after receiving a full explanation of the research according to procedures approved by the Institutional Review Boards at each institution.
Table 1 Demographic and injury-related data of the participants

Procedure
Participants were recruited and interviewed by a trained research assistant. Out of 100 people contacted by telephone or in person, 11 declined to participate. As part of an extensive long-term outcome study conducted by this group, various premorbid variables, frequently used in literature as reserve indices, were collected. Data were obtained from patients’ medical files and collected in-person during several sessions with the patient, using questionnaires, clinical interviews, and neuropsychological assessments (administered by a neuropsychologist).
Measures
Premorbid socioeconomic status
Parents’ occupation
Parents’ occupation level, considered as a SES indicator (Hauser & Sewell, Reference Hauser and Sewell1986), was classified according to Roe's (Reference Roe1956) professional, skilled, and unskilled employment categories. Occupation level index was assigned for both father and mother of each participant, selecting the parent with the higher level.
Sibling number
The number of children in the family is considered to be a strong SES indicator in Israel, with low SES level associated with having more children on average (Brosch & Peres, Reference Brosch and Peres2000).
Income
Pre-injury income, reflecting SES, was assessed by interviewing the participant. Since most of the participants were injured at young age, the data were referred to both parents’ income. In case the participant had a family of his own before injury, his/her and his/her spouse salaries were considered. The participant's report was confirmed with an estimation of the average salary of his/her or his/her parents’ occupation, according to the Central Bureau of Statistics (2012). The index used was a 4-level scale, as the following: 1 = salary up to 7,000 NIS, 2 = between 7,000 and 15,000 NIS, 3 = between 15,000 and 25,000 NIS, 4 = above 25,000 NIS.
Self-reported SES
Perceived economic state was assessed based on the following question: “How do you define your economic state before the injury?” Answers were scored on a scale from 1 (very bad) to 5 (very good).
It should be noted that since a large percentage (40.45%) of our participants were young soldiers at the time of injury (18–21 years of age), we did not include premorbid educational and occupational attainment (often used as CR indices), as these would likely under-represent their true potential.
Premorbid Intelligence
Premorbid Intelligence was assessed using three subtests from the Hebrew version of the Wechsler Adult Intelligence Scale – III (WAIS-III) (Wechsler, Reference Wechsler1997): (1) Information; (2) Vocabulary; (3) Matrix Reasoning. These subtests were selected as they are considered to be relatively resistant to a brain insult (therefore, termed as “hold” tests since they represent performance that hold the level of premorbid function) and are frequently used as proxies for premorbid intellectual functioning in TBI (e.g., Donders, Tulsky, & Zhu, Reference Donders, Tulsky and Zhu2001; Green et al., Reference Green, Melo, Christensen, Ngo, Monette and Bradbury2008; Lezak, Howieson, & Loring, Reference Lezak, Howieson and Loring2004; Russell, Reference Russell1980).
Premorbid Leisure Activity
Premorbid leisure activity was assessed using three questionnaires (cognitive activity, social activity, and physical activity sub-scales, as following). These questionnaires are partially based on the Lifetime of Experience Questionnaire (LEQ) (Valezuela & Sachdev, Reference Valezuela and Sachdev2007), which was modified and adapted for the study's population. Answers were scored on a scale from 1 (not at all or very seldom) to 3 (very frequent) with 3 indicating high involvement in premorbid complex leisure activity, as perceived by the individual.
Premorbid cognitive activity was assessed using the cognitive activity sub-scale, which includes 15 questions about the participant's complex mental leisure activity (e.g., How often did you practice or develop an artistic pastime, e.g., drawing, writing, acting?)
Premorbid social activity was assessed using the social activity sub-scale, which includes five questions regarding participant's active social involvement (e.g., How often were you seeing friends?)
Premorbid physical activity was assessed using physical activity sub-scale, which includes two questions about participant's physical leisure activity (e.g., How often did you go for a walk or ride a bicycle?)
Data Analyses
Pearson bivariate correlations (two-tailed) were calculated to examine zero-order correlations among the variables. The hypothesized underlying structure of the CR construct was examined using the SEM approach. A one-factor model (Figure 1) with one latent variable and ten indicators that represented premorbid Intelligence, SES, and Leisure activity as a single CR construct was compared to a three-factor model (Figure 2) with three latent variables that represented premorbid Intelligence, SES, and Leisure activity as three separate constructs. All models were estimated with EQS Structural Equation Package (Bentler, Reference Bentler1996), using maximum likelihood solution. We report in this study the three commonly reported indices: the χ2, the CFI, and the RMSEA. A good fitting model is typically indicated by a non-significant χ2, CFI values >0.90 and RMSEA values <0.1 (Hu & Bentler, Reference Hu and Bentler1999). Difference between the chi-square coefficients was used to compare the relative fit of the models. As some data were missing (17% of the participants had one to two missing variables), analyses were performed by first analyzing the data with listwise deletion and repeating the analyses using maximum-likelihood expectation-maximization (Jamshidian & Bentler, Reference Jamshidian and Bentler1999). As the pattern of results from the two methods was virtually identical, only the results obtained by using the maximum-likelihood expectation-maximization method are reported here.

Fig. 1 A one-factor structural equation modeling (SEM) model that represent premorbid Intelligence, Socioeconomic status (SES), and Leisure activity as a single latent variable. Circles represent latent variables, and rectangles represent measured variables. Values are standardized path coefficients. Error terms for the variables: Information = 0.53, Vocabulary = 0.48, Matrix reasoning = 0.79, Parents’ occupation = 0.93, Income = 0.94, Self-reported SES = 1.00, Siblings number = 0.99, Cognitive activity = 1.00, Physical activity = 0.99, Social activity = 1.00. *p < .05, χ 2 (35, n = 89) = 113.23, p < .01.

Fig. 2 A three-factor structural equation modeling (SEM) that represents premorbid Intelligence, Socioeconomic status (SES), and Leisure activity as three separate latent variables. Circles represent latent variables, and rectangles represent measured variables. Values are standardized path coefficients. Error terms for the variables: Information = 0.48, Vocabulary = 0.52, Matrix reasoning = 0.78, Parents’ occupation = 0.86, Income = 0.40, Self-reported SES = 0.85, Siblings number = 0.94, Cognitive activity = 0.73, Physical activity = 0.81, Social activity = 0.91. *p < .05, χ 2 (32, n = 89) = 40.42, p < .001.
Results
The means and standard deviations of the variables used in the study are displayed in Table 2. The zero-order correlations between the indicator variables are also presented (Table 3).
Table 2 Means and standard deviations of study variables
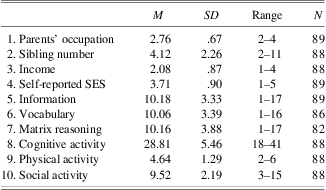
Table 3 Zero-order correlations of cognitive reserve variables

*p < .05.
**p < .01.
To examine whether CR variables represent one unitary construct or separate factors (i.e., whether they are better modeled as one, two, or three separate constructs), a series of models were evaluated. All independence models, testing whether or not the observed data fit the expected data, were rejected (The χ 2 for the independence model should always be significant, indicating that there is a relationship among the variables.)
First, a one-factor model that represented all 10 measured CR variables as a single latent variable (see Figure 1) was tested. As can be seen in Figure 1, many of the indicators did not load significantly on the respective latent variable, and overall the one-factor model provided a poor fit to the data, CFI = 0.6, χ 2 (35, n = 89) = 113.23, p < .01, RMSEA = 0.16. Next, a three-factor model that represented premorbid Intelligence, SES, and Leisure activity as three separate latent variables (see Figure 2) was examined. The three-factor model fit the data well, CFI = 0.96, χ 2 (32, n = 89) = 40.42, p < .001, RMSEA = .077. All indicators had moderate-to-high loadings on their respective latent variables, and all were significant at the 0.05 level. As can be seen in Figure 2, a moderate covariance was found between the latent variables of SES and Intelligence (standardized coefficient = 0.31; p < .05), and between SES and Leisure activity (standardized coefficient = 0.35; p < .05). Testing the difference between the chi-squares of the two models revealed that the three-factor model fit the data significantly better than the one-factor model, χ 2 diff (3,89) = 72.8, p < .001.
Because significant covariance was found between SES and Leisure activity, as well as between SES and Intelligence, we tested whether the aforementioned three-factor model would still provide the best fit for the data when compared against each of the two two-factor models (one with the combined SES and Leisure activity and the other with the combined SES and Intelligence). Thus, a two-factor model that represented both SES and Leisure activity as one latent variable, and Intelligence as another latent variable, was compared to a three-factor model that represented SES, Leisure activity, and Intelligence as three separate latent variables. The two-factor model provided a moderate fit to the data, CFI = 0.86, χ 2 (34, n = 89) = 60.58, RMSEA = .094, and the three-factor model fit the data significantly better than the two-factor model, χ 2 diff (2, n = 89) = 20.2, p < .001. Similarly, a two-factor model that represented both SES and Intelligence as one latent variable and Leisure activity as another latent variable was compared to a three-factor model that represented SES, Leisure activity, and Intelligence as three separate latent variables. This two-factor model provided a poor fit to the data CFI = 0.72, χ 2(34, n = 89) = 89.31, RMSEA = 0.14, and again, the three-factor model fit the data significantly better than the two-factor model, χ 2 diff (2, n = 89) = 48.89, p < .001.
Discussion
In the present study, we tested several models hypothesized to represent CR in TBI. Understanding the structure of CR in the context of TBI has potential implications for both TBI and pathological aging, as suggested by the proposed association between these two entities (Lye & Shores, Reference Lye and Shores2000). Using the SEM approach, we found that a three-factor model that represented premorbid Intelligence, SES, and Leisure activity as separate constructs fit the data better than a one-factor model or two-factor models.
Our findings suggest that CR is not a unitary structure but rather a multi-dimensional one, with at least three different components. The finding that SES was correlated with Intelligence and Leisure activity is not surprising, in view of evidences indicating that environmental effects of social advantage can benefit cognitive development (e.g., Carpon & Duyme, Reference Carpon and Duyme1989; Duncan, Brooks-Gunn, & Klebanov, Reference Duncan, Brooks-Gunn and Klebanov1994) and affect leisure activity patterns (e.g., Holman & Epperson, Reference Holman and Epperson1984). Notably, we did not find a covariance between Intelligence and Leisure activity. It would be informative to examine similar models in healthy aging, as well as in other clinical population, to test whether a similar dissociation between intelligence and engagement in leisure activities exists in these other groups, or rather limited to TBI.
The present findings have important implications for studies investigating the CR concept. They suggest, for example, that due to the heterogeneity of the CR construct, uninformed combination of CR indices may lead to inconsistent results. Accordingly, these findings offer guidance in the selection of relevant CR measures (i.e., questionnaires or interviews regarding premorbid SES and mental activity, as well as a neuropsychological evaluation of premorbid intelligence). Additionally, elucidating the underlying structure of CR would inform studies attempting to predict injury outcome, by identifying significant factors that might be moderating the course of other brain pathologies (e.g., Alzheimer's disease, multiple sclerosis, epilepsy), as well as healthy aging. This approach may, in turn, facilitate the development of effective rehabilitation programs that are sensitive to priorities set by specific clinical settings in accordance with patient's needs.
Several methodological limitations of the present study should be noted. Sample size limitations and heterogeneity with respect to injury characteristics limits the number of potential intervening variables that could be included in the model, as well as generalizations to other populations. Therefore, it would be informative to test these models in larger and more homogenous samples. Additionally, the present study did not include reliable brain measures (e.g., MRI). Therefore, we focused only on CR and were unable to evaluate models of BR and combined BR-CR models. It should also be noted that most of the participants were injured at a relatively young age, before having the opportunity to acquire higher education and fulfill their employment potential. Therefore, we were not able to examine premorbid educational and vocational attainment, despite the relevance of these variables in moderating the effects of aging and various neurological pathologies (e.g., Kesler et al., Reference Kesler, Adams, Blasey and Bigler2003; Legendre et al., Reference Legendre, Stern, Solomon, Furman and Smith2003; Schmand, Smit, Geerlings, & Lindeboom, Reference Schmand, Smit, Geerlings and Lindeboom1997; Snowdon et al., Reference Snowdon, Ostwald and Kane1989; Stern et al., Reference Stern, Albert, Tang and Tsai1999). In addition, the number of indices for premorbid intelligence we were able to include was somewhat limited, both by sample size and by the lack of availability of standardized measures in Hebrew. Finally, construct validation of the CR components identified in the present study requires an evaluation of the contribution of these components to functional outcome (i.e., protection against decline). This effort is currently underway in our laboratory.
These limitations notwithstanding, this study provides a basis and methodological approach for conceptualization and evaluation of premorbid cognitive traits that may protect against the consequences of head injury, thereby suggesting a coherent framework for further investigation. It would be informative, for example, to use this approach in the investigation of the various variables thought to constitute BR. The reserve literature alludes to a host of BR indices, such as total brain volume, total intracranial volume, head circumference, ventricle-to-brain-ratio (Bigler, Reference Bigler2006; Stern, Reference Stern2006), synaptic count, and dendritic branching (Stern, Reference Stern2006). Whether these indices reflect a unitary underlying construct, or separate structures is yet to be determined. Finally, the central challenge for future research will be to examine the reciprocal relationship between CR and BR structures and the respective roles these constructs play in protecting against brain damage and in moderating outcome in TBI and other brain pathologies, as well as in healthy aging.
Acknowledgments
This study was carried out as part of a PhD dissertation by Yifat Levi at Bar Ilan University, Ramat-Gan, Israel. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. No conflicts exist. The authors thank Irene Kopel for her assistance with interviewing participants and data management.







