The psychosis continuum
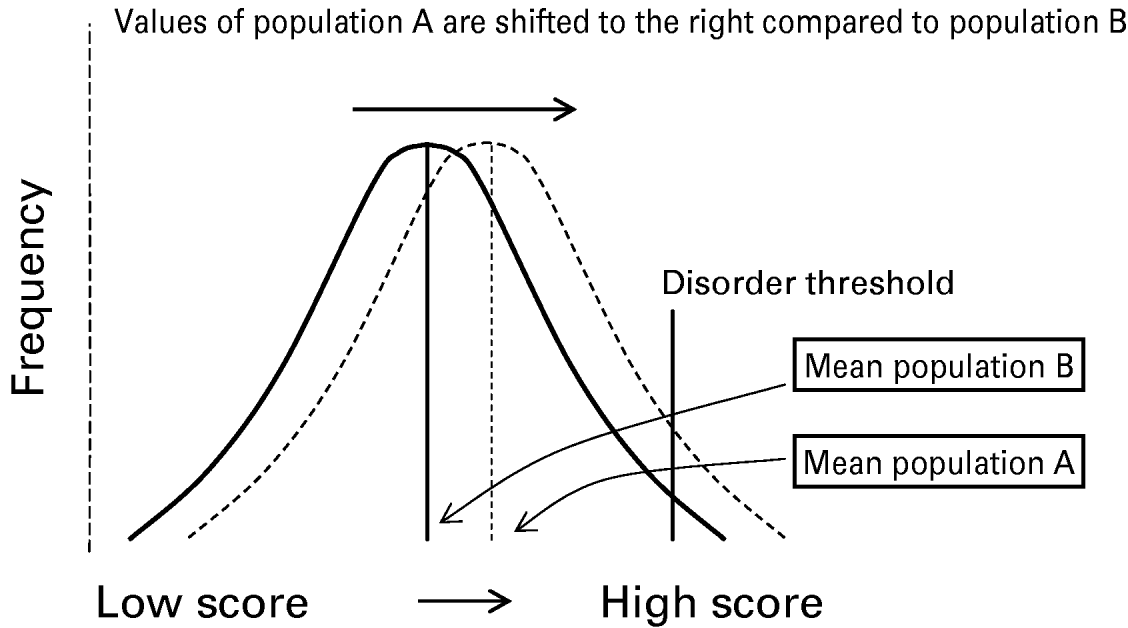
Psychiatric morbidity in a population may be seen as a function of the degree to which the distribution of a continuous phenotype, measurable in both healthy and ill individuals, is shifted towards higher values (Fig. 1). There is a long-standing notion that the psychosis phenotype is expressed at levels well below its clinical manifestation, commonly referred to as psychosis proneness, psychotic experiences, schizotypy or at-risk mental states (Meehl, Reference Meehl1962; Siever et al. Reference Siever, Kalus and Keefe1993; Chapman et al. Reference Chapman, Chapman, Kwapil, Eckblad and Zinser1994; Claridge, Reference Claridge1997; Crow, Reference Crow1998; Kwapil, Reference Kwapil1998; Verdoux et al. Reference Verdoux, Maurice-Tison, Gay, van Os, Salamon and Bourgeois1998a; van Os et al. Reference van Os, Hanssen, Bijl and Ravelli2000; Stefanis et al. Reference Stefanis, Hanssen, Smirnis, Avramopoulos, Evdokimidis, Stefanis, Verdoux and van Os2002; Vollema et al. Reference Vollema, Sitskoorn, Appels and Kahn2002; Yung et al. Reference Yung, Phillips, Yuen, Francey, McFarlane, Hallgren and McGorry2003). A psychosis continuum implies that the same symptoms that are seen in patients with psychotic disorders can be measured in non-clinical populations. The assumption of this approach is that experiencing symptoms of psychosis such as delusions and hallucinations is not inevitably associated with the presence of disorder. The latter is thought to be dependent on symptom factors such as intrusiveness, frequency and psychopathological co-morbidities on the one hand, and personal and cultural factors such as coping, illness behaviour, societal tolerance and the degree of associated developmental impairment on the other (Johns & van Os, Reference Johns and van Os2001). Thus, even though the prevalence of the clinical disorder is low, the prevalence of the symptoms can conceivably be much higher.

Fig. 1. Relationship between continuous phenotype and dichotomous disorder. The difference in prevalence of a psychotic disorder between population A (high prevalence) and population B (low prevalence) is shown in the graph as a function of differences between A and B in the population mean value of a continuous phenotype.
What constitutes proof for a psychosis continuum?
Distributional validity: a simulation
Although the distinction between health and illness makes intuitive sense, it can be readily shown that most common illnesses cannot be entirely dichotomous in nature. Diseases caused by a single dominant gene defect that is fully penetrant may exist as a truly dichotomous phenomenon. If nothing else influences the expression of the genetic defect, the disease in question will have the same distribution as the genetic defect itself (Fig. 2b). However, in the case of multi-factorial diseases, such as psychiatric disorders, where multiple interacting causes contribute to the phenotypic distribution, it can be shown, using statistical simulations (available on request), that the most likely distribution is half-normal (Fig. 2c). It may be argued that it would still be possible that multiple interacting factors contribute to an underlying continuous biological abnormality that, when a certain threshold is reached, gives rise to a dichotomous behavioural phenotype. Although this may be possible, it is unlikely given the fact that the biological and cognitive abnormalities associated with (the genetics of) schizophrenia have all been demonstrated to behave as linear risk indicators without evidence of threshold effects (Jones et al. Reference Jones, Rodgers, Murray and Marmot1994a, Reference Jones, Harvey, Lewis, Toone, Van Os, Williams and Murrayb).

Fig. 2. Expected phenotypic distribution of a disorder of multi-factorial interactive aetiology. (a) Shows a continuous and normal distribution of a trait in the general population, much as would be expected in the case of, for example, weight or IQ. (b) Shows a clear bimodal distribution, with the great majority of the population having negligible values of the trait, whereas a very small proportion has extremely high values. (c) Depicts a continuous but only half-normal distribution, with the majority of the population having very low values but a significant proportion also having progressively higher values.
Psychopathological validity
The vast majority of patients with non-affective psychotic disorder meet criteria for other DSM-IV psychiatric disorders (Kessler et al. Reference Kessler, Birnbaum, Demler, Falloon, Gagnon, Guyer, Howes, Kendler, Shi, Walters and Wu2005). Psychotic symptoms outside psychotic disorder show a similar pattern of ‘co-morbidity’ (van Os et al. Reference van Os, Hanssen, Bijl and Ravelli2000), suggesting continuity in terms of psychopathological associations. Psychotic disorders can be usefully represented as variation in several correlated psychopathological dimensions, in particular dimensions of positive, negative and affective symptoms (Kitamura et al. Reference Kitamura, Okazaki, Fujinawa, Yoshino and Kasahara1995; McGorry et al. Reference McGorry, Bell, Dudgeon and Jackson1998). Interestingly, subclinical psychotic experiences and the related concept of schizotypy show a similar pattern. Thus, subclinical positive psychotic experiences are strongly associated with the negative symptoms within the psychosis phenotype (van Os et al. Reference van Os, Hanssen, Bijl and Ravelli2000), and emerging work in general population samples suggests the existence of similar correlated affective and non-affective dimensions of the psychosis phenotype at the subclinical level (Stefanis et al. Reference Stefanis, Hanssen, Smirnis, Avramopoulos, Evdokimidis, Stefanis, Verdoux and van Os2002; Krabbendam et al. Reference Krabbendam, Myin-Germeys, De Graaf, Vollebergh, Nolen, Iedema and Van Os2004). Similarly, it has been observed that in studies using variably defined schizotypy scales to measure the subclinical manifestations of the psychosis phenotype, the dimensions of subclinical psychosis closely resemble those that have been identified in schizophrenia, thus suggesting psychopathological continuity between the clinical and subclinical phenotypes (Vollema & van den Bosch, Reference Vollema and van den Bosch1995; Gruzelier, Reference Gruzelier1996; Vollema & Hoijtink, Reference Vollema and Hoijtink2000; Mata et al. Reference Mata, Gilvarry, Jones, Lewis, Murray and Sham2003; Lewandowski et al. Reference Lewandowski, Barrantes-Vidal, Nelson-Gray, Clancy, Kepley and Kwapil2006).
Epidemiological validity
Epidemiological validity refers to the notion that evidence with respect to the distribution of a construct of interest within a population should be consistent with the propositions that stem from the theoretical model of that construct. A categorical model of psychosis does not predict that the symptoms of psychosis are more common than the clinical disorder. By contrast, a continuum model accommodates high-prevalence and high-incidence rates of psychotic and psychosis-like experiences. To address this contrast, we systematically reviewed evidence on the prevalence and incidence of psychotic symptoms and experiences in the general population.
Methods
We searched entries in the Medline database, with publication years from 1950 to (7 August) 2007, to identify the intersection of two sets of publications: (1) those papers identified using the truncated keyword search terms ‘delus’, ‘hallucinat’, ‘paranoi’, ‘psychos’, ‘psychot’, ‘schizophr’ or ‘schizotyp’; and (2) those papers identified using the keyword search terms ‘incidence’, ‘prevalence’, ‘sensitivity’ or ‘specificity’. This intersection set, containing 17 363 articles, was then reduced to those that were limited to human research and included one or more of the following key phrases:
‘general population’
‘normal population’, ‘normal individuals’, ‘normal sample’
‘healthy population’, ‘healthy individuals’, ‘healthy sample’
‘community individuals’, ‘community sample’
‘nonpsychotic’, ‘survival’, ‘screening’, ‘subclinical’
This yielded 2442 potentially relevant papers. We then searched each of these papers, first by reading the title and subsequently, as necessary, the abstract and the paper itself to identify papers that described studies of symptoms of psychosis in the general population. The following inclusion and exclusion criteria were applied:
Papers included in the meta-analysis were those that: (a) reported a study of a general population sample with complete data on at least 100 participants; (b) reported exact incidence or prevalence rates (or count data or scores from which rates could be determined) for dichotomous (at item or instrument level) psychosis outcomes (symptoms or experience of or resembling hallucinations, delusions, or both); and (c) were published as original research in or since 1950. We searched citations within papers meeting these criteria to identify other potentially eligible studies. We excluded studies for which: (a) participants were recruited through secondary or tertiary health services (e.g. ophthalmology services), prisons, or aged-care facilities; (b) there was insufficient information to determine a prevalence or incidence rate, a sample size, or that inclusion criteria were met; (c) more than 20% of the participants were (likely to have been) aged ⩾65 years; (d) outcome measures conflated psychosis outcomes with other outcomes, such as hypomania or depersonalization; and (e) psychosis outcomes were sleep-related (hypnopompic and hypnagogic) hallucinations.
From each article, we recorded a cohort name and its characteristics (sampling population, recruitment strategy, response rate, the actual or eligible age range of participants, the mean age and its standard deviation, the proportion of participants aged ⩾65 years, the proportion of males in the sample, and significant inclusion and exclusion criteria), the key outcome phenotype and the criteria used to determine outcome [the name of the measurement instrument, the administration format (self-report, lay interview, professional interview, observer ratings); the number of items of the instrument that were used; classes of excluded experience (reports attributed to misunderstanding, experience judged as realistic or plausible, experience attributed to drugs or general medical conditions, experiences judged to be inconsequential); the number of affirmative responses required to reach study threshold for outcome presence; any frequency, severity, or likelihood criterion required to reach study threshold for outcome presence; for composite phenotype outcomes, such as those collapsing outcomes across items measuring hallucinations and delusions, the number of items representing each phenotype in the measure; the outcome interval; how outcome data were handled (whether rates were weighted to compensate for the sampling strategy or not, whether the rate was of any affirmative response or a mean item endorsement frequency), the rate denominator, and the rate itself. We recorded as many rates as possible for each paper and cohort, provided these were not derived under identical conditions. The psychosis outcomes were hallucinations, delusions, and the combined or unsegregated reporting of these.
Analysis
Of the papers we searched, 47 met the inclusion and exclusion criteria. These 47 articles reported data from analyses of 35 participant cohorts and yielded 217 estimates of the prevalence or 1-year incidence of the phenotypes (Table 1). The highest number of estimates yielded by a single paper or cohort was 36 from the Zurich Study of Young Adults (Rössler et al. Reference Rössler, Riecher-Rössler, Angst, Murray, Gamma, Eich, van Os and Gross2007), a longitudinal study with rates of three types of experience (hallucinations, delusions, and unsegregated) for two severity levels (at least moderate, at least a little bit), across six waves of data collection spanning 20 years. By contrast, nine cohorts provided a single prevalence or incidence estimate. The median number of rates per cohort was 4.
Table 1. Cohorts and data sources included in the analysis of epidemiological validity

H, Hallucinations; D, delusions; H/D, unsegregated; P, prevalence; I, annual incidence.
To summarize rate data, we adopted the graphical approach to the analysis of epidemiological findings that Saha et al. (Reference Saha, Chant and McGrath2008) proposed. This approach does not yield a summary meta-analytic or weighted mean rate but has the advantage of conveying full information about the variability in findings. It also has the benefit of allowing the inclusion of more than one rate per cohort. That is, because there is no requirement that synthesized rates be independent, multiple rates derived from non-identical conditions within a single cohort (e.g. the observed rates of hallucinations across different thresholds or assessment periods) can be included in the graphical analysis.
Results
Estimates of the prevalence and 1-year incidence of psychotic symptoms and experiences vary substantially across cohorts and studies (Fig. 3; Tables 1 and 2). The median prevalence, overall, was 5.3%, but the interquartile range (IQR) was 1.9–14.4%. For incidence, the median rate was 3.1% and the IQR was 1.1–8.6%. Thus, regardless of whether phenotype assessment was based on self-report, lay interview or clinical interview, the prevalence and annual incidence rates are much higher than the clinical phenotype of non-affective psychotic disorder.

Fig. 3. Cumulative relative frequencies of (a) prevalences (n=195) and 1-year incidence (n=22) rates, collapsed across phenotypes and other variables, and (b) prevalence rates for phenotypes hallucinations (n=72), delusions (n=54), and for unsegregated hallucinations, delusions, or both (n=69).
Table 2. Prevalence and incidence percentiles and quartiles for psychosis phenotypes

The great majority of studies focused on prevalent psychotic experiences with relatively few studies being able to define truly incident cases (Tien & Eaton, Reference Tien and Eaton1992; Henderson et al. Reference Henderson, Korten, Levings, Jorm, Christensen, Jacomb and Rodgers1998; Hanssen et al. Reference Hanssen, Bak, Bijl, Vollebergh and van Os2005) or possible incident cases (Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2006b; Wiles et al. Reference Wiles, Zammit, Bebbington, Singleton, Meltzer and Lewis2006). Similarly, few studies distinguished between psychotic experiences with clinical impact (assessed, for example, measuring the level of associated distress and/or help-seeking behaviour), albeit not enough to diagnose a psychotic disorder, and psychotic experiences without clinical impact (without distress and/or help-seeking behaviour). The importance of this distinction is highlighted in several ways. First, the importance of the clinical versus subclinical distinction is evidenced by quantitative differences in associations with clinical and demographic variables (van Os et al. Reference van Os, Hanssen, Bijl and Ravelli2000) and quantitative differences in the rate of transition to complete psychiatric disorder (Hanssen et al. Reference Hanssen, Bak, Bijl, Vollebergh and van Os2005). Second, in the studies reviewed here, the median prevalence of experience that has a clinical impact was 1.5% (IQR 0.4–3.0%) whereas the median rate derived without this or similar restrictions (e.g. on the number of experiences or frequency or probability criteria) was 8.4% (IQR 3.5–20.9%). The median rate of 1.5%, however, probably represents an underestimate of the true prevalence because of the large number of methodological, design and cohort variables, in particular the use of brief screening instruments that result in fewer psychotic symptoms elicited (data not shown). Thus, in the largest two cohort studies where the clinical–subclinical distinction was specifically made, and a sufficiently large number of items for the assessment of psychotic experiences was used, the rates of clinically relevant symptoms were 4.2% and 3.8% respectively (van Os et al. Reference van Os, Hanssen, Bijl and Ravelli2000; Dominguez et al., unpublished observations).
Therefore, a distinction can be usefully made (Fig. 4) between true subclinical psychotic experiences (prevalence around 8%) and subclinical psychotic symptoms, which are associated with a degree of distress and help-seeking behaviour but do not necessarily amount to clinical psychotic disorder (prevalence around 4%). In studies where psychotic symptoms and psychotic disorder are both measured, the cut-off between psychotic symptoms and psychotic disorder co-depends on the investigator. For example, in the US National Comorbidity Study, high rates of psychotic experiences were reported by a sample of around 10 000 in the US population (28%). Nevertheless, according to the clinicians reviewing these data, the rate of non-affective psychotic disorder was only 0.7%, and based on variables such as psychiatric hospitalization, antipsychotic treatment, enduring impairment, thought disorder and long duration of illness (Kendler et al. Reference Kendler, Gallagher, Abelson and Kessler1996). A similar cut-off was reported in the Netherlands Mental Health Survey and Incidence Study (NEMESIS), where the prevalences of psychotic experiences and non-affective psychotic disorder data were 17.5% and 0.4% respectively. These data suggest that the cut-off for diagnosis, given a high prevalence of psychotic experiences in the population, is in part determined by what clinicians feel needs medical treatment. Although such a cut-off certainly has clinical validity, it is unlikely that scientific validity is determined by perception of need for treatment. A useful way to link clinical and non-clinical psychotic experiences conceptually is provided by data indicating that associated dimensions of distress and influence on behaviour discriminate between patients and non-patients (Peters et al. Reference Peters, Day, McKenna and Orbach1999a, Reference Peters, Joseph, Day and Garetyb, Reference Peters, Joseph, Day and Garety2004; Serper et al. Reference Serper, Dill, Chang, Kot and Elliot2005).
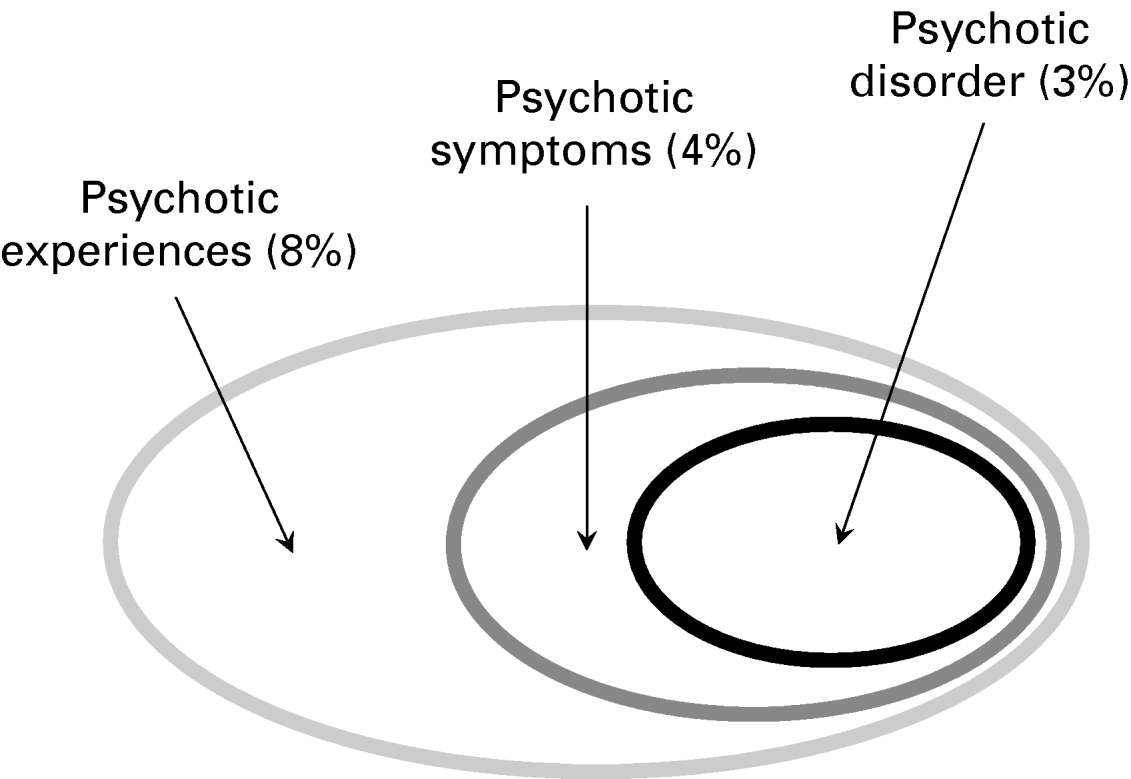
Fig. 4. Psychosis: variation along a continuum.
A factor of interest is the difference between self-report and interviewer-based assessment. Thus, in studies where the interviews were conducted by clinicians or where lay-interviews were followed by clinical reassessment at the level of symptoms, the likelihood of false positives is reduced compared to studies using direct or lay-interviewer assessed self-reports. Thus, in the Dunedin Multidisciplinary Health and Development Study, child psychiatrists interviewed 11-year-old children about hallucinatory experiences, and reported a prevalence of 8% (McGee et al. Reference McGee, Williams and Poulton2000); the proportion with any hallucinatory or delusional experience was even higher at 17.2% (Poulton et al. Reference Poulton, Caspi, Moffitt, Cannon, Murray and Harrington2000). Similarly, in the German Early Developmental Stages of Psychopathology (EDSP) study (Wittchen et al. Reference Wittchen, Perkonigg, Lachner and Nelson1998; Lieb et al. Reference Lieb, Isensee, von Sydow and Wittchen2000), the Composite International Diagnostic Interview (CIDI) was conducted by trained psychologists who were allowed to probe with follow-up clinical questions. In this sample, the prevalence of psychotic experiences was also high at 17.5% (Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2003).
In the NEMESIS, self-reports collected by lay-interviewers using the CIDI were subsequently reassessed by clinicians (van Os et al. Reference van Os, Hanssen, Bijl and Vollebergh2001). A distinction, described above, was made between psychotic symptoms that were clinically relevant (associated with distress and help-seeking behaviour) and symptoms that were subclinical (i.e. not clinically relevant because there was no distress or help-seeking behaviour). Comparing the ratings between clinicians and self-reports for clinically relevant symptoms revealed that 37% of self-reports of definite or possible clinically relevant symptoms were not rated as such by the clinicians, suggesting that these were false positives. However, when these ‘false-positive’ individuals were followed for a period of 3 years, their risk of developing later, clinician-assessed psychotic disorder was increased by a factor of 25 [odds ratio (OR) 27.5, 95% confidence interval (CI) 4.5–123.4] compared to a factor of 50 in the ‘true’ positives (OR 46.1, 95% CI 4.6–236.5) (Bak et al. Reference Bak, Delespaul, Hanssen, de Graaf, Vollebergh and van Os2003). The explanation for this result is that of the 37% that were re-rated by the clinician as not having clinical symptoms, many still had self-reports of subclinical psychotic experiences, and it was these individuals that displayed a greatly increased risk of developing future psychotic disorder. In another study, self-reported positive and negative psychotic experiences were validated against interview-based measures and found to have good concurrent validity (Konings et al. Reference Konings, Bak, Hanssen, van Os and Krabbendam2006). These findings therefore suggest that although clinician assessment and self-report of psychotic symptoms may differ on the assessment of clinical relevance, additional validity may be gained if a combination of clinician interview and self-report is applied, in terms of prediction of transition to more severe psychotic states. Nevertheless, studies using self-reports are likely to generate many false-positive reports, which may bias associations with third variables. For example, a person living in an inner-city extremely deprived area may be reporting ‘real-life’ circumstances rather than paranoid ideation.
Demographic validity
If the psychosis phenotype exists as a continuum, the relationships that are observed between clinical disorder and demographic characteristics (e.g. sex, age, ethnicity) should extend to subclinical experience. To test this hypothesis, we meta-analysed ORs reported in the body of literature identified above (Table 1).
Methods and analysis
Each paper reporting on the cohorts identified in Table 1 was examined to find reports of the odds of psychosis outcomes given the following demographic characteristics: age, education (years, qualifications), employment status, ethnicity and immigrant status, income, marital status, and sex. A number of other papers, excluded from the prevalence and incidence meta-analysis because they did not contribute unique information nevertheless met all other inclusion and exclusion criteria, reported on the effects of exposure to risk factors, and, consequently, were included in this analysis (van Os et al. Reference van Os, Hanssen, Bijl and Vollebergh2001, Reference van Os, Hanssen, Bak, Bijl and Vollebergh2003; Arseneault et al. Reference Arseneault, Cannon, Poulton, Murray, Caspi and Moffit2002; Goodwin et al. Reference Goodwin, Fergusson and Horwood2003; Janssen et al. Reference Janssen, Hanssen, Bak, Bijl, de Graaf, Vollebergh, McKenzie and van Os2003, Reference Janssen, Krabbendam, Bak, Hanssen, Vollebergh, de Graaf and van Os2004; Maric et al. Reference Maric, Krabbendam, Vollebergh, de Graaf and van Os2003; Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2004, Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2006b; Fergusson et al. Reference Fergusson, Horwood and Ridder2005).
From each paper, we recorded the key demographic exposure variable and the associated OR with its 95% CI. If multiple OR values were reported for identical exposures, the most adjusted (corrected) OR was recorded. If there were multiple contrasts for the same exposure variable (e.g. age 20–29 v. 50–59, and 30–39 v. 50–59, etc.), all OR values were recorded. If OR values were not reported, where possible we derived OR values from counts or rates that were reported. If the paper reported analysing the effects of exposure to a risk factor but no estimate of the effect was reported, we recorded the non-reporting of the effect and the result of the analysis (e.g. not significant, or significant with direction OR>1, etc.). OR values for exposure interactions (e.g. sex by age) were not recorded. We also recorded or determined relative risk (RR) ratios in the same manner for all possible cohorts and exposure conditions.
The majority of exposure effects were reported as OR values. Consequently, we used this index in all further analyses. From the collated data, we selected only one OR per cohort for each demographic variable. Where multiple OR values were available, we selected rates as follows. When a summary rate was reported, from the collapsing of multiple subdivisions of the exposure variable, this rate was used. In the absence of a summary rate, we selected the median OR. The median ratio and its 95% margin were found by taking the inverse log of the median of the log-transformed rates. Finally, when an RR but no OR was available, we used the RR as a substitute for the OR provided the reported prevalence or incidence of outcome across the whole cohort was less than 10% and provided inclusion of the RR did not significantly alter the outcome of the analysis. Weighted meta-analytic OR values, their 95% CIs, and heterogeneity statistics were calculated from log-transformed OR values and margins using the Stata metan command (StataCorp, 2007).
Results
Schizophrenia is characterized by strong associations with specific demographic characteristics including younger age, male sex, single marital status, unemployment and ethnic minority group (Driessen et al. Reference Driessen, Gunther, Bak, van Sambeek and Van Os1998; Verdoux et al. Reference Verdoux, Maurice-Tison, Gay, van Os, Salamon and Bourgeois1998b; Agerbo et al. Reference Agerbo, Byrne, Eaton and Mortensen2004; McGrath et al. Reference McGrath, Saha, Welham, El Saadi, MacCauley and Chant2004). The meta-analysis indicates that similar associations are apparent for psychotic symptoms and experiences at the subclinical level (Table 3). Specifically, the prevalence of subclinical psychosis was greater among males, migrants, ethnic minorities, unemployed, unmarried, and less educated people.
Table 3. The impact of demographic and non-genetic factors on the odds of the expression of the psychosis phenotype

OR, Odds ratio; CI, confidence interval.
a Observations gives the number of cohorts included in the analysis [excluded from the analysis due to insufficient data], using the format A-B-C [A-B-C] where A, B and C are the numbers of cohorts with the statistical outcomes OR<1, OR=1 and OR>1 respectively.
b p value heterogeneity statistic.
c Under- and unemployment.
d Excluding Asian minority comparisons.
e Relative risk.
One result was not anticipated. The available data suggest that there is no evidence of an association between the prevalence of subclinical experience and age (Table 3). However, we question the reliability of this finding. Although studies of 13 cohorts reported examining this association, sufficient data could be extracted from only five. Of the remaining eight studies, four reported significant effects in the expected direction and none reported effects in the opposite direction (i.e. greater prevalence among older participants). Of the 13 studies, eight reported at least some evidence that the prevalence of subclinical psychosis was higher among younger participants; none of the remaining tests led to the rejection of the null hypothesis. Treating the results of these tests as binomial outcomes, the probability of observing eight or more significant effects from 13 tests, given the null hypothesis and a rejection criterion of p=0.05, is 4.0×10−8.
On the whole, the evidence from meta-analyses of exposure to demographic variables strongly supports the notion of continuity between subclinical and clinical expressions of psychosis.
Aetiological validity
Non-genetic risk factors
If there is a continuum of psychosis, then it is likely that at least some of the genetic and non-genetic causes contributing to variation at the highest, disorder level of the continuum also impact at lower levels. Some of the non-genetic risk factors associated with schizophrenia such as urbanicity (Krabbendam & van Os, Reference Krabbendam, Myin-Germeys, Hanssen and van Os2005), ethnic minority status (Cantor-Graae & Selten, Reference Cantor-Graae and Selten2005), childhood trauma (Read et al. Reference Read, van Os, Morrison and Ross2005) and cannabis (Henquet et al. Reference Henquet, Murray, Linszen and van Os2005) may also impact on the rate of subclinical psychotic experiences. Studies also suggest that the same developmental window of some exposures, for example the window of childhood/adolescent development associated with exposure to cannabis and urbanicity, also applies to the subclinical domain. For example, exposure to urbanicity during adolescence increases the risk for psychotic experiences (Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2004, Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2006a), whereas adult exposure up to age 74 years does not (Wiles et al. Reference Wiles, Zammit, Bebbington, Singleton, Meltzer and Lewis2006). To test whether similar associations exist with subclinical psychosis, we meta-analysed ORs reported in the body of literature identified above (Table 1) using the same methodology as was used to explore demographic validity.
The results of the meta-analysis indicate that exposure to cannabis, alcohol or other psychoactive drugs was associated with significant higher prevalence of subclinical psychosis as well as incident experience of subclinical psychosis (Table 3). Likewise, stressful or traumatic experience (major life events, abuse, discrimination) also predicts greater odds of prevalent and incident experience, and urbanicity predicts greater odds of prevalent subclinical psychosis. The meta-analysis therefore indicates that subclinical psychotic experiences are associated with the same risk factors that apply to psychotic disorder, again suggesting that there is aetiological continuity between subclinical and clinical psychosis phenotypes.
Genetic risk factors
Given the substantial level of familial clustering of psychotic disorders (Kety et al. Reference Kety, Rosenthal, Wender and Schulsinger1971; Kendler & Gardner, Reference Kendler and Gardner1997), researchers have investigated to what degree the dimensions of the subclinical psychosis phenotype are also transmitted independently in families with one or more affected relatives. One study reported co-clustering of clinical and subclinical psychosis phenotypes in families (Kendler et al. Reference Kendler, McGuire, Gruenberg, O'Hare, Spellman and Walsh1993). Vollema et al. (Reference Vollema, Sitskoorn, Appels and Kahn2002) reported that the score on the positive dimension of a schizotypy questionnaire administered to relatives of patients with psychotic disorders corresponded to their genetic risk of psychosis. Fanous et al. (Reference Fanous, Gardner, Walsh and Kendler2001) demonstrated that interview-based positive and negative symptoms in schizophrenia predicted their equivalent subclinical symptom dimensions in non-psychotic relatives, implying an aetiological continuum between the subclinical and the clinical psychosis phenotypes.
The issue of familial clustering of the multi-dimensional, subclinical manifestation of psychosis has also been studied in general population twin samples, without selection on the basis of family history of psychotic disorder. Kendler & Hewitt (Reference Kendler and Hewitt1992) studied twins from the general population and concluded that the variance in most self-report schizotypy scales, except for perceptual aberration, involved substantial genetic contributions. MacDonald et al. (Reference MacDonald, Pogue-Geile, Debski and Manuck2001) found in their general population-based twin study only one common schizotypy factor, mainly explained by perceptual aberration, magical ideation, schizotypal cognitions and, to a lesser extent, social anhedonia. The common schizotypy factor was influenced by shared environmental, non-shared environmental and possibly genetic effects. A general population female twin study by Linney et al. (Reference Linney, Murray, Peters, MacDonald, Rijsdijk and Sham2003) showed that additive genetic and unique environmental effects influenced self-reported psychotic experiences. The multivariate structural equation model generated two independent latent factors, namely a positive (i.e. cognitive disorganization, unusual experiences and delusional ideation) and a negative dimension (i.e. cognitive disorganization and introvertive anhedonia), suggesting different aetiological mechanisms for the various scales of the subclinical psychosis phenotype (Linney et al. Reference Linney, Murray, Peters, MacDonald, Rijsdijk and Sham2003). In a recent, general population study using both self-report and interview-based measures of positive and negative dimensions of psychotic experiences in 257 subjects belonging to 82 families, significant family-specific variation for both positive and negative subclinical psychosis dimensions were demonstrated, with between-family proportions of total variance between 10% and 40%. Thus, both the positive and the negative dimensions of subclinical psychosis show familial clustering in samples unselected for psychiatric disease (Hanssen et al. Reference Hanssen, Krabbendam, Vollema, Delespaul and Van Os2006). These converging findings suggest that there is aetiological continuity between the subclinical and the clinical psychosis phenotype not only in families of patients or twin pairs but also in unselected families sampled from the general population.
Associations with cognition
Evidence that the neurocognitive deficits associated with schizophrenia are also detectable at the subclinical level, albeit to a much lesser degree, comes from studies investigating healthy individuals at genetically or psychometrically defined risk for schizophrenia. First, studies in non-affected first-degree relatives of patients with psychotic disorder have consistently shown modest alterations in neurocognitive performance (Faraone et al. Reference Faraone, Seidman, Kremen, Pepple, Lyons and Tsuang1995; Egan et al. Reference Egan, Goldberg, Gscheidle, Weirich, Rawlings, Hyde, Bigelow and Weinberger2001; Krabbendam et al. Reference Krabbendam, Marcelis, Delespaul, Jolles and van Os2001) that, according to a recent meta-analysis, predominantly affect the domains of verbal memory, executive functioning and, to a lesser extent, attention (Sitskoorn et al. Reference Sitskoorn, Aleman, Ebisch, Appels and Kahn2004). In the offspring of parents with schizophrenia, impaired cognition, particularly with regard to verbal memory, is one of the more robust findings (Owens & Johnstone, Reference Owens and Johnstone2006). The second line of studies has mainly used schizotypy instruments to define psychometric risk for schizophrenia in non-clinical populations. These studies have similarly found below-average cognitive performance (Park et al. Reference Park, Holzman and Lenzenweger1995; Dinn et al. Reference Dinn, Harris, Aycicegi, Greene and Andover2002; Bergida & Lenzenweger, Reference Bergida and Lenzenweger2006), although cognitive impairment may not be generalized (Lenzenweger & Gold, Reference Lenzenweger and Gold2000; Johnson et al. Reference Johnson, Tuulio-Henriksson, Pirkola, Huttunen, Lonnqvist, Kaprio and Cannon2003) and may occur particularly in interaction with genetic risk (Johnson et al. Reference Johnson, Tuulio-Henriksson, Pirkola, Huttunen, Lonnqvist, Kaprio and Cannon2003). Many studies have been carried out in patients with schizotypal personality disorder (Lenzenweger & Korfine, Reference Lenzenweger and Korfine1994; Voglmaier et al. Reference Voglmaier, Seidman, Niznikiewicz, Dickey, Shenton and McCarley2000; Neumann & Walker, Reference Neumann and Walker2003; Matsui et al. Reference Matsui, Sumiyoshi, Kato, Yoneyama and Kurachi2004; Siever & Davis, Reference Siever and Davis2004; Krabbendam et al. Reference Krabbendam, Myin-Germeys, Hanssen and van Os2005; Raine, Reference Raine2006), again suggesting deficits in verbal memory, executive functioning and attention, but no generalized profile of impairment. Few studies have investigated cognition in relation to subclinical psychotic or psychosis-like experiences in the general population (Lenzenweger & Korfine, Reference Lenzenweger and Korfine1994; Voglmaier et al. Reference Voglmaier, Seidman, Niznikiewicz, Dickey, Shenton and McCarley2000; Neumann & Walker, Reference Neumann and Walker2003; Matsui et al. Reference Matsui, Sumiyoshi, Kato, Yoneyama and Kurachi2004; Krabbendam et al. Reference Krabbendam, Myin-Germeys, Hanssen and van Os2005; Simons et al. Reference Simons, Jacobs, Jolles, van Os and Krabbendam2007). These studies showed weak covariation of subclinical psychosis and cognition both within subjects and between relatives.
In the realm of social cognition, the mechanisms and biases that may underlie specific symptoms of psychosis have also been shown to operate at lower levels of the psychosis continuum, where they may similarly be associated with the presence of subclinical psychotic experiences. The evidence is most robust for deficits in mentalizing (Langdon & Coltheart, Reference Langdon and Coltheart1999, Reference Langdon and Coltheart2004; Marjoram et al. Reference Marjoram, Miller, McIntosh, Owens, Johnstone and Lawrie2006; Pickup, Reference Pickup2006), as well as for probabilistic reasoning biases or jumping-to-conclusions (Linney et al. Reference Linney, Peters and Ayton1998; Colbert & Peters, Reference Colbert and Peters2002; van Dael et al. Reference van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006), both in first-degree relatives of patients with psychotic disorder and in individuals with subclinical psychotic or psychosis-like experiences or with psychometrically defined schizotypy. There is less consistent evidence for other social-cognitive mechanisms, such as the monitoring of one's own speech or actions (Laroi et al. Reference Laroi, Collignon and Van der Linden2005; Allen et al. Reference Allen, Freeman, Johns and McGuire2006; Versmissen et al. Reference Versmissen, Janssen, Johns, McGuire, Drukker, Campo, Myin-Germeys, van Os and Krabbendam2007a, Reference Versmissen, Myin-Germeys, Janssen, Franck, Georgieff, a Campo, van Os and Krabbendamb) or an externalizing attribution style (Levine et al. Reference Levine, Jonas and Serper2004; McKay et al. Reference McKay, Langdon and Coltheart2005; Janssen et al. Reference Janssen, Versmissen, Campo, Myin-Germeys, van Os and Krabbendam2006), for which both inconclusive and positive findings have been reported.
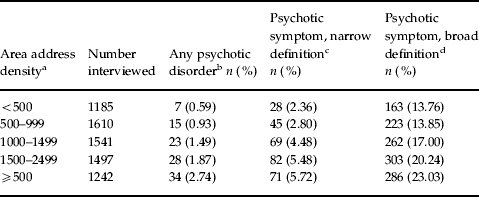
Community validity
One implication of the continuum hypothesis is that, if a true continuum exists, then the mean psychosis level of the entire population should predict the rate of cases of psychotic disorder. This issue was examined in the NEMESIS, in which the rate of psychotic experiences and the rate of psychotic disorders were assessed in a random population sample of 7076 individuals (van Os et al. Reference van Os, Hanssen, Bijl and Vollebergh2001). In that study, five different levels of urbanicity of the place of residence of the subjects were used to define five groups with differences in the rate of psychotic disorder. The study then examined to what degree the increase in the rate of psychotic disorder with greater level of urbanicity would be accompanied by a similar increase in the level of subclinical psychotic experiences. The results, depicted in Table 4, revealed that as the rate of psychotic disorder increased with greater level of urbanicity, the level of psychotic experiences in the healthy population also increased in a similar dose–response fashion. These results suggest that the rates of psychotic disorder in a population are directly related to the mean level of psychosis proneness of the healthy population, similar to the relationship between population mean blood pressure and rate of hypertension, or the mean level of neurotic symptoms and the rate of minor psychiatric disorder.
Table 4. Rate of psychotic disorder in relation to level of psychosis of the healthy population

a Greater levels of area address density indicate greater level of urbanicity.
b Any DSM psychotic disorder (affective and non-affective).
c Any psychotic experience accompanied by significant distress and/or help-seeking behaviour.
d Any psychotic experience, regardless of distress and/or help-seeking behaviour.
Predictive validity
Subclinical psychosis predicts clinical psychotic disorder
Arguably the most important aspect of the validity of subclinical psychosis involves the argument that, if there is a continuum, then dynamic transitions over time from subclinical, non-prodromal manifestations to full-scale psychotic disorder must occur over shorter and longer periods of time. Several studies have addressed this issue. The first was reported by Chapman et al. (Reference Chapman, Chapman, Kwapil, Eckblad and Zinser1994), demonstrating high rates of psychotic outcomes in individuals who had rated high on scales of magical ideation and perceptual aberration 10 years earlier. The longest prospective investigation was the follow-up of the Dunedin Multidisciplinary Health and Development Study, in which children who had reported psychotic experiences at age 11 years were clinically assessed at age 26 years. The 16-year risk of developing schizophreniform disorder associated with prevalent psychotic experiences at age 11 was increased 16-fold compared to children without psychotic experiences. In terms of absolute risk, 25% of children with psychotic experiences at age 11 developed schizophreniform disorder at age 26 over the 16-year follow-up (Poulton et al. Reference Poulton, Caspi, Moffitt, Cannon, Murray and Harrington2000). Similar results were reported by Hanssen et al. (Reference Hanssen, Bak, Bijl, Vollebergh and van Os2005), who first followed up a sample of 7076 individuals for 1 year to identify new, incident cases of psychotic experiences. In a second follow-up, individuals with incident psychotic experiences were followed for 2 years to identify transitions to psychotic disorder. In their study, the 2-year transition rate to clinical psychotic disorder was 8%, representing a greater than 60-fold increase in risk compared to those without incident psychotic experiences. The 2-year risk rose to 21% for those with multiple psychotic experiences, and to 15% for those whose psychotic experience had arisen in the context of significant lowering of mood (Hanssen et al. Reference Hanssen, Bak, Bijl, Vollebergh and van Os2005).
Most subclinical psychosis is transitory
Although the above studies suggest that subclinical expressions of psychosis are indeed predictive of future transition to clinical psychotic disorder, also important are the questions: (i) How many individuals have subclinical psychotic experiences that remain subclinical over time? and (ii) How many have subclinical psychotic experiences that disappear over time? Some studies were able to address this issue. Hanssen et al. (Reference Hanssen, Bak, Bijl, Vollebergh and van Os2005) reported that of individuals with new, incident subclinical psychotic experiences at baseline, only 8% had persistence of the psychotic experiences at the subclinical level whereas 84% no longer presented with any psychotic experiences. The remaining 8% made the transition to clinical disorder. In a later study with a more limited screen for existing, prevalent psychotic experiences at baseline and a shorter follow-up, the great majority of individuals with psychotic experiences similarly did not persist (18-month persistence rate of 30%; Wiles et al. Reference Wiles, Zammit, Bebbington, Singleton, Meltzer and Lewis2006).
The data therefore suggest that subclinical psychotic experiences are prevalent, but mostly self-limiting and of good outcome, although a small proportion go on to develop a clinical psychotic disorder.
Clinical implications: the psychosis proneness–persistence–impairment model
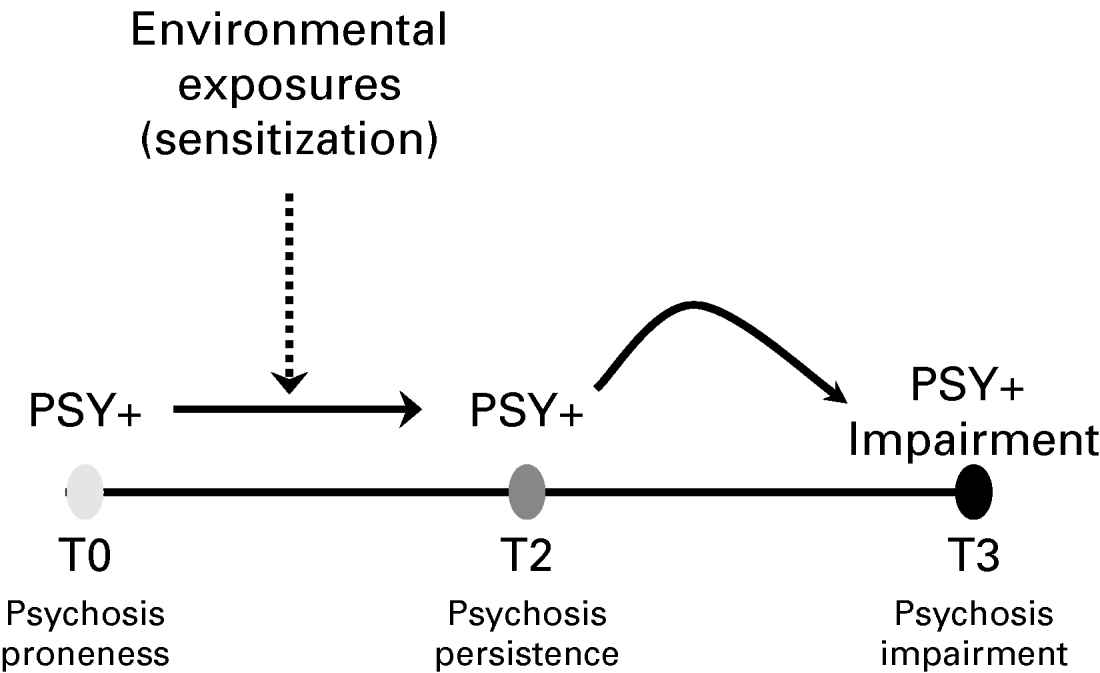
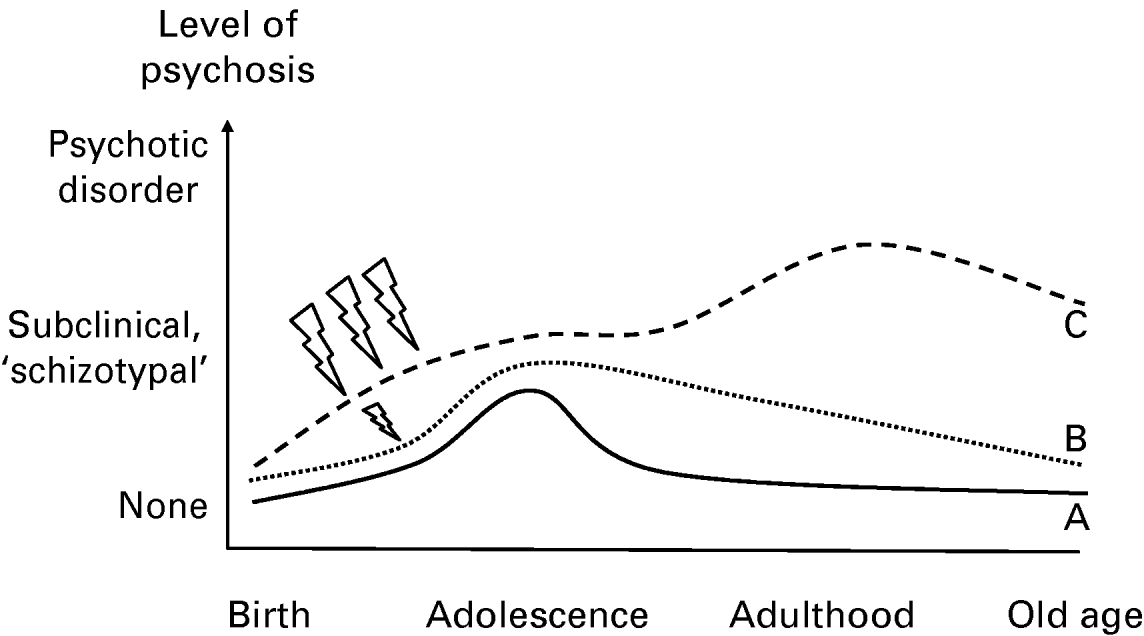
An important observation is that studies have demonstrated that the generally good (because the symptoms are only transitory as described above) outcome of subclinical psychotic experiences can be modified to poorer outcomes of persistence and clinical need for care if subjects are exposed to additional (proxy) environmental risk factors. Examples of these are trauma (Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2006b), cannabis (Henquet et al. Reference Henquet, Murray, Linszen and van Os2005) and urbanicity (Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2004, Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2006a). This fact, together with the above discussed findings of a high prevalence of psychotic experiences, their familial clustering, age-associated expression and low rate of transition to psychotic disorder, suggests a model of psychosis that considers genetic background factors impacting on a broadly distributed and transitory population expression of psychosis during development, the poor prognosis of which, in terms of persistence and clinical need, is predicted by environmental exposures interacting with genetic risk. In other words, transitory developmental expression of psychosis may become abnormally persistent and clinically relevant depending on the degree of environmental risk the person is additionally exposed to (Fig. 5). The phenomenon of persistence and subsequent development of impairment and need for care, that is: a diagnosable psychotic disorder, may be related to processes of biological and psychological sensitization, reviewed elsewhere (Collip et al. Reference Collip, Myin-Germeys and Van Os2008), and explain differences in longitudinal trajectories of psychosis proneness as depicted in Fig. 6. Two studies to date have attempted to specifically falsify elements of the psychosis proneness–persistence–impairment model. One focused on the stage from psychosis proneness to psychosis persistence (Cougnard et al. Reference Cougnard, Marcelis, Myin-Germeys, De Graaf, Vollebergh, Krabbendam, Lieb, Wittchen, Henquet, Spauwen and Van Os2007), and the other on the stage from psychosis persistence to psychosis impairment (Dominguez et al., unpublished observations). The findings of both studies are in agreement with the proneness–persistence–impairment model of the onset of psychotic disorder; a significant proportion of psychotic disorder may be conceptualized as the rare poor outcome of a common developmental phenotype characterized by persistence of psychometrically detectable subclinical psychotic experiences. The causes of psychotic disorder may thus be traced to the factors that make the common and transitory developmental expression of subclinical psychosis persist, highlighting the importance of existing efforts at early detection and intervention.

Fig. 5. Psychosis proneness–persistence–impairment model. According to this model, developmental expression of psychotic experience is common and mostly transitory. However, psychotic experiences may become persistent through a mechanism of psychological and biological sensitization. Persistence in turn increases the probability of onset of impairment and need for care.

Fig. 6. Sensitization and onset of psychotic disorder. Person A has ‘normal’ developmental expression of subclinical psychotic experiences (psychosis proneness) that are transient. Person B has similar expression but longer persistence due to additional but mild environmental exposure. Person C has longer persistence due to severe repeated environmental exposure and transition to clinical psychotic disorder with significant impairment.
Declaration of Interest
None.