Introduction
Three-dimensional conformal radiotherapy (3D-CRT) is still commonly used in the treatment of different cancer sites in both curative and palliative settings. In 3D-CRT, beam arrangements are designed to enter the patient using different angles designed to minimise radiation exposure to organ at risk (OAR) and healthy tissues. This can be challenging if the treatment site or the target volume is located in a place where beam arrangements cannot be straightforward or standardised. Reference Starkschall and Eifel1,Reference Ezzel2 For example, a lesion where multiple critical structures are adjacent to the target. Therefore, in order to produce an optimal plan, a number of planning parameters Reference Sherouse3,Reference Dai and Zhu4 (i.e., beam angles, beam weights and beam modifiers) are manually adjusted until the plan satisfies the desired dose coverage. Overall, manual adjustment of planning parameters (forward planning) is performed using an iterative method which is a time-consuming trial and error procedure. For novice planners, this process can take considerably longer. Additionally, it is more difficult to achieve the required dose distribution in complicated cases. To overcome these issues, a number of studies have developed models on the selection and optimisation of the beam geometry for 3D-CRT. Reference Xing, Hamilton, Pelizzari and Chen5–Reference Bortfeld and Schlegel7 Bortfeld and Schlegel investigated the optimisation of the beam orientation for irradiation using multiple fixed beams. Reference Bortfeld and Schlegel7 The study concluded that using an equiangular distribution is optimal for a sufficient number of beams. Sherouse et al. proposed a mathematical method for selection of wedge angle and orientation. Reference Sherouse3 While the method had the potential for automatic selection of the beam parameters, it was found that pre-selecting the wedge angle in the optimisation process increased the computation time. Reference Xing, Hamilton, Pelizzari and Chen5 An alternative method for dose calculation can be achieved by using inverse planning. In inverse planning, clinical objectives defined in the planning system by the user which the calculation algorithm will automatically determine the most optimal treatment parameters which best match the clinical objectives. Inverse planning employs a beam angle optimiser (BAO) tool that is integrated into the treatment planning system (TPS) software, to optimise the beam angle for intensity-modulated radiotherapy (IMRT) by interactively optimising the gantry angle. Reference Pesola8 The selection of beam geometry for IMRT has been studied extensively in the literature. Reference Rocha, Dias, Ferreira and Lopes9–Reference Ghanbarzadeh, Pouladian, Shabestani Monfared and Mahdavi13 While the BAO tool has been extensively used for IMRT BAO, it is not commonly used for 3D-CRT plan optimisation. The authors could not locate a published study that has been conducted on the use of the Eclipse BAO for 3D-CRT planning. The primary aim of this study was to see if using the BAO for 3D-CRT planning would produce better plans as compared to conventional methods. Secondary aims were to assess whether there was a reduction in the planning time, and the quality of the plan produced, irrespective of the planner’s skill set.
Materials and Methods
Eleven 3D-CRT lung patients who were previously planned using a manual BAO method (non-BAO) were retrospectively replanned using the Eclipse BAO method. All patients were planned following the standard departmental protocol. The patients were immobilised using standard site specific equipment and then scanned using a Phillips Big Bore computed tomography (CT) scanner (Philips Healthcare, Amsterdam, Netherlands) with a 2-mm slice thickness. CT datasets were exported to the Eclipse TPS (Version 13.6, Varian Medical Systems, Palo Alto, CA, USA) for planning. The radiation oncologist (RO) contoured the clinical target volume and then expanded this volume to create the planning target volume (PTV). The final dose calculations were performed using Eclipse’s anisotropic analytical algorithm, with a grid size of 0·25 cm with inhomogeneity corrections applied. The treatment plans were delivered on a Varian iX linear accelerator (Varian Medical Systems) equipped with a Millennium 120 multi-leaf collimator. In the non-BAO method, all plans were created by experienced planners using forward planning processes based on the target and OAR objectives specified by the RO and in accordance with critical structure dose tolerances. The beam angles selected were based on the planner’s experience and departmental protocols and were adjusted through a trial and error process to produce a plan that best met the desired plan objectives as per RO specifications. This manual method is driven by the treatment planner and usually depends heavily on the visual dosimetric evaluation performed by the planner and oncologist. The BAO method was performed using the BAO tool in the Eclipse TPS. BAO uses only coplanar beams and the total number of beams used ranged between 5 and 9 depending on the beam selection process by the optimisation algorithm. The prescribed dose to target volume and OAR constraints were kept the same as for the original plans. After the optimal gantry angle determination, the plans were adjusted minimally by selecting appropriate collimator angles, wedges and shielding. The final dose calculations were performed using similar clinical plan settings. The generated plans using both methods were evaluated by comparing the isodose distributions and dose volume histograms (DVHs) for the PTVs and OARs, comparing the conformity index (CI) and homogeneity index (HI) for the PTV, and other dosimetric parameters for OARs. For the dosimetric comparisons, the constraints were set as: spinal cord maximum dose < 45 Gy, doses to 67% and 33% volumes of the heart were 45 and 60 Gy, respectively and lung dose (lung V20). The total monitor units (MUs) and total planning time used for each type of plan were also compared.
Results and Discussion
The generated dose distributions for the 3D-CRT plans using the non-BAO and BAO methods achieved acceptable coverage for the target volume as depicted in the DVH in Figure 1. The results in Figure 1 revealed similar DVHs for OARs, thus confirming that both methods achieved comparable outcomes. Overall, the BAO method produced slightly better results as compared to non-BAO method.
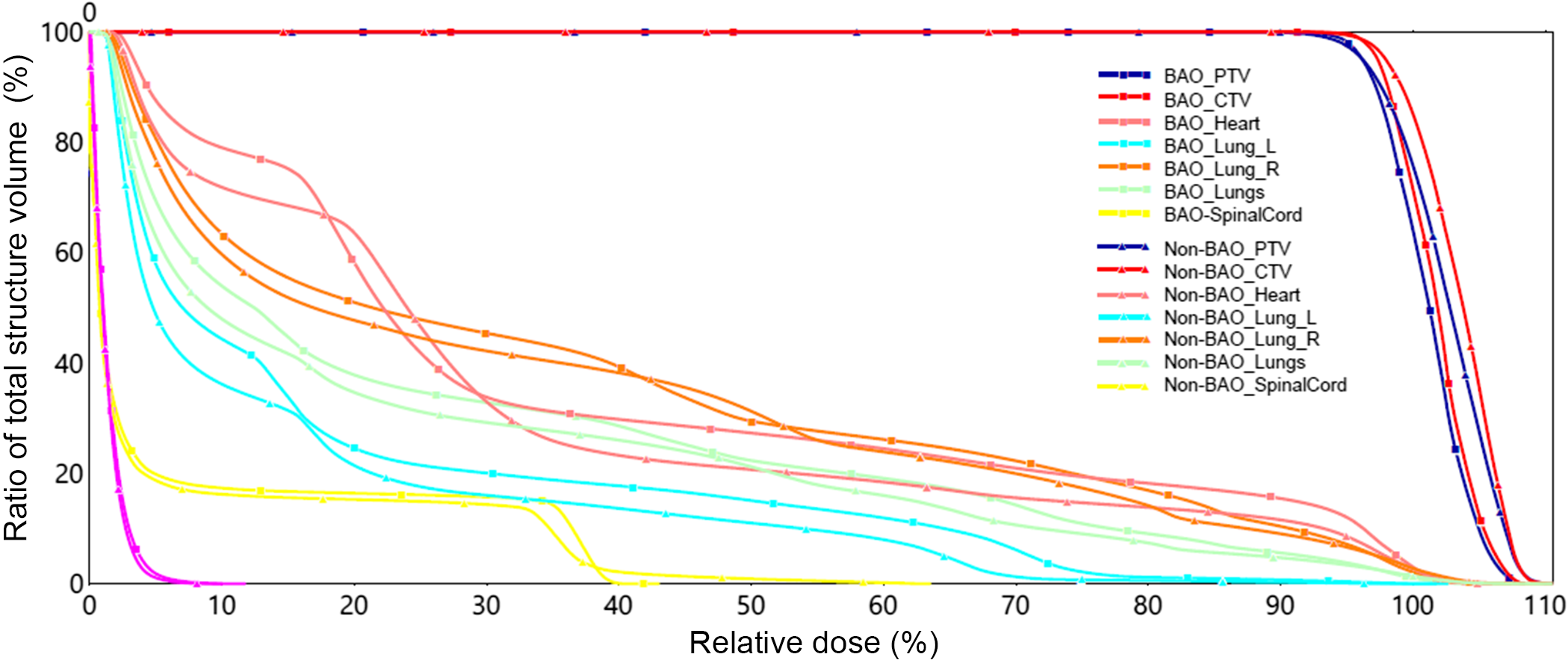
Figure 1. DVH comparison for Non-BAO and BAO methods.
Table 1 shows comparisons of CI and HI indices for both non-BAO and BAO methods for all the 11 cases studied. The CI and HI indices were used to evaluate the PTV dose coverage for each plan. A CI is a measure of how well the volume of a dose distribution conforms to the size and shape of a target volume. CI and HI indices were compared using t-test, and the numerical values are listed in Table 1. The results showed that the indices values for both non-BAO and BAO methods are not significantly different (p > 0·05).
Table 1. Comparison of conformity and homogeneity indices for non-BAO and BAO methods

The most remarkable results are observed by comparing and analysing the total MUs and total planning time calculated for both methods. Table 2 shows comparisons of the MUs between non-BAO and BAO method for the 11 plans. The non-BAO method showed a higher number of MU required for a plan compared to BAO method. The average MUs for the non-BAO and BAO were 376 ± 108 and 335 ± 98, respectively. The results were analysed using two-tailed t-test method, and the average difference between non-BAO and BAO method was 11 ± 9% (p = 0·02) which was statistically significant. Table 3 compares the total planning time for the 11 patients using both methods. It can be clearly seen that the estimated planning times for non-BAO method ranged between 25 and 90 minutes with an average of 65 minutes, while the BAO method ranged between 15 and 35 minutes with an average of approximately 25 minutes. These comparisons revealed that there is a significant reduction in planning time when applying the BAO method. This can largely be attributed to the elimination of the time-consuming trial and error process used in the non-BAO method. Figure 2 shows the selected gantry angle deviations between the two methods. Using the BAO method results in selection of gantry angles that would not have been traditionally selected. There was no correlation in beam angle selection between the non-BAO and BAO methods.
Table 2. Comparison of MU between non-BAO and BAO for 11 lung plan cases
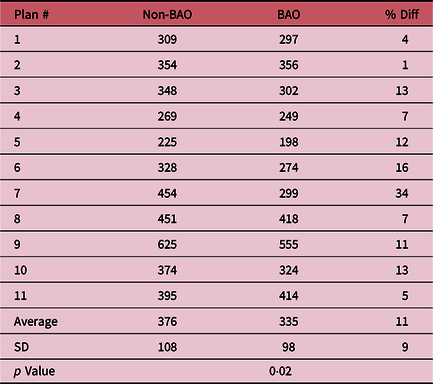
Table 3. Estimated total planning time
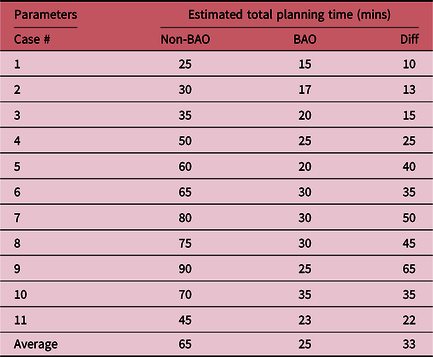
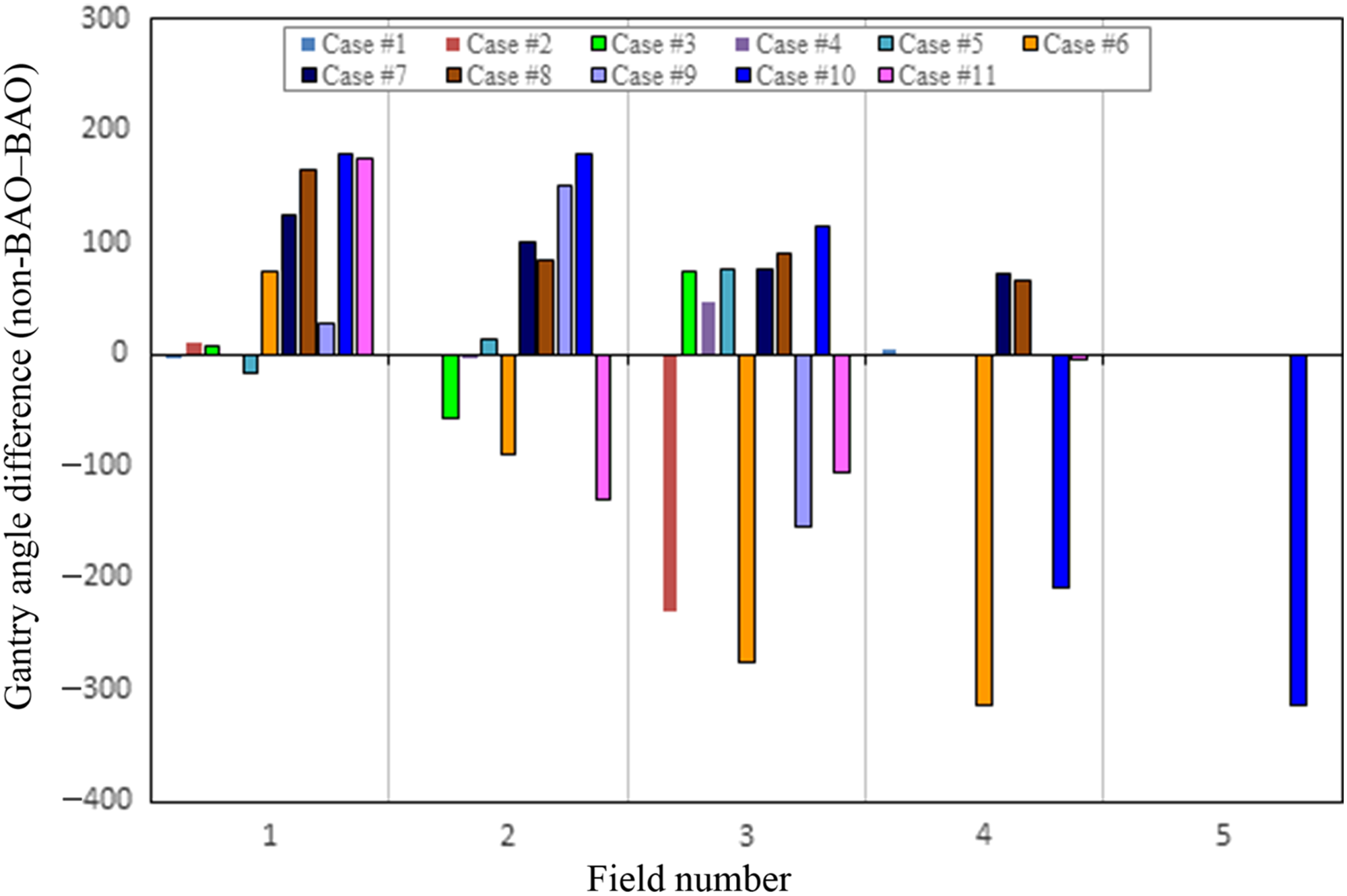
Figure 2. Differences in gantry angles between randomly spaced Non-BAO and equally spaced BAO.
Conclusion
The study on the use of the BAO method for conformal planning showed that the method could produce similar plans or slightly improved PTV coverage while keeping the critical structures under their known dose tolerances when compared to the non-BAO method. The BAO method demonstrated the potential of reducing MUs as well as the time required to produce an acceptable conformal plan compared to the non-BAO method. When using BAO method, the average MU reduction was 11% and total estimated planning time was also reduced by as much as 50%. The reduction in MUs and planning time is important especially in a busy department. Additionally, this tool can help novice planners to achieve comparable quality plans as their more experienced counterparts. Therefore, the authors conclude that the BAO tool can be utilised to produce good quality conformal plans and reduce MUs and planning time. A limitation of the tool is that the planner is required to manually enter shielding and select collimator angles. If the BAO method incorporates these processes automatically, then even better results could be achieved.
Acknowledgements
The authors wish to thank the Radiation Oncology Department of the North West Cancer Centre, NSW, Australia, for the opportunity to conduct this study. The authors specially thank Mrs. Katelyn Wall, the radiation therapy educator at the North West Cancer Centre, Tamworth Hospital, for her assistance in technical, language editing and reviewing the paper.
Conflict of Interest
The authors declare no conflict of interest.
Ethics
No human or animal testing is required for this study.
Funding Information
This manuscript was not supported by any funding.








