Introduction
Soil-transmitted helminthiasis (STH) is a common parasitic infection and ranks among the most widespread tropical diseases caused by intestinal nematodes, namely Ascaris lumbricoides (roundworm), Trichuris trichiura (whipworm), and Ancylostoma duodenale and Necator americanus (hookworms). STH may cause symptoms ranging from diarrhoea, abdominal pain, malaise and weakness, to intestinal blood loss and subsequent iron-deficiency anaemia in the case of chronic hookworm infections. Furthermore, chronic infection may also cause impairment in cognitive and physical development in children (Bethony et al., Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006).
It is estimated that more than 1.5 billion people worldwide are at risk for infection with STH, most widely in tropical and subtropical areas. In 2015, the World Health Organization (WHO) estimated that about 270 million pre-school and 600 million school-aged children were infected or were at an increased risk for parasitic infections (WHO, 2015). The estimated global burden of STH ranges between 43 and 128 disability-adjusted life-years per 100,000 population (Murray et al., Reference Murray, Vos and Lozano2012). WHO has classified STH infections as one of the neglected tropical diseases (WHO, 2016).
The WHO has endorsed preventive chemotherapy to control the morbidity and transmission of STH infections, i.e. regular administration of anthelmintic drugs (WHO, 2006). Mebendazole is one of the recommended anthelmintics in the WHO list of essential medicines for the treatment and control of STH (WHO, 2011). Recently a phase 3 clinical study was conducted with a new 500 mg chewable tablet of mebendazole for the treatment of A. lumbricoides and T. trichiura in paediatric patients (Silber et al., Reference Silber, Diro, Workneh, Mekonnen, Levecke, Steinmann, Umulisa, Alemu, Baeten, Engelen, Hu, Friedman, Baseman and Mrus2017). We undertook this literature review to summarize the available efficacy data from studies with the currently recommended single-dose 500 mg mebendazole treatment regimen against all STH types, including hookworms, in all patient populations. This review would be particularly important because of the recent increase in use of benzimidazoles in mass drug administration (MDA) to achieve the WHO goal of 75% national coverage before 2020 (WHO, 2013a). This literature review also aimed to compare the efficacy outcomes of single-dose 500 mg mebendazole for the treatment of A. lumbricoides and T. trichiura with those from the recent phase 3 clinical study (Silber et al., Reference Silber, Diro, Workneh, Mekonnen, Levecke, Steinmann, Umulisa, Alemu, Baeten, Engelen, Hu, Friedman, Baseman and Mrus2017).
Methods
Literature search
The published literature reporting the efficacy data of mebendazole 500 mg as a single-dose regimen was identified in MEDLINE using PubMed search. The search strategy utilized the following terms: Mebendazole (focusing on 500 mg single dose), soil-transmitted helminth, nematodes, Ascaris lumbricoides, roundworm, Trichuris trichiura, whipworm, Necator americanus, Ancylostoma duodenale, hookworm and efficacy (effectiveness, cure rate and egg reduction rate). The bibliography of the identified literature was also searched for relevant studies. The language was limited to English and no restriction was set on year. Data from unpublished internal reports of studies conducted by Janssen Research & Development and the bibliographies of safety updates (Clinical Overviews, Periodic Safety Update Reports and Safety Bridging Reports) of Janssen Research & Development since 1991 were also reviewed for relevant information.
Study selection
Studies that were conducted in humans and reported the efficacy outcomes of single-dose mebendazole (500 mg) for the treatment of at least one intestinal helminthic infection were selected. Only studies in which the primary outcome measures were cure rate (defined as the percentage of patients who had no detectable eggs (i.e. egg counts of 0) in stool samples after treatment) or egg reduction rate (calculated as [(initial egg count – final egg count)/initial egg count] × 100) were retained. Studies having these outcomes reported within the first month after treatment for a defined population were included in the analysis. Studies reporting these outcomes after longer periods of time were not included in the analysis, but are discussed.
Analysis
The efficacy outcomes by each STH type for patients treated with single-dose 500 mg mebendazole, as well as sample sizes, were abstracted from each publication. The efficacy outcomes and number of patients treated for comparators were also captured (not reported here), including for placebo in placebo-controlled studies. Efficacy results (cure rate and egg reduction rate) for mebendazole and placebo by each worm type were pooled by weighting the treatment group results obtained in each of the studies by the number of subjects treated, thus obtaining a weighted mean. A subgroup analysis focusing only on the studies that were placebo-controlled was performed as these studies are thought to be of the highest quality and less likely to be biased (Keiser & Utzinger, Reference Keiser and Utzinger2008).
Results
Ascaris lumbricoides
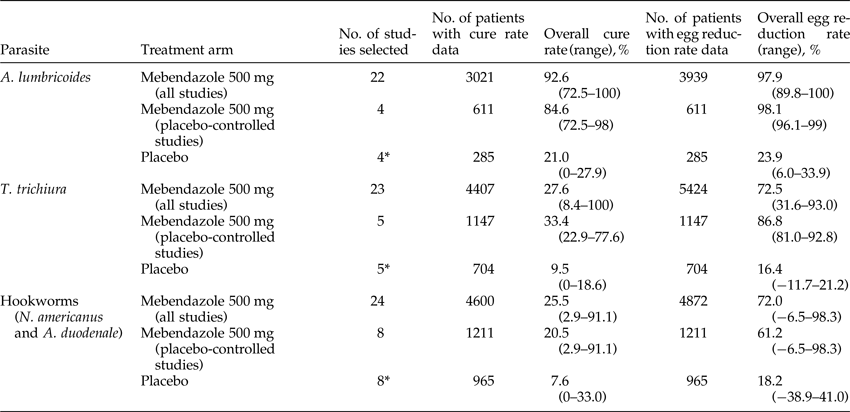
A total of 22 studies were identified for the treatment of A. lumbricoides. The cure rate was evaluated from 3021 patients in 21 of 22 studies and egg reduction rate was evaluated from 3939 patients in 19 of 22 studies. The mean cure rate for a 500 mg single-dose mebendazole regimen was 92.6% (range: 72.5–100%), with 17 studies reporting >90% cure rate. The mean egg reduction rate was 97.9% (range: 89.8–100%) (tables 1 and 2).
Table 1. Summary of efficacy results of single-dose mebendazole 500 mg against Ascaris lumbricoides, Trichuris trichiura and hookworms (Necator americanus and Ancylostoma duodenale).
* One study reported the results in graphical format without numerical values.
Note: Egg reduction rates were determined inconsistently across studies by using individual mean, group geometric mean and group arithmetic mean. Negative values represent increases in egg counts.
Table 2. Literature references of single-dose mebendazole (MBZ) 500 mg showing efficacy against Ascaris lumbricoides.
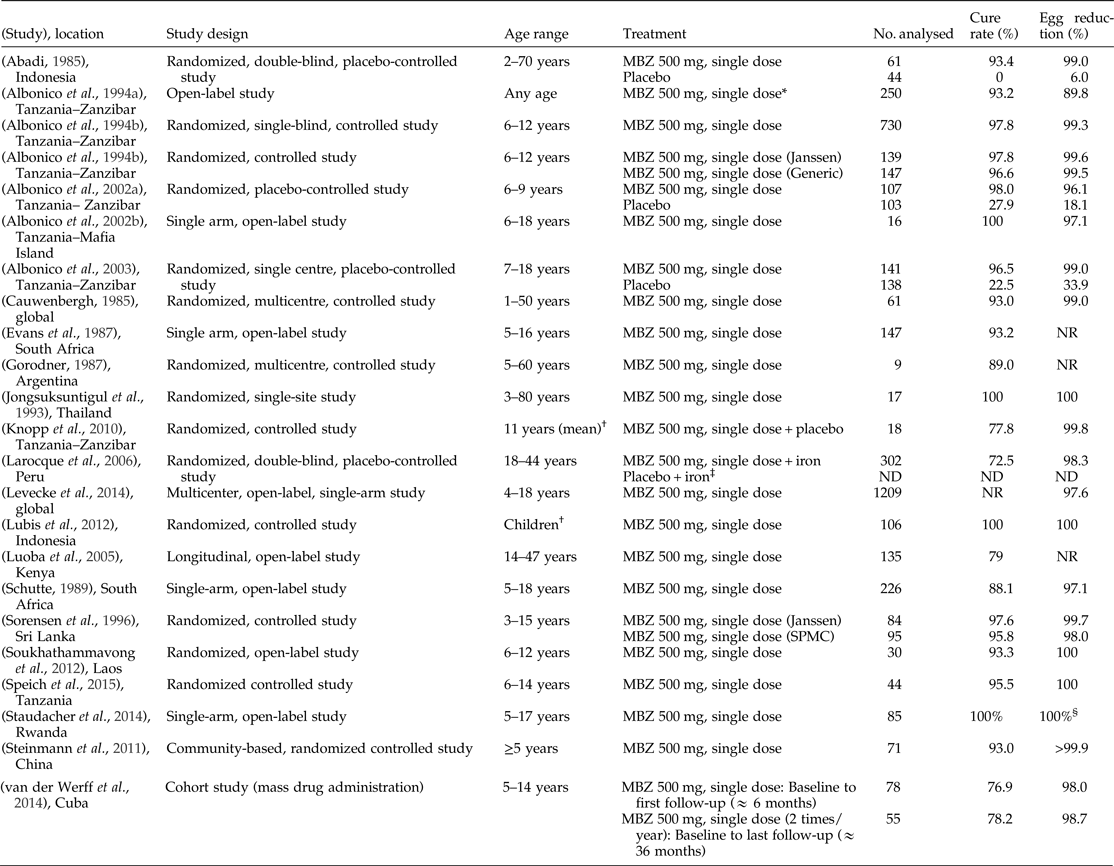
NR, Not reported; ND, data not available; SPMC, State Pharmaceuticals Manufacturing Corporation.
* Data includes MBZ 250 mg, which was administered for 42 children (<2 years).
† Age range not specified.
‡ Results for placebo arm were presented in graphical format without numerical values.
§ Egg reduction rate was assumed to be 100% as cure rate was 100%.
Note: Egg reduction rates were determined inconsistently across studies by using individual mean, group geometric mean and group arithmetic mean.
Of 22 studies identified, four were placebo-controlled studies. The mean cure rate calculated in placebo-controlled studies for mebendazole 500 mg (611 patients) was 84.6% (range: 72.5–98%) compared with 21.0% (range: 0–27.9%) for placebo regimen (285 patients). The mean egg reduction rate for the mebendazole regimen was 98.1% (range: 96.1–99%) compared with 23.9% (6.0–33.9%) for the placebo regimen (tables 1 and 2).
In another study conducted in patients treated with mebendazole 500 mg twice a year for A lumbricoides as part of an MDA programme in Cuba, (van der Werff et al., Reference van der Werff, Vereecken, van der Laan, Campos Ponce, Junco Diaz, Nunez, Rojas Rivero, Bonet Gorbea and Polman2014) a cure rate of 76.9% and egg reduction rate of 98.0% at about 6 months (n = 78) after the first dose and a cure rate of 78.2% and egg reduction rate of 98.7% after 3 years (n = 55) were reported.
Trichuris trichiura
For the treatment of T. trichiura, 23 studies were identified, of which five were placebo-controlled studies (results were not provided for the placebo arm of one study). The mean cure rate calculated from 4407 patients in 21 of 23 studies was 27.6% (range: 8.4–100%) and the mean egg reduction rate calculated from 5424 patients in 22 of 23 studies was 72.5% (range: 31.6–93.0%) (tables 1 and 3).
Table 3. Literature references of single-dose mebendazole (MBZ) 500 mg showing efficacy against Trichuris trichiura.
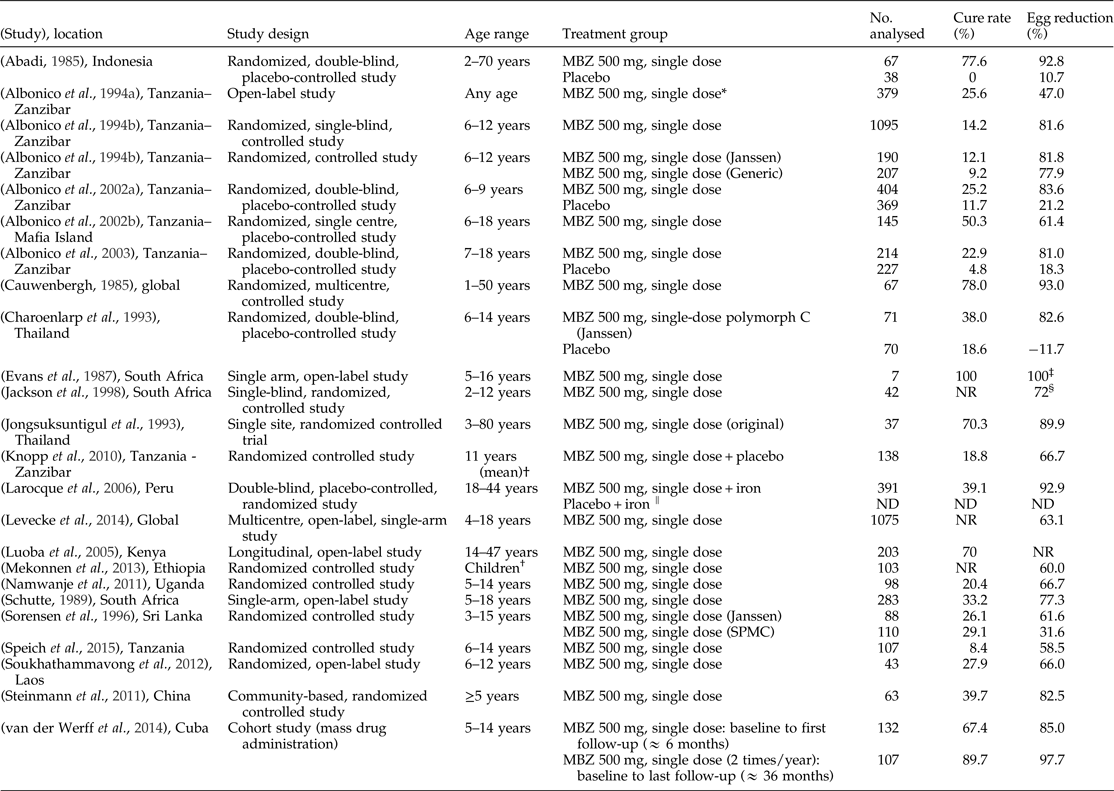
NR, not reported; ND, data not available; SPMC, State Pharmaceuticals Manufacturing Corporation.
* Data includes MBZ 250 mg, which was administered for 42 children (<2 years).
† Age range not specified.
‡ Egg reduction rate was assumed to be 100% as cure rate was 100%.
§ Median value; all other egg reduction rate values are means, although there were variations in assessment (individual means, geometric means and arithmetic means).
‖ Results for placebo arm were presented in graphical format without numerical values.
Note: The negative value represents an increase in egg count.
In the five placebo-controlled studies, from 1147 patients treated with 500 mg single-dose mebendazole, the mean cure rate evaluated was 33.4% (range: 22.9–77.6%) versus 9.5% (range: 0–18.6%) for placebo regimen (704 patients). The mean egg reduction rate calculated was 86.8% (range: 81.0–92.8%) versus 16.4% (range: –11.7% (denoting an increase in mean egg count) to 21.2%) for placebo regimen (tables 1 and 3).
In addition to these single-dose studies, there was one study conducted in Cuba, in which evaluated patients were treated with mebendazole 500 mg twice a year for T. trichiura as part of an MDA programme (van der Werff et al., Reference van der Werff, Vereecken, van der Laan, Campos Ponce, Junco Diaz, Nunez, Rojas Rivero, Bonet Gorbea and Polman2014). At 6 months after the first dose of mebendazole 500 mg (n = 132), the cure rate was 67.4% and egg reduction rate was 85.0% and after 3 years (n = 107), the cure rate was 89.7% and egg reduction rate was 97.7%.(van der Werff et al., Reference van der Werff, Vereecken, van der Laan, Campos Ponce, Junco Diaz, Nunez, Rojas Rivero, Bonet Gorbea and Polman2014).
Hookworms (Necator americanus and Ancylostoma duodenale)
A total of 24 studies were identified that reported the use of a 500 mg single-dose mebendazole regimen for hookworm (N. americanus and A. duodenale) infections. The mean cure rate evaluated from 4600 patients in 23 of 24 studies was 25.5% (range: 2.9–91.1%). The mean egg reduction rate evaluated from 4872 patients in 21 of 24 studies was 72.0% (range: −6.5% (denoting an increase in egg count) to 98.3%).
Of 24 studies, eight were placebo-controlled studies, in which 1211 patients receiving mebendazole treatment and 965 patients receiving placebo treatment were evaluated. However, only seven studies reported the results for placebo. The mean cure rate evaluated in placebo-controlled studies for the 500 mg single-dose mebendazole regimen was 20.5% (range: 2.9–91.1%) versus 7.6% (range: 0.0–33.0%) for the placebo regimen. The mean egg reduction rate for 500 mg single-dose mebendazole regimen was 61.2% (range: −6.5% to 98.3%) versus 18.2% (range: −38.9% to 41.0%) for the placebo regimen (tables 1 and 4).
Table 4. Literature references of single-dose mebendazole (MBZ) 500 mg showing efficacy against hookworms (N. americanus and A. duodenale).
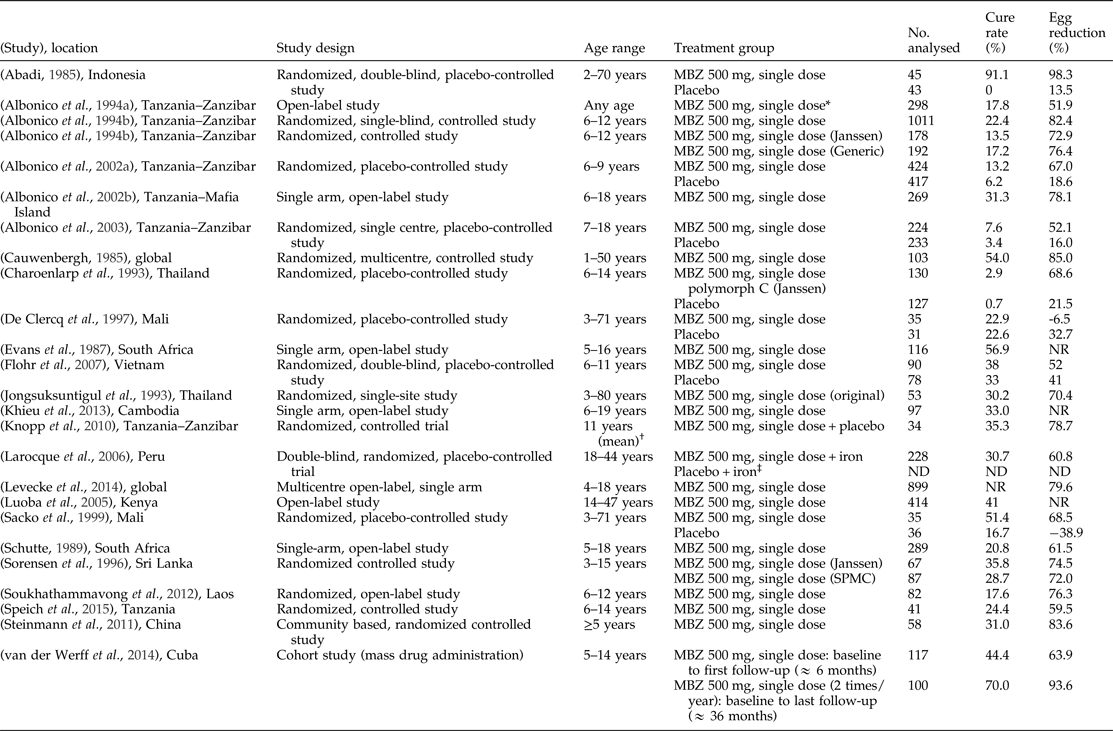
NR, Not reported; ND, data not available; SPMC, State Pharmaceuticals Manufacturing Corporation.
* Data includes MBZ 250 mg, which was administered for 42 children (<2 years).
† Age range not specified.
‡ Results for the placebo arm were presented in graphical format without numerical values.
Note: Egg reduction rates were determined inconsistently across studies by using individual mean, group geometric mean and group arithmetic mean. Negative value represents increase in egg count.
The study conducted in patients treated with mebendazole 500 mg twice a year for hookworms as a part of an MDA programme in Cuba showed a cure rate of 44.4% and egg reduction rate of 63.9% at about 6 months (n = 117) after the first dose and a cure rate of 70.0% and egg reduction rate of 93.6% after about 3 years (n = 100) (van der Werff et al., Reference van der Werff, Vereecken, van der Laan, Campos Ponce, Junco Diaz, Nunez, Rojas Rivero, Bonet Gorbea and Polman2014).
Discussion
This literature review examined the efficacy of single-dose 500 mg mebendazole in the treatment of STH, a neglected tropical disease. In this review, mebendazole 500 mg was found to be highly efficacious against A. lumbricoides infection, with a cure rate of more than 90% and egg reduction rate more than 95%. Although mebendazole 500 mg yielded a lower cure rate in the treatment of T. trichiura and hookworms compared with A. lumbricoides, a substantial egg reduction rate (>70%) was reported for both species. The reduction of egg burden is crucial in order to decrease morbidity and overall transmission of infections (Charoenlarp et al., Reference Charoenlarp, Waikagul, Muennoo, Srinophakun and Kitayaporn1993; Albonico et al., Reference Albonico, Bickle, Ramsan, Montresor, Savioli and Taylor2003; Bethony et al., Reference Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez2006), thus implying a beneficial effect on overall disease control (Montresor, Reference Montresor2011).
In a recently conducted phase 3, randomized, placebo-controlled study (Silber et al., Reference Silber, Diro, Workneh, Mekonnen, Levecke, Steinmann, Umulisa, Alemu, Baeten, Engelen, Hu, Friedman, Baseman and Mrus2017), the efficacy of a 500 mg single dose of a new, rapidly disintegrating, chewable tablet of mebendazole was evaluated in a paediatric population (age 1–15 years). The efficacy results for A. lumbricoides infection (cure rate, 83.7% (95% confidence interval (CI): 74.2%, 90.8%); egg reduction rate, 97.9% (95% CI: 94.4, 99.9)) and for T. trichiura infection (cure rate, 33.9% (95% CI: 25.6%, 42.9%); egg reduction rate, 59.7% (95% CI: 33.9, 78.8)) in the phase 3 study were similar to the results of the overall cohort and the placebo-controlled subset in this literature review. The only exception was egg reduction rate for T. trichiura in the present review (overall: 72.5%; placebo-controlled studies: 86.8%), which was higher than in the phase 3 study (59.7%) and may be, at least partly, explained by the use of differing methods for calculating egg reduction rates among the studies in the literature and the phase 3 study. Overall, the results of this review are consistent with the earlier reviews for the treatment of mebendazole 500 mg against STH (Keiser & Utzinger, Reference Keiser and Utzinger2008; Levecke et al., Reference Levecke, Montresor, Albonico, Ame, Behnke, Bethony, Noumedem, Engels, Guillard, Kotze, Krolewiecki, McCarthy, Mekonnen, Periago, Sopheak, Tchuem-Tchuente, Duong, Huong, Zeynudin and Vercruysse2014). Both albendazole and mebendazole are widely used in the treatment of STH and their efficacy has been discussed and compared previously (Keiser & Utzinger, Reference Keiser and Utzinger2008; Steinmann et al., Reference Steinmann, Utzinger, Du, Jiang, Chen, Hattendorf, Zhou and Zhou2011).
A few studies have indicated treatment failures with anthelmintic drugs (De Clercq et al., Reference De Clercq, Sacko, Behnke, Gilbert, Dorny and Vercruysse1997). Although there is some genetic evidence indicating the appearance of drug resistance in STH, the impact on efficacy of various anthelmintics has not been demonstrated conclusively (Diawara et al., Reference Diawara, Halpenny, Churcher, Mwandawiro, Kihara, Kaplan, Streit, Idaghdour, Scott, Basanez and Prichard2013; Kotze et al., Reference Kotze, Hunt and Skuce2014; Rashwan et al., Reference Rashwan, Scott and Prichard2017). Therefore, the WHO recommends continuous monitoring of efficacy of current drugs used in MDA (WHO, 2013b), which might help to elucidate whether resistance is one of the causes responsible for decreased efficacy.
A potential limitation of this review is that only the articles written in English were selected. The identified studies had differences in study design, definitions and methodology utilized for cure rates and mean percent egg reduction rate, geographical location, time from treatment to assessment, intensity of infection, and other factors that could influence the primary efficacy measures. Also, the calculation of egg reduction rate was not consistent across the literature identified (use of individual means, group means, arithmetic means or geometric means) and thus limited the ability to compare among studies. Moreover, for hookworm infections, in most studies efficacy by specific species (N. americanus and A. duodenale) was not reported.
In conclusion, the present review confirms that single-dose 500 mg mebendazole is effective in the management of STH infections. Higher cure rates were observed for A. lumbricoides than for T. trichiura and hookworms. However, for all species, there was a considerable reduction in the egg burden, which implies the significance of the single-dose 500 mg regimen in controlling STH infections through MDA programmes. Overall, the pooled results of this review, especially the results of placebo-controlled studies, are consistent not only with results reported in earlier reviews of the 500 mg solid tablet (Keiser & Utzinger, Reference Keiser and Utzinger2008; Levecke et al., Reference Levecke, Montresor, Albonico, Ame, Behnke, Bethony, Noumedem, Engels, Guillard, Kotze, Krolewiecki, McCarthy, Mekonnen, Periago, Sopheak, Tchuem-Tchuente, Duong, Huong, Zeynudin and Vercruysse2014), but also with the results of the phase 3 study with a new, chewable 500 mg mebendazole tablet (Silber et al., Reference Silber, Diro, Workneh, Mekonnen, Levecke, Steinmann, Umulisa, Alemu, Baeten, Engelen, Hu, Friedman, Baseman and Mrus2017).
Acknowledgements
Writing support was provided by Ramji Narayanan (SIRO Clinpharm Pvt. Ltd) funded by Janssen Research & Development, LLC, USA. Bradford Challis (Janssen Research & Development, LLC) provided additional editorial support for the development of this review.
Author contributions
J.M., B.B., M.E. and S.A.S. contributed to the concept, data interpretation, drafting and editing of the manuscript. J.M. was involved in the statistical analysis.
All authors contributed to the data interpretation, development and review of this manuscript, and confirm that they have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. All authors met International Committee of Medical Journal Editors (ICMJE) criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the study data and made the final decision about where to publish these data and approved submission to this journal.
Conflict of interest
The study presented in this report was sponsored by Janssen Research & Development, LLC. J.M., S.A.S., B.B. and M.E. were employees of Janssen Research & Development, LLC at the time of the study, and may hold stock or stock options in Johnson & Johnson. J.M. is currently employed at ViiV Healthcare, Research Triangle Park, North Carolina, USA.






