Introduction
Glutenins can be classified into two groups: high-molecular-weight glutenin subunits (HMW-GS) and low-molecular-weight glutenin subunits (LMW-GS). HMW-GS are encoded by Glu-1 loci on the long arm of group 1 chromosomes and LMW-GS are encoded by Glu-3 loci on the short arm of the same chromosomes (Payne, Reference Payne1987; Singh and Shepherd, Reference Singh and Shepherd1988). The allelic variations at Glu-1 loci mainly determine the bread baking characteristics of dough, and Glu-3 alleles also play an important role in the determination of dough properties and bread baking quality (Gianibelli et al., Reference Gianibelli, Larroque, Mac-Ritchie and Wrigley2001; D'Ovidio and Masci, Reference D'Ovidio and Masci2004). LMW-GS have not been studied as intensively as HMW-GS due to their complex banding and mobility patterns, which overlap with those of gliadins in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) (D'Ovidio and Masci, Reference D'Ovidio and Masci2004). Allele-specific DNA markers for glutenin have been developed recently and widely used to select wheat lines with the required quality attributes by wheat breeders (Goutam et al., Reference Goutam, Kukreja, Tiwari, Chaudhury, Gupta, Dholakia and Yadav2013).
Korean wheat breeding programmes have mainly focused on improving grain yield and early maturation. Quality improvement, especially of bread baking, has recently gained attention from wheat breeders in Korea. The objective of this study was to evaluate allelic variations at Glu-1, Glu-A3 and Glu-B3 loci in Korean wheat landraces using gene-specific PCR markers for obtaining useful information to improve the bread baking quality in wheat breeding programmes.
Experimental design
A total of 222 Korean wheat landraces were obtained from the National Agrobiodiversity Center, National Academy of Agricultural Science of Rural Development Administration (Suwon, Korea). Genomic DNA was extracted from young leaf tissue (100 mg) using a genomic DNA preparation kit (Solgent Co., Ltd, Korea) according to the manufacturer's instructions.
Allelic variations at Glu-1 loci were determined using the procedure described by Lafiandra et al. (Reference Lafiandra, Tucci, Pavoni, Turchetta and Margiotta1997) and Liu et al. (Reference Liu, Chao and Anderson2008) for the Glu-A1 allele, that described by Ma et al. (Reference Ma, Zhang and Gale2003) and Lei et al. (Reference Lei, Gale, He, Gianibelli, Larroque, Xia, Butow and Ma2006) for the Glu-B1 allele, and that described by Liu et al. (Reference Liu, Chao and Anderson2008) and De Bustos et al. (Reference De Bustos, Rubio, Soler, García and Jouve2001) for the Glu-D1 allele. Allelic variations at Glu-A3 and Glu-B3 loci were determined using the procedure described by Wang et al. (Reference Wang, Li, Peña, Xia and He2010) and Wang et al. (Reference Wang, Zhao, He, Ma, Appels, Peña and Xia2009), respectively. HMW-GS compositions were determined according to the protocol of Payne and Lawrence (Reference Payne and Lawrence1983). The sequence of primers, expected PCR fragment sizes and HMW-GS compositions in SDS–PAGE are summarized in Table S1 and Figs. S1, S2 and S3, respectively (available online). The statistical analysis of genetic diversity and variation was conducted using GenALEx version 6.501 (Peakall and Smouse, Reference Peakall and Smouse2006).
Discussion
The genetic diversity and variation at Glu-1, Glu-A3 and Glu-B3 loci of the 222 Korean wheat landraces are summarized in Table 1. Three alleles were identified at Glu-A1 and Glu-D1 loci and five alleles were identified at Glu-B1 loci. Seven alleles were identified at Glu-A3 loci and eight alleles were identified at Glu-B3 loci. Glu-A1c (86.5 %), Glu-B1b (87.8 %) and Glu-D1a (61.7 %) alleles were found at high frequencies at Glu-1 loci, and Glu-A3c (77.0 %) and Glu-B3i (37.8 %) alleles were commonly found at the respective loci. The mean number of effective alleles (N e), Shannon's information index (I) and Nei's gene diversity (h) of the Korean wheat landraces were 1.99, 0.85 and 0.42, respectively. Glu-B3 alleles had higher N e (3.70) and genetic diversity (1.59 for I and 0.73 for h) values than other alleles, while Glu-B1 alleles had lower N e and h values and Glu-A1 alleles had lower I values than other alleles.
Table 1 Genetic diversity and variation of the 222 Korean wheat landraces at Glu-1, Glu-A3 and Glu-B3 loci
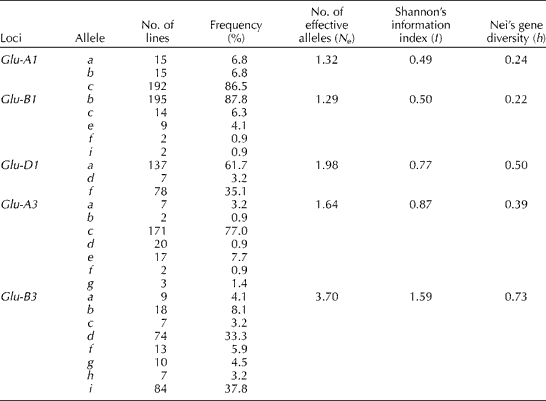
Glu-A1c and Glu-B1b alleles were also found at high frequencies in Asian and French wheat lines (Branlard et al., Reference Branlard, Dardevet, Amiour and Igrejas2003; Terasawa et al., Reference Terasawa, Takata, Hirano, Kato, Kawahara, Sasakuma and Sasanuma2011). Shewry et al. (Reference Shewry, Halford and Tatham1992) proposed that wheat cultivars with good bread baking quality might require Glu-A1a or Glu-A1b, Glu-B1b or Glu-B1i, and Glu-D1d alleles. Only seven Korean wheat landraces carried the Glu-D1d allele (Table S2, available online), which was found to occur at a low frequency in Korean wheat cultivars (Park et al., Reference Park, Kang, Jeung and Woo2011). The Glu-D1f allele was predominantly found in Korean and Japanese wheat cultivars (Nakamura et al., Reference Nakamura, Inazu and Hirano1999; Park et al., Reference Park, Kang, Jeung and Woo2011). Eleven Korean wheat landraces carried the Glu-B1e or Glu-B1i allele; these alleles were not found in Korean wheat cultivars (Park et al., Reference Park, Kang, Jeung and Woo2011). Among the 222 Korean wheat landraces, only one Korean wheat landrace (IT173162) achieved 10 points for the Glu-1 score, which is the sum of the assigned scores of Glu-1 alleles proposed by Payne et al. (Reference Payne, Nightingale, Krattiger and Holt1987) based on the significant relationship between allelic composition at Glu-1 loci and bread baking quality to predict the potential of bread baking quality in wheat breeding programmes.
The Glu-A3c allele was predominantly found in Australian, Argentinean and US wheat lines (Eagles et al., Reference Eagles, Hollamby, Gororo and Eastwood2002; Shan et al., Reference Shan, Clayshulte, Haley and Byrne2007; Lerner et al., Reference Lerner, Kolman and Rogers2009). The Glu-B3i allele was rarely found in foreign wheat lines, but was frequently found in French and US wheat cultivars and Chinese landraces, the Glu-B3j allele was predominantly found in Argentinean and Chinese cultivars, and the Glu-B3a or Glu-B3b allele was commonly found in Australian and Japanese wheat lines (Eagles et al., Reference Eagles, Hollamby, Gororo and Eastwood2002; Branlard et al., Reference Branlard, Dardevet, Amiour and Igrejas2003; Takata et al., Reference Takata, Nishio, Iriki, Tabiki, Funatsuki and Yamauchi2005; Shan et al., Reference Shan, Clayshulte, Haley and Byrne2007; Lerner et al., Reference Lerner, Kolman and Rogers2009; Li et al., Reference Li, Huang, Sui, Fan, Li and Chu2009). Zhang et al. (Reference Zhang, Jin, Zhang, Liu, Li, Xia, He and Zhang2012) reported that Glu-A3e and Glu-B3c represented inferior alleles for bread baking quality, whereas Glu-A3d and Glu-B3b, Glu-B3g or Glu-B3i alleles were correlated with superior bread baking quality and Glu-D3 alleles played minor roles in the determination of quality variation. Peña et al. (Reference Peña, Gonzalez-Santoyo, Cervantes, Lafiandra, Masci and D'Ovidio2004) proposed that wheat lines with Glu-D1d and Glu-B3d alleles had the greatest gluten strength followed by groups possessing the Glu-D1d allele combined with the Glu-B3b, Glu-B3f or Glu-B3g allele. Twenty Korean wheat landraces carried the Glu-A3d allele and these lines also carried the Glu-B3b, Glu-B3d, Glu-B3f, Glu-B3g or Glu-B3i allele. Among these wheat lines, two landraces (IT59787 and IT236544) carried the Glu-D1d allele. It should be noted that the frequency of Glu-A1a or Glu-A1b, Glu-B1i and Glu-D1d genotypes at Glu-1 loci and that of Glu-A3d and Glu-B3b, Glu-B3f or Glu-B3g alleles at Glu-3 loci should be increased in Korean wheat lines with greater gluten strength to improve the bread baking quality.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262114000926
Acknowledgement
This work was supported by a grant from the Next-Generation BioGreen 21 Program (Plant Molecular Breeding Center no. PJ008006), Rural Development Administration, Republic of Korea.



