1. Introduction
Radiodonts are lower stem-group euarthropods (sensu Ortega-Hernández, Reference Ortega-Hernández2016; see Daley et al. Reference Daley, Budd, Caron, Edgecombe and Collins2009; Cong et al. Reference Cong, Ma, Hou, Edgecombe and Strausfeld2014; Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; Van Roy et al. Reference Van Roy, Daley and Briggs2015; Lerosey-Aubril & Pates Reference Lerosey-Aubril and Pates2018; Moysiuk & Caron, Reference Moysiuk and Caron2019) characterized by a sclerotization of the body wall that is restricted to a few elements of the head region, namely the frontal appendages and discrete cephalic sclerites. For decades, these animals were depicted as the fearsome apex-predators of the Early Palaeozoic seas, as best incarnated by the Burgess Shale taxon Anomalocaris (Whittington & Briggs, Reference Whittington and Briggs1985; Chen et al. Reference Chen, Ramsköld and Zhou1994; Vannier & Chen, Reference Vannier and Chen2005; Daley et al. Reference Daley, Paterson, Edgecombe, García-Bellido and Jago2013a; Daley & Edgecombe, Reference Daley and Edgecombe2014). However, in recent years it has become clear that radiodonts were a taxonomically and ecologically diverse group, which played a key role in the emergence of complex marine trophic webs. Adult radiodonts measured from 10 cm (Cong et al. Reference Cong, Ma, Hou, Edgecombe and Strausfeld2014) to 2 m in length (Van Roy et al. Reference Van Roy, Daley and Briggs2015), spent most of their lives roaming the upper (Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; Van Roy et al. Reference Van Roy, Daley and Briggs2015; Lerosey-Aubril & Pates, Reference Lerosey-Aubril and Pates2018) or lower layers (e.g. Vannier & Chen, Reference Vannier and Chen2005; Daley & Budd, Reference Daley and Budd2010) of the water column, or foraging the seafloor (Daley et al. Reference Daley, Budd, Caron, Edgecombe and Collins2009; Moysiuk & Caron, Reference Moysiuk and Caron2019), and occupied trophic positions ranging from raptorial predators (e.g. Chen et al. Reference Chen, Ramsköld and Zhou1994; Liu et al. Reference Liu, Lerosey-Aubril, Steiner, Dunlop, Shu and Paterson2018) to filter feeders (Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; Van Roy et al. Reference Van Roy, Daley and Briggs2015; Lerosey-Aubril & Pates, Reference Lerosey-Aubril and Pates2018).
The sclerotized body parts of radiodonts, especially their distinctive frontal appendages, are ubiquitous in Cambrian Konservat-Lagerstätten. In fact, radiodont frontal appendages are often amongst the first (and sometimes are the only) non-biomineralized fossils found in exceptional Cambrian deposits (e.g. Whiteaves, Reference Whiteaves1892; Briggs & Mount, Reference Briggs and Mount1982; Lieberman, Reference Lieberman2003; Daley & Legg, Reference Daley and Legg2015; Pates & Daley, Reference Pates and Daley2017; Pates et al. Reference Pates, Daley and Butterfield2019), owing to their large size, heavily sclerotized construction and the fact that they belonged to free-swimming organisms. More than 25 species of radiodonts have been formally described to date (see analyses by Vinther et al. Reference Vinther, Stein, Longrich and Harper2014; Van Roy et al. Reference Van Roy, Daley and Briggs2015; Lerosey-Aubril & Pates, Reference Lerosey-Aubril and Pates2018), and the number of new taxa keeps increasing at a steady rate (e.g. Pates & Daley, Reference Pates and Daley2017; Cong et al. Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018; Zeng et al. Reference Zeng, Zhao, Yin and Zhu2018; Guo et al. Reference Guo, Pates, Cong, Daley, Edgecombe, Chen and Hou2019; Moysiuk & Caron, Reference Moysiuk and Caron2019; Pates et al. Reference Pates, Daley and Butterfield2019, Reference Pates, Daley, Edgecombe, Cong and Liebermanin press). Recent studies have shown that most exceptionally-preserved Cambrian biotas contain more than one representative of the group (Daley & Budd, Reference Daley and Budd2010; Lerosey-Aubril et al. Reference Lerosey-Aubril, Hegna, Babcock, Bonino and Kier2014; Pates et al. Reference Pates, Daley and Lieberman2018; Pates & Daley, Reference Pates and Daley2019; Lerosey-Aubril et al. Reference Lerosey-Aubril, Kimmig, Pates, Skabelund, Weug and Ortega-Hernándezin press; Pates et al. Reference Pates, Daley, Edgecombe, Cong and Liebermanin press), and typically members of two or more of the four families of radiodonts. All of this is clear evidence that radiodonts, like many metazoan groups at that time, underwent a major adaptive radiation during the Cambrian period (e.g. Paterson et al. Reference Paterson, Edgecombe and Lee2019).
Cambroraster falcatus, the latest addition to the group, was recently described from the Wuliuan Burgess Shale Formation of British Columbia, Canada (Moysiuk & Caron, Reference Moysiuk and Caron2019). This taxon is characterized by a unique horseshoe-shaped carapace central element, which superficially resembles the cephalic shields of some euarthropods with a benthic mode of life, and thus offers new insights into the ecological variability of Cambrian radiodonts. In this contribution, we report the discovery of a similar form in the lower Cambrian (Stage 3) Chengjiang Lagerstätte of South China. This new occurrence of Cambroraster – the second oldest occurrence of the Hurdiidae after Peytoia infercambriensis Daley & Legg, Reference Daley and Legg2015 – demonstrates that some early representatives of this family had already evolved a highly derived carapace morphology adapted for sediment foraging with a eudemersal habitus (i.e. obligate swimmers restricted to near-benthic settings; see Whalen & Briggs, Reference Whalen and Briggs2018).
2. Material and methods
The new material of Cambroraster consists of a single central carapace element (Fig. 1), which has been recovered from the Yu’anshan Member of the Chiungchussu Formation (Cambrian Series 2, Stage 3) at Ercaicun village, near Haikou town, Yunnan province, China. Detailed accounts of the geochemical and sedimentological contexts that have facilitated the preservation of the Chengjiang biota can be found in many authoritative contributions (e.g. Hammarlund et al. Reference Hammarlund, Gaines, Prokopenko, Qi, Hou and Canfield2017; Hou et al. Reference Hou, Siveter, Siveter, Aldridge, Cong, Gabbott, Ma, Purnell and Williams2017 and references therein). Like most soft-bodied fossils recovered in Chengjiang, the studied specimen is preserved as iron oxides likely resulting from the weathering of pyrite during surface alteration of the shales (Gabbott et al. Reference Gabbott, Xian-Guang, Norry and Siveter2004). The material is deposited in the collections of the Yunnan Key Laboratory for Palaeobiology (YKLP), Yunnan University, Kunming.

Fig. 1. Cambroraster sp. nov. A (YKLP 11420) from the Yu’anshan Member of the Chiungchussu Formation (Chengjiang biota), Cambrian Stage 3 (Eoredlichia–Wutingaspis Zone), Ercaicun Village, Haikou town, Kunming, Yunnan, South China. Images (a) and (c) are photographs of the specimen dry taken using cross-polarized light, (b) is a 3D reconstruction from CT data using the transfer function of Drishti (Limaye, Reference Limaye2012), (d) and (f) are photographs in non-polarized light, and (e) is a photograph taken using fluorescence microscopy under blue light. (a–c) General views (a, b) and detail of the ocular notch area (c) of YKLP 11420a (part). (d–f) General views (d, e) and detail of the putative oral cone (c) of YKLP 11420b (counterpart). Note the putative oral cone (arrowheads). Abbreviations: cw, compaction wrinkles; m?, putative position of the mouth; nr, nuchal region; ns, nuchal spine; on, ocular notch; ple, posterolateral extension; sm, smooth margin. Scale bars = 2 mm for (a), (b), (d), (e), 1 mm for (c), (f).
Part and counterpart were photographed dry under cross-polarized or normal light with a digital single lens reflex camera and stereomicroscope. Fluorescence photographs in blue light and ultraviolet light were also obtained with a fluorescence microscope to increase the contrast between the autofluorescent matrix and the non-autofluorescent fossil. The specimen was also scanned using a Zeiss Xradia Versa 520 X-ray microscope. The computed tomography (CT)-scan data were investigated and 3D models generated with Drishti (see methods in Chen et al. Reference Chen, Ortega- Hernández, Wolfe, Zhai, Hou, Chen, Mai and Liu2019; Zhai et al. Reference Zhai, Ortega-Hernandez, Wolfe, Hou, Cao and Liu2019) and segmented slice by slice with Mimics 20.0® (Materialize NV). 3D models were exported as raster images by using Drishti and Meshlab (meshlab.net) screenshot functions. For comparative purposes, photographs of a harpetid trilobite and an incomplete dorsal exoskeleton of Limulus polyphemus (juvenile) belonging to the Laboratory for Invertebrate Paleobiology and Evolution at Harvard University (no numbers) were taken using a Nikon D5500 camera fitted with a Nikon 40 mm DX Micro-Nikkor lens.
3. Systematic palaeontology
Terminology. The terminology used is that of Lerosey-Aubril & Pates (Reference Lerosey-Aubril and Pates2018), except for the terms ‘nuchal region’ and ‘posterolateral extensions’, which refer to extensions of the central element postero-medially and postero-laterally, respectively (‘posterior axial area’ and ‘lateral areas’, respectively, of Moysiuk & Caron, Reference Moysiuk and Caron2019). Abbreviations used in the main text: exs., exsagittally, sag., sagittally, tr., transverse.
Superphylum Panarthropoda Nielsen, Reference Nielsen1995
Order Radiodonta Collins, Reference Collins1996
Family Hurdiidae Lerosey-Aubril & Pates, Reference Lerosey-Aubril and Pates2018
Genus Cambroraster Moysiuk & Caron, Reference Moysiuk and Caron2019
Type species. Cambroraster falcatus Moysiuk & Caron, described from the Wuliuan Burgess Shale Formation, British Columbia, Canada.
Cambroraster sp. nov. A
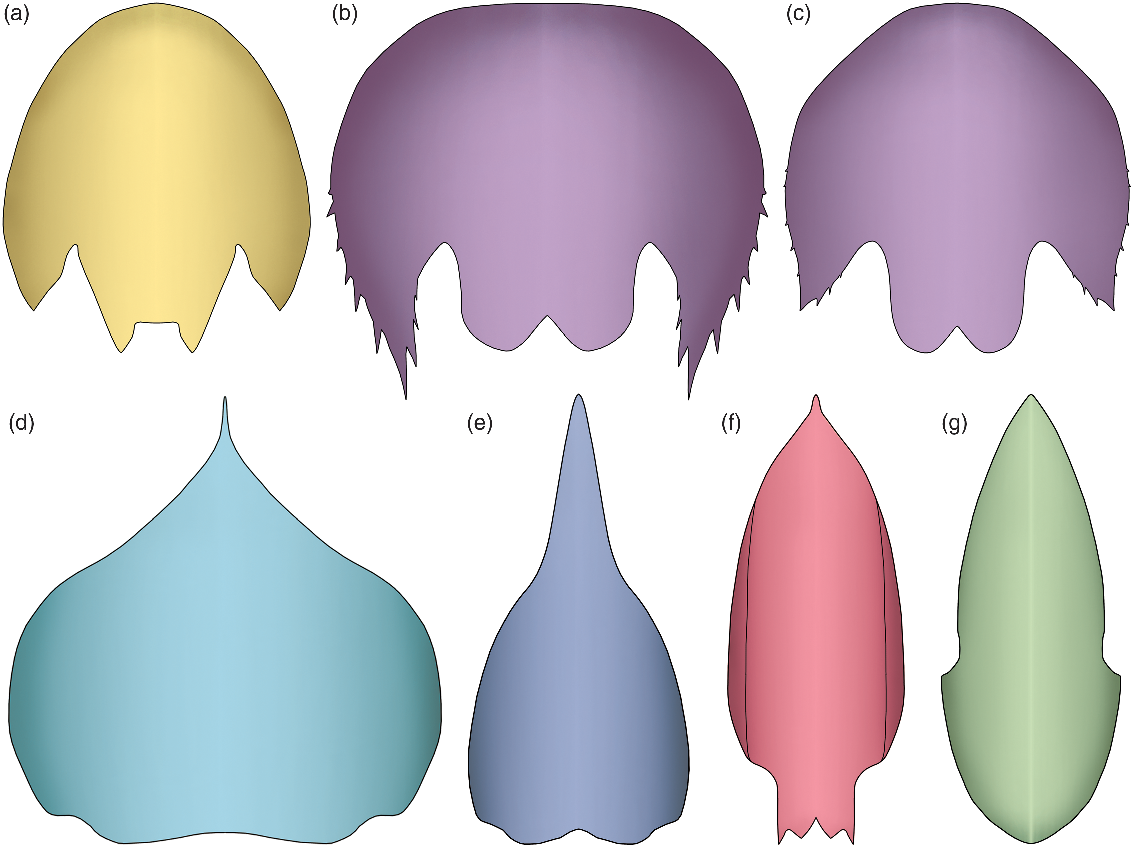
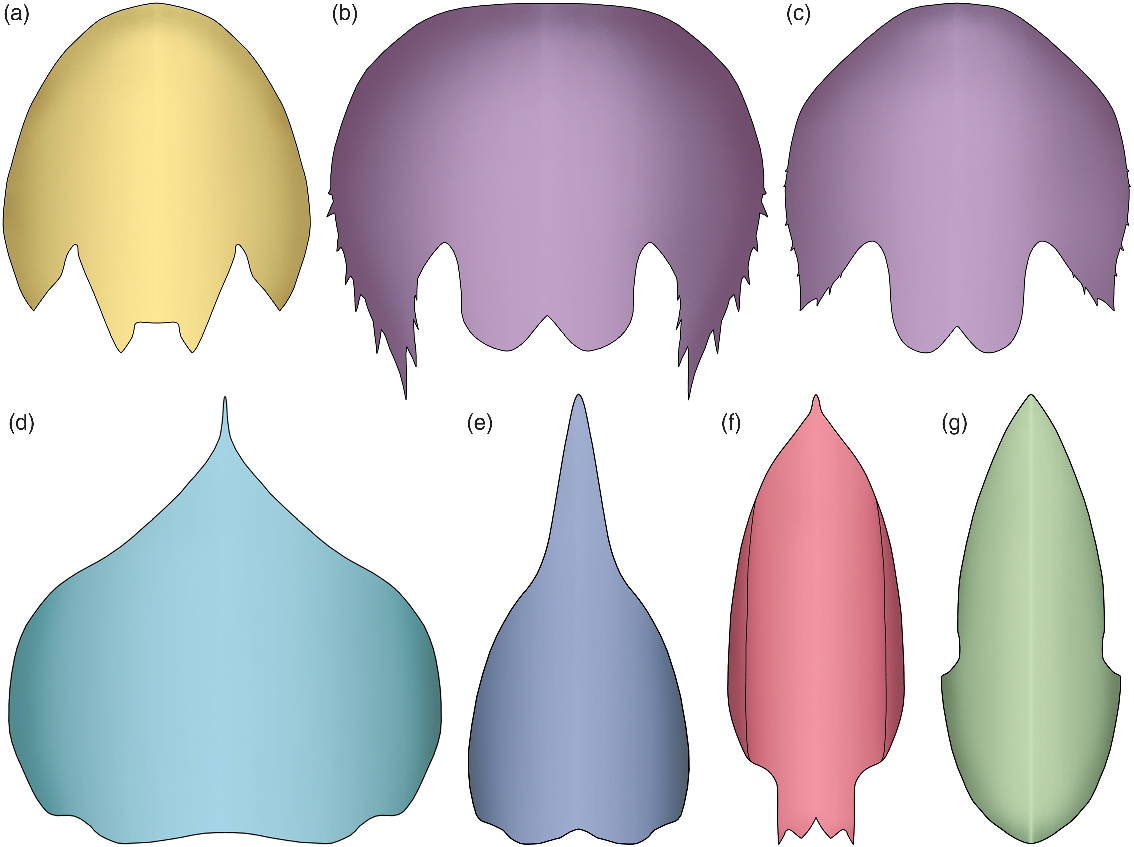
Fig. 2. Morphological disparity of the cephalic carapace central element in hurdiids. (a) Juvenile specimen of Cambroraster sp. nov. A (Cambrian Stage 3; Chengjiang). (b, c) Juvenile (b) and mature (c) specimens of Cambroraster falcatus (Wuliuan; Burgess Shale). (d) Hurdia triangulata (Wuliuan; Burgess Shale). (e) H. victoria (Wuliuan; Burgess Shale). (f) Pahvantia hastata (Drumian; Wheeler Shale). (g) Aegirocassis benmoulai (Tremadocian; Fezouata Shale).
Material. A single isolated central element of the cephalic carapace (YKLP 11420a/b).
Locality and horizon. Yu’anshan Member of the Chiungchussu Formation (Cambrian Series 2, Stage 3) at Ercaicun village, Haikou town, Kunming, Yunnan province, China. Eoredlichia-Wutingaspis trilobite biozone.
Description. The specimen is particularly small – c. 8.1 mm and c. 6.9 mm in maximum length (exs.) and maximum width (tr.), respectively – and has been flattened dorso-ventrally parallel to bedding, although it retains some three-dimensionality. It is narrowly semi-elliptical in outline (Fig. 1a, b, d, e), with a pair of deep ocular notches extending from the posterior margin (Fig. 1c). These notches separate a well-differentiated nuchal region from long (exs.) acuminate posterolateral extensions. The nuchal region has the shape of an isosceles trapezium, with the straight posterior margin being flanked by short triangular spines that point backwards (Fig. 1a–e). The posterolateral extensions are devoid of marginal spines, but exhibit acute terminations. Numerous compaction wrinkles running subparallel to the antero-lateral margin are visible in the lateral and anterior regions (e.g. Fig. 1b, d); these indicate that unlike the somewhat flat or gently convex central and nuchal regions, the marginal areas dipped ventrally at a steep slope. A ring-like structure on the counterpart is tentatively interpreted as the remains of an oral cone (Fig. 1d–f), based on its central location, size and shape. Owing to its poor preservation, some details of its morphology (e.g. its anterior margin) cannot be described with confidence. Yet, it exhibits numerous furrows converging towards the depressed central area that it surrounds (Fig. 1f), which suggests that it was made of radially arranged elements similar to the plates of a radiodont oral cone. On the other hand, this seems to be an impression on the carapace element only, for no hidden structure could be identified underneath the element using the three-dimensional CT model.
Remarks. The identification of the unique specimen of Cambroraster sp. nov. A was possible thanks to the context provided by the recent description of C. falcatus from the Wuliuan Burgess Shale in British Columbia (Moysiuk & Caron, Reference Moysiuk and Caron2019). The distinctive outline of the central carapace element in this new species from China superficially resembles the cephalic shields of horseshoe crabs or some trilobites (see Section 4b below), but the details of the ocular notches and nuchal region are incompatible with the organization of the dorsal exoskeleton in Palaeozoic Xiphosura (e.g. Bicknell et al. Reference Bicknell, Amati and Ortega-Hernández2019) or in any trilobites.
4. Discussion
4.a. Ontogenetic variant or new species?
The genus Cambroraster was recently described from the Wuliuan Burgess Shale of British Columbia, Canada (Moysiuk & Caron, Reference Moysiuk and Caron2019). The type species occurs with confidence at the Marble Canyon and Tokumm Creek localities only, but a couple of poorly preserved remains from Mount Stephen (Collins Quarry) and Mount Field have also been tentatively assigned to this genus (Moysiuk & Caron, Reference Moysiuk and Caron2019). Cambroraster shows typical hurdiid characters, such as a well-developed tripartite cephalic carapace, an oral cone with smooth, tetraradially arranged large plates with extra circlets of small plates, and frontal appendages that bear five unpaired plate-like endites. However, this taxon is also easily distinguished from any other representatives of the family by the morphology of its carapace central element. The atypical horseshoe shape of this sclerite (see Section 4.b. below), its deep ocular notches and its well-developed nuchal region are all features that readily differentiate this taxon from other hurdiids (cf. Fig. 2a–c and Fig. 2d–g). These characteristics are all displayed by the minute Chinese specimen described herein, allowing its confident assignment to the genus Cambroraster (Fig. 1).
The carapace central element from Chengjiang also shows obvious morphological differences compared to that of the type species C. falcatus, such as the absence of marginal spines and the straight, rather than bilobed, posterior margin of the nuchal region that is flanked with short spines (Figs. 2a–c). Considering the small size of our specimen, it could be argued that these morphological differences are ontogenetic in origin, but this is not supported by the variously sized central elements of the type species illustrated by Moysiuk & Caron (Reference Moysiuk and Caron2019, cf. fig. 1g and fig. 1k). Indeed, their illustrations allow the description of the following ontogenetic changes: a shortening (exs.) of the posterolateral extensions, a decrease of their marginal spinosity and a modification of the element outline from semicircular to narrowly elliptical (Fig. 2b, c). These changes produce a morphology in the larger Cambroraster individuals that increasingly resembles that of the minuscule specimen from Chengjiang, not the other way around. In other words, the differences between the Chinese specimen and C. falcatus are not consistent with ontogenetic changes, and are instead better interpreted as differences between species. This conclusion comes as no surprise considering the fact that the Canadian and Chinese Cambroraster are found in beds deposited on separate palaeocontinents (Laurentia vs South China), and 13 million years apart from each other.
The phylogenetic analysis of Moysiuk & Caron (Reference Moysiuk and Caron2019) recovered Cambroraster falcatus as the sister taxon of the Chengjiang species Zhenghecaris shankouensis Vannier et al., Reference Vannier, Chen, Huang, Charbonnier and Wang2006. Originally described as a thylacocephalan euarthropod (Vannier et al. Reference Vannier, Chen, Huang, Charbonnier and Wang2006), Z. shankouensis was recently reassigned to the Hurdiidae based on the observation of a bilateral symmetry and a two-layered structure in better-preserved material (Zeng et al. Reference Zeng, Zhao, Yin and Zhu2018). Although Cong et al. (Reference Cong, Edgecombe, Daley, Guo, Pates and Hou2018) rejected radiodont affinities for the latter species, we are inclined to follow Zeng et al. (Reference Zeng, Zhao, Yin and Zhu2018) and Moysiuk & Caron (Reference Moysiuk and Caron2019) in regarding the known specimens (‘Z-elements’) of this taxon as possible radiodont central carapace elements. However, the discoidal outlines and reduced wing-like lateral extensions of Z. shankouensis significantly differ from the posteriorly deeply incised, horseshoe-shaped carapace element described above and the central elements of C. falcatus. The affinities of Z. shankouensis within the Radiodonta are uncertain, and in the absence of complete individuals it remains unknown whether the carapace element of this species was part of a well-developed tripartite cephalic carapace (a hurdiid synapomorphy). Of more modest significance, Z. shankouensis is not known from the Ercaicun section where the fossil described herein has been found. Accordingly, we believe that this new fossil from Chengjiang does not belong to Zhenghecaris, but instead represents a new species of Cambroraster. The small size (<10 mm in length, sag.) suggests that YKLP 11420 (Fig. 1) most likely represents a particularly immature individual (see hurdiid adult sizes in Lerosey-Aubril & Pates, Reference Lerosey-Aubril and Pates2018, fig. 5). As such, it would be inappropriate as a holotype, which explains our reluctance to formally define this new species of Cambroraster in the present contribution.
4.b. Hurdiid ecomorphotypes
The wide horseshoe-shaped cephalic carapace of Cambroraster (Fig. 3a) is closely reminiscent of the strongly vaulted cephalic shields of euarthropods that feed on infaunal organisms, such as horseshoe crabs (Fisher, Reference Fisher1975; Botton & Shuster, Reference Botton, Shuster, Shuster, Barlow and Brockmann2003; Fig. 3b, c) or trilobites with filtering chambers (e.g. Fortey & Owens, Reference Fortey and Owens1999; Fig. 3d). In all these taxa, the cephalic shield forms a protective dome that provides ample space for the movement of appendages, allowing them to probe the sediment (Botton & Shuster, Reference Botton, Shuster, Shuster, Barlow and Brockmann2003), or put it in suspension and sieve it (Campbell, Reference Campbell1975; Fortey & Owens, Reference Fortey and Owens1999; Moysiuk & Caron, Reference Moysiuk and Caron2019) to extract prey/food particles. This feeding behaviour implies that the animal spent a considerable amount of time on the seafloor, which in the case of Cambroraster must have represented a major departure from the nektonic lifestyle of most known radiodonts. Other characteristics of the anatomy of Cambroraster also point towards a eudemersal lifestyle (sensu Whalen & Briggs, Reference Whalen and Briggs2018): (1) the single row of reduced trunk flaps suggestive of reduced swimming capabilities; (2) the short body comprising a massive horseshoe-shaped head carapace with little to no streamlining; (3) the dorsal position of the eyes. Interestingly, a large and highly vaulted cephalic shield evolved independently in radiodonts, trilobites and xiphosurids. The central carapace element of hurdiids is homologous to the dorsal/anterior sclerite of other radiodonts and some Cambrian euarthropods, and therefore belongs to the anteriormost (protocerebral) cephalic segment (Cong et al. Reference Cong, Ma, Hou, Edgecombe and Strausfeld2014; Ortega-Hernández, Reference Ortega-Hernández2015; Ortega-Hernández et al., Reference Ortega-Hernández, Janssen and Budd2017). Conversely, the cephalon of trilobites and the prosomal shield of horseshoe crabs result from the fusion of the dorsal exoskeleton encompassing several segments (five and seven/eight, respectively; Dunlop & Lamsdell, Reference Dunlop and Lamsdell2017; Park & Kihm, Reference Park and Kihm2017). A parallel can also be drawn with the iterative convergent evolution of similarly shaped cephalic shields in some galeaspid (e.g. Laxaspis, Nochelaspis) and osteostracean (e.g. Zenaspis) jawless fish in the middle Palaeozoic (e.g. Donoghue & Keating, Reference Donoghue and Keating2014, fig. 3D, F), once again in relation to the adaptation to an epibenthic lifestyle (Radinsky, Reference Radinsky1987, pp. 29–33).
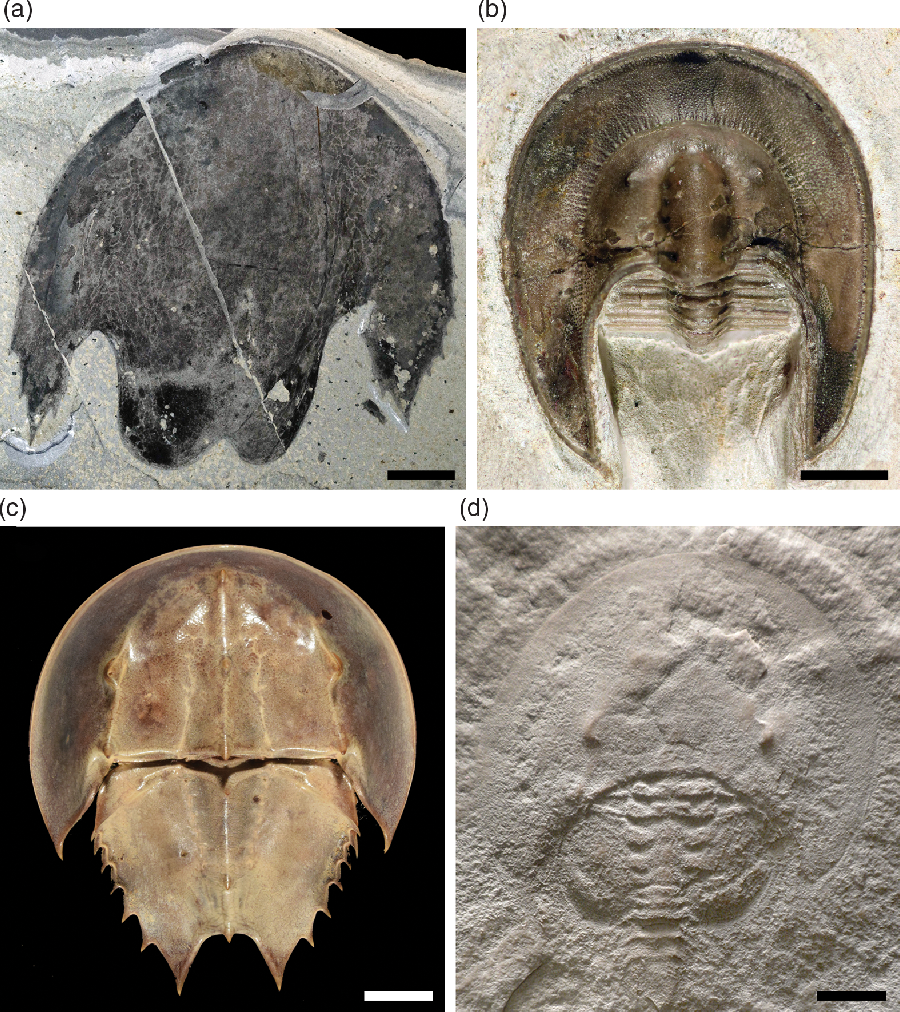
Fig. 3. Convergent evolution of a large horseshoe-shaped cephalic shield in stem-group and crown-group euarthropods. (a) Carapace central element of the hurdiid radiodont Cambroraster falcatus from the Wuliuan Burgess Shale of Canada (ROMIP65316; image: J-B Caron). (b) Cephalon (and enrolled trunk) of an indeterminate harpetid trilobite from the Devonian of Morocco. (c) Dorsal exoskeleton (telson missing) of the American horseshoe crab Limulus polyphemus (juvenile). (d) Dorsal exoskeleton (telson not illustrated) of the Ordovician horseshoe crab Lunataspis aurora (image: David Rudkin/The Manitoba Museum). Scale bars = 2 cm for (a), 1 cm for (c), 5 mm for (b), (d).
The discovery of Cambroraster (Moysiuk & Caron, Reference Moysiuk and Caron2019) has revealed a more intimate relationship between the shape of the cephalic carapace and feeding ecology in hurdiids than hitherto realized, as independently confirmed by the characteristics of the frontal appendages. Considering these morphological traits, the family Hurdiidae illustrates particularly well the morphological and ecological diversification of radiodonts during the Cambrian Epoch 2–Miaolingian time interval (mostly). Except for Peytoia, representatives of this family typically feature a well-developed tripartite cephalic carapace, which can be broadly subdivided into two distinct morphotypes. In suspension-feeding representatives, the carapace is elongate and narrow in dorsal view (Fig. 2f, g), which contributed to the streamlining of the body of these pelagic forms (Van Roy et al. Reference Van Roy, Daley and Briggs2015; Lerosey-Aubril & Pates, Reference Lerosey-Aubril and Pates2018). The frontal appendages of suspension-feeding taxa are composed of low (dorso-ventrally), possibly weakly sclerotized podomeres, which bear endites fringed with numerous setae. By contrast, essentially demersal hurdiids which are thought to have fed on infaunal organisms, such as Cambroraster and possibly Hurdia species, have a carapace that is wide and short (Daley et al. Reference Daley, Budd and Caron2013b; Moysiuk & Caron, Reference Moysiuk and Caron2019; Fig. 2a–e). The podomeres of their frontal appendages are high (dorso-ventrally), short (proximo-distally) and more strongly sclerotized, and they bear endites equipped with spines that greatly vary in size and number between taxa. In this context, the more elongate central carapace element of Hurdia victoria is atypical, and might be interpreted as evidence for a more pelagic lifestyle compared to its congeneric species, H. triangulata.
5. Conclusions
The discovery of Cambroraster in Chengjiang demonstrates that radiodonts had already evolved the phylogenetically highly derived cephalic morphology characterizing this genus (Moysiuk & Caron, Reference Moysiuk and Caron2019), and most likely the feeding ecology (sediment forager) and lifestyle (eudemersal) it was associated with, by the early Cambrian Age 3. Added to the presence of representatives hyperspecialized for raptorial predation (Amplectobelua and especially Lyrarapax; Cong et al. Reference Cong, Ma, Hou, Edgecombe and Strausfeld2014, Reference Cong, Daley, Edgecombe, Hou and Chen2016, Reference Cong, Daley, Edgecombe and Hou2017; Liu et al. Reference Liu, Lerosey-Aubril, Steiner, Dunlop, Shu and Paterson2018) or filter feeding (Tamisiocaris; Vinther et al. Reference Vinther, Stein, Longrich and Harper2014) in the same (Chiungchussu Formation) or slightly younger (Buen Formation) strata, this discovery provides strong evidence for an early (Terreneuvian?) adaptive radiation among radiodonts. This observation adds to an ever-growing body of evidence that the Terreneuvian period must have seen not only the emergence of total-group euarthropods, but also the rapid establishment of their ecological dominance within marine animal communities (Daley et al. Reference Daley, Antcliffe, Drage and Pates2018; Paterson et al. Reference Paterson, Edgecombe and Lee2019).
Acknowledgements
This research was supported by NSFC grant (41861134032), Yunnan Provincial grants 2018FA025 and 2018IA073, the Strategic Priority Research Program of Chinese Academy of Sciences, Grant No. XDB26000000, and the Harvard China Fund. C Haug, JT Haug, S Pates, and PA. Selden discussed the affinities of the fossil described herein. H Chen, X Li, and T Zhao assisted with photography and electronic microscopy. D Rudkin and the Manitoba Museum (Winnipeg), and J-B Caron provided us with pictures of Lunataspis aurora and Cambroraster falcatus, respectively. Two anonymous colleagues helped to improve this contribution with particularly detailed reviews. We are grateful to all of these persons for their invaluable help during the course of our study.
Authors’ contributions
Y.L. collected the fossil and designed the research. R.L.-A. wrote the text with inputs from J.O.-H., D.A. and Y.L.; all authors discussed and approved the manuscript. Y.L., D.Z. and H.M. acquired the tomographic data, and D.A. and R.L.-A. performed light photography. R.L.-A. produced the line drawings in Figure 2 and prepared all the figures.
Declaration of interest
We declare no conflict of interest.