Introduction
Cognitive dysfunction is a highly relevant symptom dimension of major depressive disorder (MDD) (Millan et al., Reference Millan, Agid, Brune, Bullmore, Carter, Clayton and Young2012) because it severely compromises quality of life and complicates remission. Cognitive domains that are predominantly affected include memory, executive function, and attention (Rock, Roiser, Riedel, & Blackwell, Reference Rock, Roiser, Riedel and Blackwell2014).
Interestingly, evidence has been accumulated that cognitive symptoms of MDD, especially impairments in memory and executive function, might be associated with elevated cortisol levels (Behnken et al., Reference Behnken, Bellingrath, Symanczik, Rieck, Zavorotnyy, Domschke and Zwanzger2013; Gomez et al., Reference Gomez, Fleming, Keller, Flores, Kenna, DeBattista and Schatzberg2006; Hinkelmann et al., Reference Hinkelmann, Moritz, Botzenhardt, Riedesel, Wiedemann, Kellner and Otte2009, Reference Hinkelmann, Muhtz, Dettenborn, Agorastos, Moritz, Wingenfeld and Otte2013; O'Hara et al., Reference O'Hara, Schroder, Mahadevan, Schatzberg, Lindley, Fox and Hallmayer2007; Schlosser et al., Reference Schlosser, Mensebach, Rullkotter, Schaffrath, Driessen, Beblo and Wingenfeld2011). Maladaptive changes in the stress systems have been found frequently in MDD patients, including alterations of the hypothalamus pituitary adrenal (HPA) axis with higher basal cortisol release and reduced feedback sensitivity. Reduced glucocorticoid receptor (GR) functions have been suggested as an underlying mechanism (Calfa et al., Reference Calfa, Kademian, Ceschin, Vega, Rabinovich and Volosin2003; Holsboer, Reference Holsboer2000; Pariante & Lightman, Reference Pariante and Lightman2008; Parker, Schatzberg, & Lyons, Reference Parker, Schatzberg and Lyons2003; Waters et al., Reference Waters, Rivalan, Bangasser, Deussing, Ising, Wood and Summers2015; Webster, Knable, O'Grady, Orthmann, & Weickert, Reference Webster, Knable, O'Grady, Orthmann and Weickert2002).
In accordance with the hypothesis of reduced GR sensitivity in MDD patients, previous studies from our group demonstrated that acute cortisol administration took effect on cognitive performance, with lower performance in working memory and retrieval and better performance in response inhibition in healthy control participants but not in MDD patients (Schlosser et al., Reference Schlosser, Wolf, Fernando, Riedesel, Otte, Muhtz and Wingenfeld2010; Schlosser et al., Reference Schlosser, Wolf, Fernando, Terfehr, Otte, Spitzer and Wingenfeld2013; Terfehr et al., Reference Terfehr, Wolf, Schlosser, Fernando, Otte, Muhtz and Wingenfeld2011a, Reference Terfehr, Wolf, Schlosser, Fernando, Otte, Muhtz and Wingenfeld2011b). To some extent, these pharmacological manipulations also mimic parts of the biological stress response.
However, a whole stress response is a complex interplay of related hormone and immune system factors. This explains why divergent effects of stress induction versus cortisol administration on cognitive performance (e.g., executive functions) were also found in previous studies (Shields, Sazma, & Yonelinas, Reference Shields, Sazma and Yonelinas2016). Since stress-related factors such as socioenvironmental events play an important role for the risk and outcome of MDD (Otte et al., Reference Otte, Gold, Penninx, Pariante, Etkin, Fava and Schatzberg2016), it is of importance to understand the effects of acute stress on cognitive performance in these patients. Several studies investigated the effects of acute psychosocial stress on cognitive performance in healthy participants (Domes, Heinrichs, Reichwald, & Hautzinger, Reference Domes, Heinrichs, Reichwald and Hautzinger2002; Jiang & Rau, Reference Jiang and Rau2017; Luethi, Meier, & Sandi, Reference Luethi, Meier and Sandi2008; Olver, Pinney, Maruff, & Norman, Reference Olver, Pinney, Maruff and Norman2015; Schoofs, Preuss, & Wolf, Reference Schoofs, Preuss and Wolf2008). The results of these studies vary between different cognitive domains and show impairing effects of acute psychosocial stress on working memory (Jiang & Rau, Reference Jiang and Rau2017; Luethi, et al., Reference Luethi, Meier and Sandi2008; Olver, et al., Reference Olver, Pinney, Maruff and Norman2015; Schoofs, et al., Reference Schoofs, Preuss and Wolf2008) and attention (Olver, et al., Reference Olver, Pinney, Maruff and Norman2015), improvement of spatial memory (Luethi, et al., Reference Luethi, Meier and Sandi2008) and mixed results for verbal memory (Domes, et al., Reference Domes, Heinrichs, Reichwald and Hautzinger2002; Luethi, et al., Reference Luethi, Meier and Sandi2008; Olver, et al., Reference Olver, Pinney, Maruff and Norman2015).
Comparable studies are missing in MDD patients. However, since important changes in the stress systems have been found in these patients, an acute stress induction, such as psychosocial stress, might have different effects on MDD patients than on healthy control participants as well.
One important factor that might contribute to maladaptive changes in the stress systems is severe early stress experiences, or adverse childhood experiences (ACE) such as physical or sexual abuse. Such experiences increase the risk for MDD, but might also have persistent effects on the stress systems (Otte et al., Reference Otte, Gold, Penninx, Pariante, Etkin, Fava and Schatzberg2016). Furthermore, ACE have been associated with diminished cognitive performance later in life (Hedges & Woon, Reference Hedges and Woon2011; Lovallo et al., Reference Lovallo, Farag, Sorocco, Acheson, Cohoon and Vincent2013; Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011; Pesonen et al., Reference Pesonen, Eriksson, Heinonen, Kajantie, Tuovinen, Alastalo and Raikkonen2013). Therefore, it seems of great importance to take the potential role of ACE into account when associations of stress and cognitive functions are investigated in MDD patients.
With the current study, we intended to systematically investigate the effect of a psychosocial stress induction on cognitive performance in different cognitive domains in individuals with MDD and ACE. To disentangle potential effects of MDD and ACE, MDD patients with and without ACE and healthy participants with and without ACE were included using a full factorial design with the factors MDD and ACE as predictors for cognitive performance after stress. For psychosocial stress induction, we chose the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, Reference Kirschbaum, Pirke and Hellhammer1993) to administer to experimental groups to compare their cognitive performance with participants in a Placebo-TSST group. We expected the largest effects of the stress intervention on cognitive performance for the combination of MDD and ACE factors.
Methods and material
Participants
To investigate the potential effects of MDD and ACE, we recruited women with MDD and ACE (MDD + / ACE +; N = 32); with MDD but without ACE (MDD + / ACE -; N = 52); with ACE but no current or lifetime MDD (MDD - / ACE +; N = 22); and women who had never had any mental disorder and did not report sexual or physical abuse (MDD - / ACE -; N = 37). ACE was defined as repeated sexual or physical abuse at least once a month over a period of one year or more (Heim, Ehlert, & Hellhammer, Reference Heim, Ehlert and Hellhammer2000) before the age of 18 years.
On the first study day, all participants underwent a comprehensive clinical assessment. This assessment included the Structured Clinical Interview for DSM-IV axis I and II to validate psychiatric diagnoses and a semistructured interview, the Early Trauma Inventory (ETI; Bremner, Vermetten, & Mazure, Reference Bremner, Vermetten and Mazure2000; Wingenfeld et al., Reference Wingenfeld, Driessen, Mensebach, Rullkoetter, Schaffrath, Spitzer and Heim2011) and the Childhood Trauma Questionnaire (CTQ; Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia and Zule2003; Wingenfeld et al., Reference Wingenfeld, Spitzer, Mensebach, Grabe, Hill, Gast and Driessen2010) to assess ACE.
All patients of the MDD groups met the diagnostic criteria for current major depressive disorder according to DSM-IV, assessed by SCID-I (Structured Clinical Interview for DSM-IV Axis I disorders). Exclusion criteria for the MDD groups were bipolar disorder, depressive disorder with psychotic features, schizophrenia, schizoaffective disorder, anorexia, and alcohol or drug dependence. Current depressive symptoms were assessed by a clinical interview, the Montgomery Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, Reference Montgomery and Asberg1979; Williams & Kobak, Reference Williams and Kobak2008), and a self-rating questionnaire (Beck Depression Inventory (BDI; Beck, Steer, & Brown, Reference Beck, Steer and Brown1996). Healthy participants with and without ACE were free of any current mental disorder.
Additional exclusion criteria for all participants were severe somatic diseases, CNS or autoimmune diseases, metabolic or endocrine diseases, current infections, pregnancy, or a body mass index (BMI) above 30.
Participants were recruited from our affective disorder clinics and by public posting. Healthy controls were recruited via (online) advertisement.
All participants provided written informed consent. Healthy control participants and outpatients received monetary compensation (€200) for their participation. The study was approved by the local ethical committee.
Procedures
The study protocol included two experimental sessions beginning at 5 p.m. At baseline, salivary samples for analyses of cortisol and alpha-amylase were collected (Salivettes®, blue cap; Sarstedt, Germany) and blood pressure as well as heart rate were measured with an automatic blood pressure monitor for upper arm measurement (Boso Medicus Uno, Bosch + Sohn GmbH u. Co KG) at 0 and +15 minutes. In quasi-randomized order, a psychosocial stress test (TSST) or a control task (P-TSST) was conducted on the first or the second day, respectively. Afterwards, two post-stress salivary samples (+30 and +40 minutes) were collected and blood pressure as well as heart rate were measured. Thirty minutes after the TSST or P-TSST, neuropsychological testing took place and saliva samples were collected before and after neuropsychological testing (+60 and +75 minutes). Blood pressure and heart rate were measured as well. During the break between TSST and neuropsychological testing, participants were invited to consume food from a standardized buffet as a separate part of this study, with results reported elsewhere (Wingenfeld et al., Reference Wingenfeld, Kuehl, Boeker, Schultebraucks, Ritter, Hellmann-Regen and Spitzer2017). There was no difference in the number of consumed calories between groups or between TSST and P-TSST conditions. Apart from the buffet, participants were instructed not to eat after 1 p.m.
Stress induction
We used the Trier Social Stress Test (TSST) as a standardized psychosocial stress test and the Placebo-TSST (P-TSST) as the control condition (Het, Rohleder, Schoofs, Kirschbaum, & Wolf, Reference Het, Rohleder, Schoofs, Kirschbaum and Wolf2009). The TSST reliably induces activation of the HPA axis and consists of three phases: preparation, a speech in front of a trained audience, and an arithmetic task (5 minutes each). The P-TSST is designed to be as similar as possible to the TSST without being stressful to the participant. In an empty room, the participant is asked to talk aloud about a topic of his/her choice after a preparation phase and to do an easy arithmetic task afterwards. We have described the TSST procedure and important outcome variables in more detail in Wingenfeld et al. (Reference Wingenfeld, Kuehl, Boeker, Schultebraucks, Ritter, Hellmann-Regen and Spitzer2017).
Neuropsychological testing
Neuropsychological tests were conducted 30 minutes after the TSST or Placebo-TSST and comprised the Auditory Verbal Learning Test, the Trail Making Test A and B (TMT-A, TMT-B), the letter cancellation test (d2), and the Digit Span Test. All neuropsychological tests were administered by trained psychologists.
Auditory verbal learning test (AVLT; Lezak, Howieson, Loring, Hannay, & Fischer, Reference Lezak, Howieson, Loring, Hannay and Fischer1995)
The AVLT measures short- and long-term verbal memory. For five times, the experimenter reads a list of 15 words (list A), which the participant is requested to repeat in loose order (verbal learning). Then, the participant is asked to reproduce words from a newly presented list (list B) as a distractor. Following this, the participant is asked to recall the words from list A without renewed presentation (immediate recall). After 30 min, the participant is instructed to repeat the words from list A again without renewed presentation (delayed recall). Outcome measures were the amount of recalled words of Trial 5 (measure of verbal learning) and the percentage of recalled words based on the number of recalled words in the delayed trial (delayed recall after 30 min) compared with Trial 5.
Trail-making test (TMT; Reitan, Reference Reitan1992)
With the TMT part A, psychomotoric speed is assessed. In this task, the participant has to connect encircled numbers in ascending order as quickly as possible. With part B, executive function is also assessed. This test requires the alternation between numbers and letters in ascending order. The score of each part is represented by the time needed to complete the task. Additionally, the difference score (B minus A) reflects switch costs, a relatively pure indicator of executive function (Sanchez-Cubillo et al., Reference Sanchez-Cubillo, Perianez, Adrover-Roig, Rodriguez-Sanchez, Rios-Lago, Tirapu and Barcelo2009).
Forward and backward digit span (Tewes, Reference Tewes1991)
This test is part of the Wechsler Adult Intelligence Scale (WAIS). During the forward digit span task, participants are asked to remember a series of digits and to repeat them in the same order. During the backward digit span task, participants are asked to repeat the digits in reverse order. The number of correctly remembered digits is a measure of working memory.
Test d2 (Brickenkamp, Reference Brickenkamp1978)
The Test d2 is a letter cancellation test that measures selective and sustained attention. On a paper with rows of target and distractor stimuli, the participant is instructed to cross out the letter “d” whenever it is accompanied by two small lines; d's with more or less than two lines or any stimuli containing the character “p” serve as distracters. After a practice trial, there are 14 rows with target and distracter stimuli. The number of correctly identified items serves as an indicator of selective attention, the difference of correctly identified items minus wrongly identified items is a measure of sustained attention.
Data analysis
Demographic and clinical characteristics were analyzed with ANOVA for continuous variables or a chi-squared test for dichotomous variables.
We analyzed whether the TSST evoked an increased stress response compared with the P-TSST. For cortisol and alpha-amylase, we additionally computed the area under the curve with respect to increase (AUCi) (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). Because both variables were not normally distributed, we log-transformed raw values and conducted 2 × 4 repeated measures ANOVAs with the factors Stress (TSST vs. P-TSST) and Group (MDD-/ ACE-; MDD-/ ACE+; MDD+/ ACE-; MDD+/ ACE+) for statistical analyses. The two AUCi variables served also as predictors in the models described below. Further analyses of biological and subjective stress responses are presented in detail in Wingenfeld et al. (Reference Wingenfeld, Kuehl, Boeker, Schultebraucks, Ritter, Hellmann-Regen and Spitzer2017).
We used mixed-model linear regression analyses (unstructured covariance type, maximum likelihood method) to analyze the influence of the factors MDD and ACE on neuropsychological performance after the stress (TSST) and non-stress (P-TSST) induction conditions. The outcome measure of each neuropsychological test served as the dependent variable. For the computations, MDD, ACE, and Stress (repeated factor) were defined as fixed factors in model I. To control for cortisol and alpha-amylase response, we included the AUCi of cortisol and alpha-amylase as covariates in addition to the fixed factors MDD and ACE in model II. Akaike's Information Criterion (AIC) was used to assess model fit for each outcome variable.
In a follow-up analysis, we investigated whether antidepressant medication had an effect on cognitive performance in our study. Within the depressive participants, we calculated a 2 × 2 ANOVA for each dependent variable with the factors ACE (yes/no) x Medication (yes/no). For explorative purposes, we calculated bivariate correlations between neuropsychological outcome variable and childhood trauma (CTQ sum score) measures and depressive symptoms (BDI score).
Data analysis was performed using SPSS statistical software (SPSS 22.0, Inc., Chicago, IL, USA). A p-value smaller than .05 was considered to indicate statistical significance.
Results
Sample characteristics
Demographic and clinical data are shown in Table 1. The groups did not differ in age, years of education, smoking, and intake of oral contraceptives. None of the healthy participants (MDD - / ACE -, MDD - / ACE +) took any psychotropic medication, whereas several patients with MDD (MDD + / ACE +; MDD + / ACE -) received antidepressant medication (see also Table 1). However, the MDD patients with ACE did not differ in current medication intake from MDD patients without ACE (MDD + / ACE + 37% with antidepressant medication; MDD + / ACE -, 44% with antidepressant medication), χ 2 = .37, df = 1, p = .543.
Table 1. Demographic and clinical characteristics of patients with MDD with and without ACE and healthy participants with and without ACE

MDD = Major depressive disorder; ACE = Adverse childhood experiences; MDD + / ACE + = MDD patients with ACE; MDD + / ACE - = MDD patients without ACE; MDD - / ACE + = participants with ACE but no MDD; MDD - / ACE - = healthy control participants; SNRI = selective serotonin and noradrenaline reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; NDRI = dopamine and noradrenergic reuptake inhibitor; MADRS = Montgomery Asberg Depression Rating Scale; BDI = Beck Depression Inventory; ETI = Early Trauma Inventory; CTQ = Childhood Trauma Questionnaire; BMI = body mass index; OC = oral contraceptives; SD = standard deviation
Following our inclusion and exclusion criteria, none of the controls (MDD - / ACE -) and none of the ACE group (MDD - / ACE +) met any mental disorder diagnoses as assessed with the SCID interview. Of the MDD patients with ACE (MDD + / ACE +), eight patients met criteria for a comorbid anxiety disorder, one patient met criteria for an obsessive-compulsive disorder, two patients met criteria for a somatic symptom disorder, and one patient met criteria for borderline personality disorder. Of the MDD patients without ACE (MDD + / ACE -), 14 showed a comorbid anxiety disorder, four a somatic symptom disorder and one met criteria of borderline personality disorder or avoidant personality disorder.
In accordance with our recruitment, MDD patients with ACE (MDD + / ACE +) and healthy participants with ACE (MDD - / ACE +) had significantly higher ETI and CTQ scores compared with the MDD patients without ACE (MDD + / ACE -) and the MDD - / ACE - group (ETI: F (3, 139) = 29.30, p < .001; CTQ: F (3, 137) = 55.63, p < .001). MDD patients with and without ACE (MDD +/ ACE +, MDD + / ACE -) had higher depression scores compared with the MDD - / ACE + group and the MDD - / ACE – group, as expected (MADRS: F (3, 139) = 318.55, p < .001; BDI: F (3, 135) = 43.65, p < .001). For ETI and CTQ sum scores, post hoc tests (Bonferroni) revealed significantly higher scores in both ACE groups (MDD +/ ACE +, MDD - / ACE +) compared with patients and participants without ACE (MDD +/ ACE -, MDD - / ACE -), but no significant difference between both ACE groups (MDD +/ ACE +, MDD - / ACE +). More detailed information is provided in (Wingenfeld et al., Reference Wingenfeld, Kuehl, Boeker, Schultebraucks, Ritter, Hellmann-Regen and Spitzer2017).
Stress response
For area under the curve (AUC) values, separate 2 (Stress) x 4 (Group) ANOVAs were computed for AUCi of cortisol and alpha-amylase levels. For AUCi of cortisol levels, a significant main effect of Stress occurred, F (1, 139) = 37.4, p < .001, indicating successful stress induction by the TSST compared with the P-TSST. Concerning alpha-amylase levels, we found no significant effects (see Table 2). Results of further biological and subjective stress responses (saliva cortisol and alpha-amylase, blood pressure, heart rate, subjective stress ratings) are presented in detail in (Wingenfeld et al., Reference Wingenfeld, Kuehl, Boeker, Schultebraucks, Ritter, Hellmann-Regen and Spitzer2017).
Table 2. Area under the curve with respect to increase (AUCi) for cortisol and alpha-amylase levels (log mean) in the Placebo-TSST (no stress) and TSST (stress) condition

MDD = Major depressive disorder; ACE = Adverse childhood experiences; MDD + / ACE + = MDD patients with ACE; MDD + / ACE - = MDD patients without ACE; MDD - / ACE + = participants with ACE but no MDD; MDD - / ACE - = healthy control participants; TSST = Trier Social Stress Test; P-TSST = Placebo Trier Social Stress Test; SD = Standard deviation; n.s. = not significant.
Neuropsychological testing after TSST and Placebo-TSST
We used mixed-models linear regression analysis to study the relationship between MDD, ACE, stress induction, and the performances in the different neuropsychological tests. For all test outcomes, the basic model I with MDD, ACE, and Stress as fixed factors was the best fitting model according to the minimum AIC (see Table 3). Increase of cortisol and alpha-amylase did not contribute significantly to the model.
Table 3. Overview of the neuropsychological test results of the best fitting model (according to the minimum Akaike's Information Criterion)
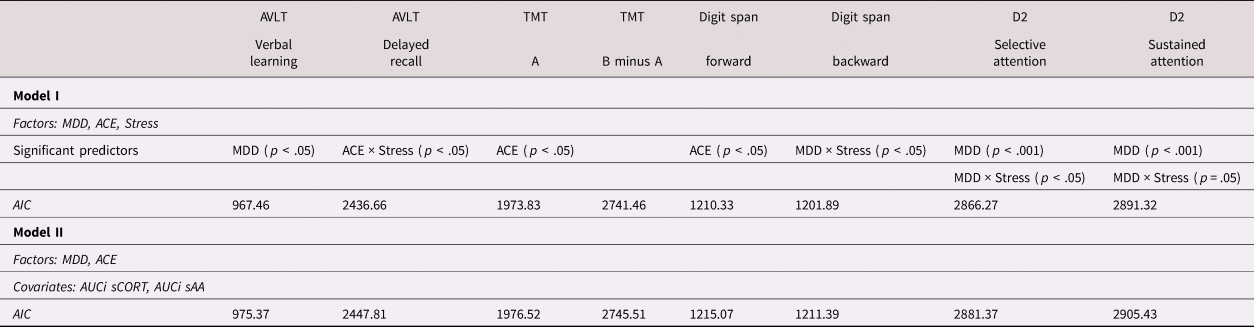
AVLT: Auditory verbal learning test; TMT: Trail-making test; digit span: Forward and backward digit span test; d2: Test d2, MDD: Major depressive disorder; ACE: Adverse childhood experiences; AUCi: Area under the curve with regard to increase; sCORT: saliva cortisol levels; sAA: saliva alpha-amylase levels; AIC: Akaike's Information Criterion
Auditory verbal learning test (AVLT)
For the AVLT, verbal learning, calculated by the recalled words from Trial 5, served as the dependent variable. As an additional dependent variable, we calculated the percentage of delayed recalled words based on the number of recalled words in the delayed trial compared with those from Trial 5.
Concerning verbal learning, MDD was a significant predictor (Trial 5: F (1, 143) = 5.16, p = .025; AIC = 967.46). That is, participants with MDD recalled fewer words than did participants without MDD (see also Figure 1a). We found no influence of medication as a main effect, F (1, 80) = 0.01, p = .924, or via interaction with ACE, F (1, 80) = 1.17, p= .283).

Figure 1. (a) Recalled words of Trial 5 of the AVLT (Auditory Verbal Learning Test), according to the factors MDD and ACE as well as the stress conditions and P-TSST. Here, MDD was a significant predictor. (b) Percentage of recalled words in the delayed trial (delayed recall) of the AVLT, according to the factors MDD and ACE as well as the stress conditions TSST and P-TSST. Here, ACE × Stress was a significant predictor. Error bars indicate standard errors. MDD, major depressive disorder. ACE, adverse childhood experiences. TSST, Trier Social Stress Test. P-TSST, placebo-TSST.
Concerning delayed recall, the ACE x Stress interaction was a significant predictor (F (1, 143) = 6.19, p = .014, AIC = 2436.66), indicating that participants with ACE were more likely to recall fewer words in the delayed Trial 7 (as a percentage compared with Trial 5) after stress (TSST) than after non-stress (P-TSST) conditions compared with participants without ACE (see also Figure 1b).
We found no influence of medication as a main effect, F (1, 80) = 1.63, p = .205) or via interaction with ACE (F (1, 80) = 2.18, p = .144).
Trail-making test (TMT)
For the TMT, the speed (in seconds) to complete part A, as a measure of psychomotoric speed, and the difference score B minus A, as a measure of executive function, served as dependent variables.
For part A, ACE was a significant predictor, F (1, 142) = 4.00, p = .048; AIC = 1973.83. That means participants with ACE were more likely to complete part A more slowly than participants without ACE (see Figure 2a). We found no influence of medication as a main effect, F (1, 80) = 0.33, p = .569, or via interaction with ACE (F (1, 80) = 2.38, p = .127).

Figure 2. Mean reaction times to complete (a) Part A and (b) the difference score of B minus A of the TMT (Trail-making test) according to the factors MDD and ACE as well as the stress conditions TSST and P-TSST. For Part A, ACE was a significant predictor, while there was no significant result for the difference score. Error bars indicate standard errors. MDD, major depressive disorder, ACE, adverse childhood experiences. TSST, Trier Social Stress Test. P-TSST, placebo-TSST.
For the difference score B minus A, there was no significant result (AIC = 2741.46; see Figure 2b). We found no influence of medication as a main effect, F (1, 80) = 0.03, p = .871, or via interaction with ACE, F (1, 80) = 1.44, p = .234.
Forward and backward digit span
Here, the number of correctly remembered digits of the digit span forward and the digit span backward as measures of working memory served as dependent variables.
For digit span forward, ACE was a significant predictor, F (1, 142.52) = 6.47, p = .012; AIC = 1210.33). That is, participants with ACE were more likely to remember fewer digits compared with participants without ACE (see Figure 3a). We found no influence of medication as a main effect, F (1, 80) = 1.30, p = .257, or via interaction with ACE, F (1, 80) = 1.07, p = .304.

Figure 3. Mean numbers of correctly remembered digits of (a) digit span forward and (b) digit span backward as measures of working memory according to the factors MDD and ACE as well as the stress conditions TSST and P-TSST. For digit span forward, ACE was a significant predictor. For digit span backward, MDD × Stress was a significant predictor. Error bars indicate standard errors. MDD, major depressive disorder. ACE, adverse childhood experiences. TSST, Trier Social Stress Test. P-TSST, placebo-TSST.
For digit span backward, the MDD x Stress interaction was a significant predictor, F (1, 142.29) = 4.28, p = .040; AIC = 1201.89. This means that participants with MDD were more likely to remember fewer digits after the stress (TSST) than after the non-stress (P-TSST) condition compared with participants without MDD (see Figure 3b). We found no influence of medication as a main effect, F (1, 80) = 2.63, p = .109, or via interaction with ACE, F (1, 80) = 0.16, p = .689.
Test d2
For the d2, the score for selective attention (number of correctly identified items) and the score for sustained attention (difference of correctly minus wrongly identified items) served as dependent variables.
For selective attention, MDD (F (1, 141.86) = 19.21, p < .001; AIC = 2866.27) and the MDD x Stress interaction were significant predictors, F (1, 140.73) = 4.97, p = .027. This means that participants with MDD were more likely to identify fewer items and even fewer after stress (TSST) than after non-stress (P-TSST) conditions compared with participants without MDD (see Figure 4a). We found no influence of medication as a main effect, F (1, 80) = 0.44, p = .510, or via interaction with ACE, F (1, 80) = 0.00, p = .987.

Figure 4. Mean numbers of (a) correctly identified items of the d2 test (measure of selective attention, and of (b) the difference score of correctly minus wrongly identified items (measure of sustained attention according to the factors MDD and ACE as well as the stress conditions TSST and P-TSST. For selective attention, MDD and MDD × Stress were significant predictors. For sustained attention, MDD was a significant predictor and MDD × Stress almost a significant predictor. Error bars indicate standard errors. MDD, major depressive disorder. ACE, adverse childhood experiences. TSST, Trier Social Stress Test. P-TSST, placebo-TSST.
For sustained attention, MDD was also a significant predictor (F (1, 142.11) = 19.39, p < .001; AIC = 2891.32), and the MDD x Stress interaction was almost a significant predictor, F (1, 142.11) = 3.89, p = .050, both results tending in the same direction as the results for selective attention (see Figure 4b). We found no influence of medication as a main effect, F (1, 80) = 0.76, p = .385, or via interaction with ACE, F (1, 80) = 0.01, p = .915.
Relationship between degree of MDD or ACE and neuropsychological measures
We found a negative association between CTQ and AVLT delayed recall after TSST, r = −.17, p = .04, and a positive association between CTQ sum and TMT A after TSST, r = .24, p = .004. After the TSST, there were negative associations between BDI and AVLT verbal learning, r = −.22, p = .01, selective attention, r = −.22, p = .02, and sustained attention, r = −.19, p = .03. Furthermore, BDI correlated positively with TMT B-A after TSST, r = .25, p = 005. In the P-TSST condition there was a positive association between BDI and TMT B-A, r = .23, p = 008. These explorative results fit to our mixed-model analyses.
Discussion
With the current study, we intended to systematically investigate the effects of an acute psychosocial stress induction on cognitive performance in individuals with MDD and ACE. MDD patients with and without ACE and healthy participants with and without ACE were included to disentangle potential effects of MDD and ACE on the association between stress and cognitive performance. Overall, the diagnosis of MDD and the experience of childhood adversity were significant predictors for a lower performance in specific cognitive tasks. MDD could predict a lower performance in verbal learning (measured with the AVLT) and in selective and sustained attention (measured with the test d2), while ACE predicted a lower performance in working memory (measured with the digit span test) and psychomotoric speed (measured with part A of the TMT).
Deficits in learning and memory, attention, working memory, and psychomotoric speed have previously been demonstrated in MDD patients (Lee, Hermens, Porter, & Redoblado-Hodge, Reference Lee, Hermens, Porter and Redoblado-Hodge2012; Roca, Vives, Lopez-Navarro, Garcia-Campayo, & Gili, Reference Roca, Vives, Lopez-Navarro, Garcia-Campayo and Gili2015; Rock et al., Reference Rock, Roiser, Riedel and Blackwell2014) and in individuals with ACE (Hedges & Woon, Reference Hedges and Woon2011; Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011). However, potential confounding effects of MDD and ACE cannot be ruled out in many studies. Because of the four-group design of the current study, we could systematically investigate the effects of MDD and ACE. The results revealed only one of the factors, MDD or ACE, as a significant predictor for different test outcomes, which might indicate that each factor influences distinct cognitive domains. Of course, these findings need further replication. So far, only a few studies have investigated the effects of MDD and ACE on cognitive performance systematically. For example, Saleh et al. (Reference Saleh, Potter, McQuoid, Boyd, Turner, MacFall and Taylor2017) investigated the effects of ACE on depression, cognitive performance, and brain morphology in depressed patients and never-depressed individuals. In line with our results, individuals with ACE—or more specifically with the subtypes emotional and sexual abuse and severe family conflicts—showed lower performance in processing speed and working memory independently of an MDD diagnosis. Other cognitive domains were not affected. Furthermore, associations of cognitive function and brain structure including smaller volumes of orbitofrontal cortex and decreased cortical thickness in multiple areas were found in these individuals, which might at least partly explain the results. In a recent review, Chen and Baram (Reference Chen and Baram2016) explain how ACE in the sense of early life stress can influence the maturation of brain networks underlying cognitive functions such as hippocampal circuitry. As one important mechanism, alterations of the hypothalamic-pituitary-adrenal axis are described. For MDD, accumulated research suggests that stress-associated alterations in inflammatory and glucocorticoid signaling are associated with corresponding functional changes in multiple brain networks that might contribute to cognitive dysfunction (Otte et al., Reference Otte, Gold, Penninx, Pariante, Etkin, Fava and Schatzberg2016). However, confounding effects of MDD and ACE might play a role in some of these studies as well.
Interestingly, stress alone had no predictive value in the current study. Indeed, the Stress condition (TSST vs. P-TSST) predicted cognitive performance only in interaction. In depressed patients, stress predicted the lowest performance of working memory and selective attention (measured with the digit span and d2 test). In individuals with ACE, only verbal memory (measured with the AVLT) was affected by stress. Our results might indicate that patients with MDD or individuals with ACE were more susceptible to the negative effects of acute psychosocial stress, which could reflect alterations in signaling pathways or brain networks as mentioned above. Of note, the cortisol stress response to the TSST was rather blunted in the MDD and ACE groups compared with healthy controls, which is in line with previous research (Bunea, Szentagotai-Tatar, & Miu, Reference Bunea, Szentagotai-Tatar and Miu2017; Zorn et al., Reference Zorn, Schur, Boks, Kahn, Joels and Vinkers2017). Still, we found an increase of cortisol across groups after the TSST, thereby validating the experimental stress manipulation (for detailed results of biological and subjective responses to the TSST and P-TSST, please see Wingenfeld et al. (Reference Wingenfeld, Kuehl, Boeker, Schultebraucks, Ritter, Hellmann-Regen and Spitzer2017). However, the statistical models that included measures of cortisol and alpha-amylase increase did not provide additional informational value. In previous studies using exogenous cortisol administration, we did not find effects in healthy participants (Deuter, Wingenfeld, Schultebraucks, Otte, & Kuehl, Reference Deuter, Wingenfeld, Schultebraucks, Otte and Kuehl2019) or MDD patients (Schlosser et al., Reference Schlosser, Wolf, Fernando, Riedesel, Otte, Muhtz and Wingenfeld2010; Schlosser et al., Reference Schlosser, Wolf, Fernando, Terfehr, Otte, Spitzer and Wingenfeld2013; Terfehr et al., Reference Terfehr, Wolf, Schlosser, Fernando, Otte, Muhtz and Wingenfeld2011a, Reference Terfehr, Wolf, Schlosser, Fernando, Otte, Muhtz and Wingenfeld2011b). In individuals with ACE, such studies are missing. However, we have investigated patients with PTSD caused by childhood trauma and found improved memory performance after hydrocortisone administration (Wingenfeld et al., Reference Wingenfeld, Driessen, Terfehr, Schlosser, Fernando, Otte and Wolf2012). Of course, psychosocial stress induction differs markedly from exogenous cortisol administration and has been shown to affect cognitive processes differently (Shields et al., Reference Shields, Sazma and Yonelinas2016). Thus, other than stress-associated cortisol, related biological processes such as further hormone and immune system factors or psychological processes such as rumination might have played an important role as well in our study. Future studies should further investigate which factors mediate stress induced effects on cognition in patients with MDD and individuals with ACE.
While there is a lack of studies investigating the effects of acute psychosocial stress on cognitive performance in MDD patients and traumatized individuals, several studies have investigated this topic in healthy participants. For the cognitive domains of working memory, attention and delayed verbal recall, some studies show impairments in healthy participants after the TSST (Jiang & Rau, Reference Jiang and Rau2017; Luethi et al., Reference Luethi, Meier and Sandi2008; Olver et al., Reference Olver, Pinney, Maruff and Norman2015; Schoofs et al., Reference Schoofs, Preuss and Wolf2008), while other studies found no effects of acute psychosocial stress (Hidalgo et al., Reference Hidalgo, Villada, Almela, Espin, Gomez-Amor and Salvador2012; Luettgau, Schlagenhauf, & Sjoerds, Reference Luettgau, Schlagenhauf and Sjoerds2018; Vrshek-Schallhorn, Velkoff, & Zinbarg, Reference Vrshek-Schallhorn, Velkoff and Zinbarg2018), at least not in younger (Hidalgo, Almela, Villada, & Salvador, Reference Hidalgo, Almela, Villada and Salvador2014) or female subsamples (Espin et al., Reference Espin, Almela, Hidalgo, Villada, Salvador and Gomez-Amor2013; Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, Reference Wolf, Schommer, Hellhammer, McEwen and Kirschbaum2001). The latter findings are in line with our results, which showed no main effect of the stress intervention. Such heterogeneous findings might be due to differences in the paradigms or in the study protocols. In addition, gender differences might play a role. Especially for effects of the TSST on cognitive performance, several studies explicitly emphasize the importance of gender (Cornelisse, van Stegeren, & Joels, Reference Cornelisse, van Stegeren and Joels2011; Dos Santos, Leyendecker, Costa, & de Souza-Talarico, Reference Dos Santos, Leyendecker, Costa and de Souza-Talarico2018; Espin et al., Reference Espin, Almela, Hidalgo, Villada, Salvador and Gomez-Amor2013; Schoofs, Pabst, Brand, & Wolf, Reference Schoofs, Pabst, Brand and Wolf2013; Zandara et al., Reference Zandara, Garcia-Lluch, Pulopulos, Hidalgo, Villada and Salvador2016). Our sample was restricted to females, thus it is unclear whether our results are applicable to men. This seems particularly important in the light of studies showing differences of acute stress effects on cognitive performance between men and women. As order of task was not randomized, we could not rule out that rising tiredness or reduced attention might have influenced our results. Furthermore, we only measured cortisol and alpha-amylase, but not ACTH release or other factors in response to stress which limits our interpretations of the stress response effects. It is possible that taking antidepressant medication has an influence on the cognitive measures we assessed. Accordingly, we took care to ensure that the proportion of medicated patients in the groups with/without ACE was not different. In order to check the influence of medication, additional post hoc analyses were calculated. There were no differences between patients with or without medication in all dependent measures, both for the main effects Medication and in the interactions Medication x ACE.
Besides these limitations, our study had several strengths. We used a stress task as well as a control condition to investigate the endocrine stress response and effects on cognitive performance in ACE and MDD. Additionally, the presence of any mental disorder was an exclusion criterion in the ACE group, which is a major strength of the study because it allowed us to systematically differentiate between the factors MDD and ACE.
Conclusions
With the current study, we investigated the differential effects of a psychosocial stress induction on cognitive performance by using established neuropsychological tests in a four-group factorial design in which the factors MDD and ACE were fully crossed. In sum, our results indicate a negative effect of MDD and ACE on distinct cognitive domains. Thereby, our results underline the importance of systematically studying the effects of MDD and ACE on cognitive impairments to understand underlying mechanisms. Furthermore, the presence of MDD and/or ACE seemed to enhance susceptibility for stress-related impairments of cognitive performance. Future studies should further investigate which factors mediate such stress-induced effects on cognition and should additionally include male participants to investigate potential gender differences.
Acknowledgements
The study was supported by grant of the Deutsche Forschungsgemeinschaft (WI 3396/6-1& SP 579/3-1) awarded to Dr. Wingenfeld, Dr. Spitzer, and Dr. Otte.










