Introduction
The climate and the ecosystem diversity in the North African countries support the habitat of various wild species but urbanization, desertification, pollution, over exploitation of some wild species and the lack of knowledge on the pathogens of wild animals are under increased threat of wild life. In Tunisia a national strategy has been developed to preserve wild Bovidae and Cervidae, the only forms of wild ruminants in the Artiodactyla order and the most threatened (Anonymous, 2013). These species are classified in the International Union for Conservation of Nature (IUCN) red list of threatened species with different conservation status: the Dorcas gazelle (Gazella dorcas) and Barbary sheep (Ammotragus lervia) as vulnerable, the Addax (Addax nasomaculatus) as critically endangered, the Scimitar-horned Oryx (Oryx dammah) as extinct in the wild and the Slender-horned gazelle (Gazella leptoceros) as endangered. The Barbary red deer (Cervus elaphus barbarus), protected under Appendix III of the Bern Convention, is a rare subspecies of the red deer present only in Northern Africa (Tunisia and Algeria) (Gharaibeh, Reference Gharaibeh1997; Hajji et al. Reference Hajji, Zachos, Charfi-Cheikrouha and Hartl2007) and its population has been increasingly declining (Gharaibeh, Reference Gharaibeh1997; Lovari et al. Reference Lovari, Lorenzini, Masseti, Pereladova, Carden and Brook2016). The national strategy involved the instauration of legislation prohibiting catching and hunting, creation of fenced protected areas, participation in the Convention on Migratory Species (CMS) action plan for the conservation and restoration of sahelo-saharan Antelopes and the cooperation with international organization to reintroduce some wild ruminants that were extinct as the Addax and the Scimitar-horned Oryx (Gilbert and Woodfine, Reference Gilbert and Woodfine2008; Woodfine et al. Reference Woodfine, Chetoui, Zahzah, Gilbert and Soorae2011). In the protected areas (nature reserves and national parks) only wild animals are present and there is no possible contact with domestic animals, which could be reservoirs of infectious diseases and parasites. Available literature on parasite cross-transmission between domestic and wild host is limited (Walker and Morgan, Reference Walker and Morgan2014) but this transmission is possible and even when there is no direct contact between hosts, it could occur from the environment, water, or grazing areas.
The Nematodes play an ubiquitous part of the ecosystem and many of them are generalist (a parasite who survives and reproduces in more than one type of host) (Walker and Morgan, Reference Walker and Morgan2014). In livestock, the clinical signs of Nematode-infection are non-specific and dependent on the number of parasites in the host (Brooker, Reference Brooker2010). They strongly affect health, production, population dynamics and susceptibility to other diseases (Perry and Randolph, Reference Perry and Randolph1999; Steele et al. Reference Steele, Orsel, Cuyler, Hoberg, Schmidt and Kutz2013; Thumbi et al. Reference Thumbi, Bronsvoort, Poole, Kiara, Toye, Ndila, Conradie, Jennings, Handel, Coetzer, Hanotte and Woolhouse2013). In fact, wild ruminants are more resistant and often parasite infection are subclinical but this infection can affect body condition, weight gain, fecundity, population density (Arneberg et al. Reference Arneberg, Folstad and Karter1996; Albon et al. Reference Albon, Stien, Irvine, Langvatn, Ropstad and Halvorsen2002; Stien et al. Reference Stien, Irvine, Ropstad, Halvorsen, Langvatn and Albon2002; Hughes et al. Reference Hughes, Albon, Irvine and Woodin2009) and has the potential to impede conservation efforts (Dobson and Hudson, Reference Dobson and Hudson1986).
In Tunisia and most generally in Northern Africa, there is a lack of knowledge on parasite biodiversity of these threatened wild hosts and there are not any studies on their gastrointestinal nematodes. Any scientific research in this field will be important to understand the dynamic relationship between populations of threatened hosts and their parasites and to be more effective in the conservation strategy. The study of parasite biodiversity based only on morphology misidentified cryptic species (Hoberg et al. Reference Hoberg, Monsen, Kutz and Blouin1999). Thus the use of molecular genetic tools is necessary to unequivocally identify parasite species and improves the understanding of multi-host/multi-parasite systems and parasite distribution (Morgan et al. Reference Morgan, Clare, Jefferies and Stevens2012; Walker and Morgan, Reference Walker and Morgan2014).
It is difficult to study nematodes on wild hosts based on sporadic necropsy of naturally dead animals. Thus the aim of the present study was to identify the gastrointestinal nematode fauna present in the Tunisian wild ruminants faecal samples using faecal examination and polymerase chain reaction (PCR).
Materials and methods
Sampling and coprological examination
From May 2015 until February 2016, a total of 262 faecal samples were collected from domestic sheep and goat, and 6 wild ruminant species [Addax (A. nasomaculatus) (Bovidae, Hippotraginae), Barbary sheep (A. lervia) (Bovidae, Caprinae), Barbary red deer (C. e. barbarus) (Cervidae, Cervinae), Dorcas gazelle (G. dorcas) (Bovidae, Antilopinae), Slender-horned gazelle (G. leptoceros) (Bovidae, Antilopinae) and Scimitar-horned Oryx (O. dammah) (Bovidae, Hippotraginae)] from 7 national parks (El Feidja, Bouhedma, Haddaj, Orbata, Dghoumes, Sidi Toui, Djbil) and one nature reserve (Oued Dekouk) (Fig. 1) and from domestic sheep and goat flocks living near these protected areas (Table 1). Samples collected from the wild ruminants were picked from the ground in the grazing areas after looking for each specie's herd, then they were transported in cooling boxes and stored at −20 °C until examination.
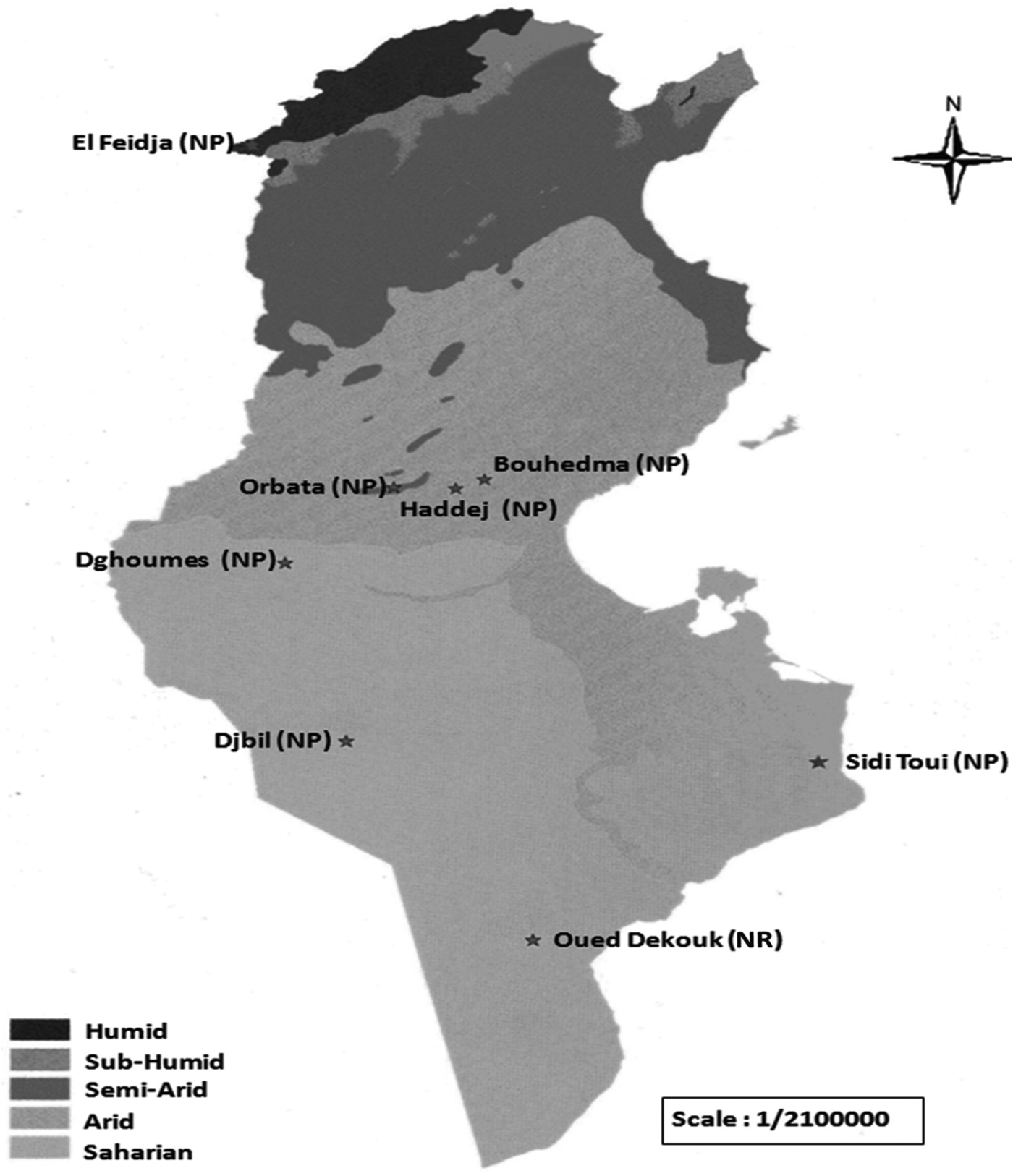
Fig. 1. Bioclimatic map of Tunisia representing studied National parks (NP) and Nature reserve (NR).
Table 1. Faecal samples collected from various animal species and localities
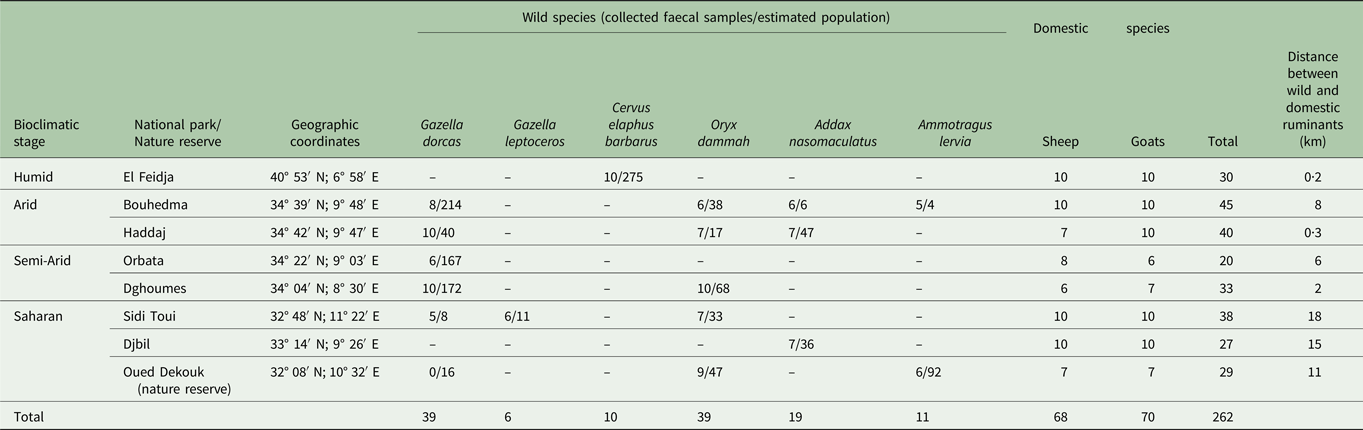
(–): Animal species absent in the park or reserve.
Flotation test was used to detect nematodes’ eggs in the faecal samples. It was performed using 5 g of feces and saturated sodium chloride solution (specific gravity 1.20 at 20 °C) (Thienpont et al. Reference Thienpont, Rochette and Vanparijs1979).
Molecular analyses
DNA isolation and PCR
Genomic DNA was isolated from faecal samples (n = 262) after performing the flotation technique using the modified protocol described by Bott et al. (Reference Bott, Campbell, Beveridge, Chilton, Rees, Hunt and Gasser2009). Briefly, 5 g of feces were used instead of 4 g without performing the McMaster step. DNA was purified from the collected eggs using the Wizard Genomic DNA Purification kit (Promega, Madison, USA) according to the manufacturer's protocol. To amplify the complete sequence of the second internal transcribed spacer (ITS2) regions of the ribosomal DNA, three consecutive PCR were performed. In the first PCR (PCR 1), the amplification of the parasites’ DNA was performed with the forward (NC5: 5′-GTAGGTGAACCTGCGGAAGGATCATT-3′) and reverse primers (NC2: 5′-TTAGTTTCTTTTCCTCCGCT-3′) corresponding to the conserved 3′–5′ ends of the ITS1-5.8S-ITS2 flanking the 18S and 28S gene regions of the ribosomal DNA (Gasser et al. Reference Gasser, Nansen and Guldberg1996). PCR mix consisting of 1× PCR buffer (50 mm Tris–HCl, pH 8·5; 50 mm NaCl), 0·2 mm of each dNTP (dATP, dCTP, dGTP, dTTP), 1.5 mm MgCl2, 0.5 mm of each primer mix, 1.25 µL template DNA, 0.024 units Taq DNA polymerase (Vivantis, USA) and sterile distilled water to 25 µL. The PCR was carried out in applied Biosystem, SimpliAmp™ Thermal Cycler using the following cycling conditions: an initial denaturation step at 94 °C for 3 min, 35 cycles (94, 55 and 72 °C for 1 min each) and a final extension step at 72 °C for 10 min. The second and the third PCR were performed as the protocol of nested PCR described by Sim et al. (Reference Sim, Hoar, Kutz and Chilton2010). In the second PCR (PCR 2), the ITS2 of the rDNA were amplified using 5 µL of the amplicons generated from the PCR 1 as template, 0·8 mm of each NC13aF (5′-ATCGATGAAAAACGCAGC-3′) and NC2 (5′-TTAGTTTCTTTTCCTCCGCT-3′) primers in 25 µL volume containing the same concentrations of PCR buffer, dNTP, MgCl2 and Taq polymerase (Vivantis, USA) as in the PCR 1. The PCR conditions were an initial denaturation step at 95 °C for 5 min, 30 cycles (95, 50 and 72 °C for 1 min each), and a final extension step of 72 °C for 5 min. The PCR products were visualized in 1·5% agarose gels. In the third PCR (PCR 3), all PCR products were amplified using two internal primers, NC14-Fn (5′-GAACGCATAGCGCCGTTGGGTT-3′) and NC2-Rn (5′-TGATATGCTTAAGTTCAGCGGG-3′). The third PCR was conducted as in PCR 2, except that 2 µL of PCR 2 products were used and the annealing temperature was increased to 58 °C (Sim et al. Reference Sim, Hoar, Kutz and Chilton2010). The final amplification corresponded to the ITS2 region of the rDNA.
DNA sequencing and phylogenetic analysis
All the PCR amplicons (positive) were purified (n = 92) and sequenced using NC14-Fn and NC2-Rn primers. A conventional Big Dye Terminator v3·1 cycle sequencing ready reaction kit (Applied Biosystems, Foster City, CA) and an ABI3730XL automated DNA sequencer were used. The chromatograms and sequence alignments were evaluated with the MEGA 6 software program (Tamura et al. Reference Tamura, Stecher, Peterson, Filipski and Kumar2013) and the network server of the National Center for Biotechnology Information (NCBI) using BLAST. Ninety two sequences were deposited into GenBank database and phylogenetic tree was constructed by the neighbour-joining method (NJ) after 1000 bootstraps (Saitou and Nei, Reference Saitou and Nei1987). The evolutionary distances were computed using Kimura 2-parameter method (Kimura, Reference Kimura1980) and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated. Identical sequences in the alignments were treated as single terminal taxa.
Data analysis
The prevalence of positive samples and the standard error (s.e.) were calculated. The differences in prevalence were analysed using χ 2 test and Fisher's exact test with the Statistical Package for Social Sciences (SPSS). Version 20 (IBM SPSS Statistics, Armonk, NY) and Epi Info version 7 (Centers for Disease Control and Prevention, Atlanta, GA, USA). Differences were considered statistically significant at a 0.05 threshold value. The comparison and the evaluation of the agreement between coproscopic and molecular tests were performed using Cohen κ analysis and χ 2 test.
Results
Coprological examination
Using the coproscopic flotation test, the overall prevalence of positive samples from both domestic and wild ruminants was 16 ± 0.02% (42/262). In wild ruminants, the prevalence of positive samples was 20.96 ± 0.03% (26/124), while in domestic ones the prevalence was estimated to 11.5 ± 0.02% (16/138) (P = 0.038) (Table 2). All positive samples have a low number of parasite eggs (1 to 3 eggs) by flotation and only Nematodirus eggs were found.
Table 2. Faecal samples’ examination results by laboratory technique, animal species and localities
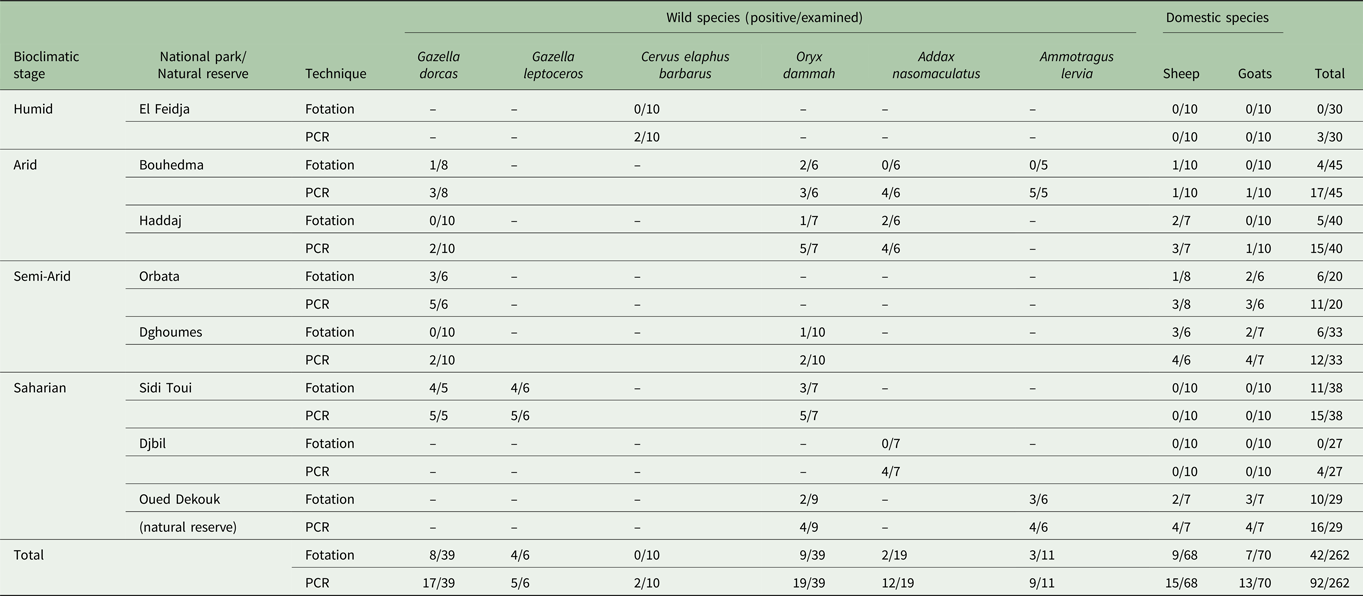
(–): Animal species absent in the park or reserve.
Molecular analyses
All the 262 faecal samples were amplified by the PCR. Amplicons were visualized by electrophoresis on a 1.5% agarose gel and produced a single amplification band (≈300 pb in length). The amplification of the ITS2 region of the rDNA only by PCR 2 followed by PCR 3 showed a weak PCR bands on agarose gel. The association of three PCRs allowed to get a higher concentration of PCR products.
The prevalence estimated by PCR (35.1 ± 0.02%; 92/262) was significantly higher than coprological examination (P = 0.000001) (Table 2). There was a moderate agreement between the two techniques (k = 0.52). The molecular prevalence in wild ruminants (51.6 ± 0.04%; 64/124) was significantly higher than the domestic ruminants (20.28 ± 0.03%; 28/138) (P = 0.000001). The infection prevalence in Slender-horned gazelle, Barbary sheep, addax, Scimitar-horned Oryx, Dorcas gazelle and Barbary red deer samples was 83 ± 0.15% (5/6), 81 ± 0.1% (9/11), 63 ± 0.1% (12/19), 48 ± 0.08% (19/39), 43 ± 0.07% (17/39) and 20 ± 0.1% (2/10), respectively.
Nematodes identification
All 92 produced ITS2 sequences were deposited in GenBank (Table 3). The multiple sequences alignment and the comparison with the GenBank database reveal that 66 isolates (71 ± 0.04%) (286 bp length) were Nematodirus helvetianus, 21 isolates (22 ± 0.04%) (286 bp length) Nematodirus spathiger, 3 isolates (3 ± 0.01%) (291 bp length) Marshallagia marshalli, 1 isolate (1 ± 0.01%) (292 bp length) Camelostrongylus mentulatus and 1 isolate (1 ± 0.01%) (288 bp length) Chabertia ovina. All the sequences showed 100% similarities with those deposited in GenBank database except for C. mentulatus and C. ovina, which had a similarity of 99.57 and 99.65%, respectively.
Table 3. Host, geographic origins and GenBank accession number of the complete ITS2 region of the rDNA sequences of parasite species isolated from Tunisian wild and domestic ruminants’ faecal samples
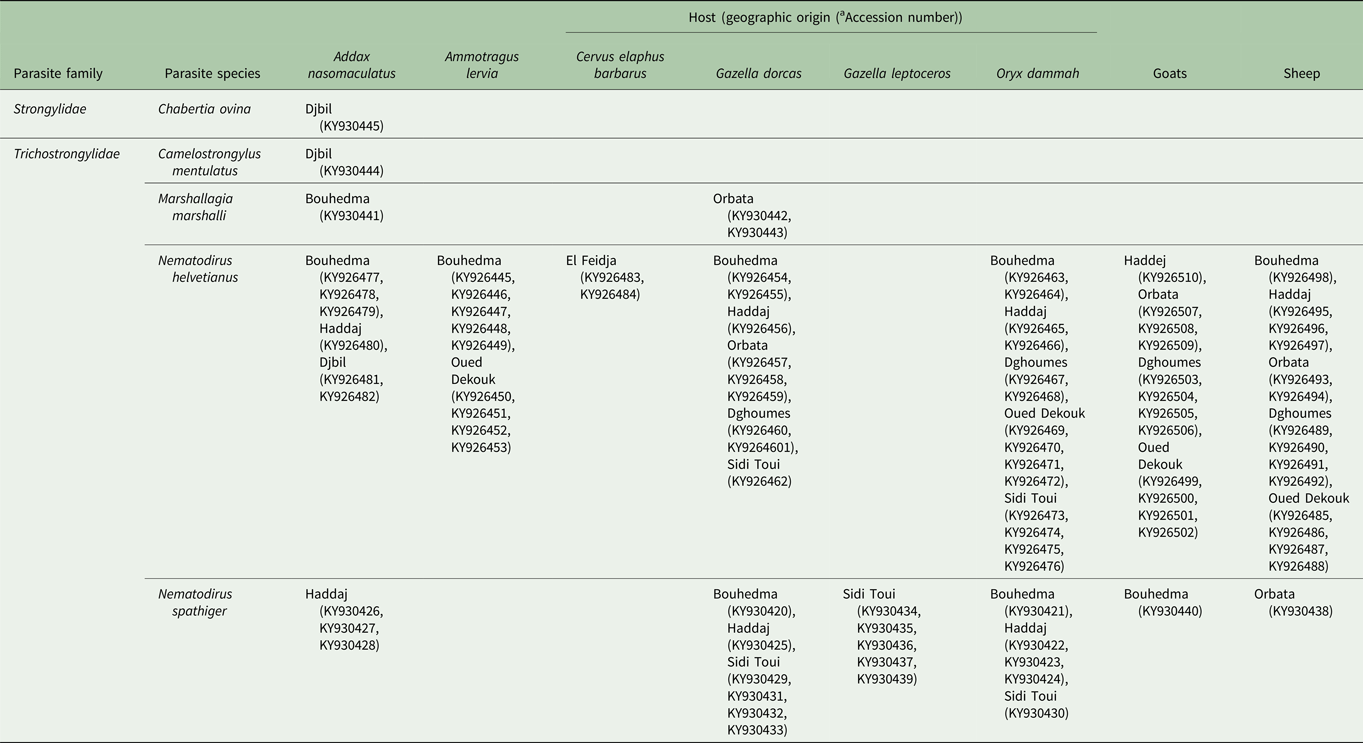
a GenBank Accession number.
Host and nematodes species
Five nematode species were identified in wild ruminant samples; N. helvetianus (62 ± 0.06%; 40/64), N. sphatiger (29 ± 0.05%; 19/64), M. marshalli (4 ± 0.02%; 3/64), C. mentulatus (1 ± 0.01%; 1/64) and C. ovina (1 ± 0.01%; 1/64) while only two nematode species were present in sheep and goat samples; N. helvetianus (92 ± 0.04%; 26/28) and N. sphatiger (7 ± 0.04%; 2/28). In sheep, the infection prevalence by N. helvetianus and N. spathiger represents 14/15 and 1/15, respectively; while, in goats they represented 12/13 and 1/13, respectively. In addax, five parasite species were identified: N. helvetianus (50 ± 0.14%), N. sphatiger (25 ± 0.12%), M. marshalli, C. mentulatus and C. ovina (with 8 ± 0.08% for each). In Dorcas gazelle, 3 parasite species were identified; N. helvetianus (52 ± 0.1%), N. sphatiger (35 ± 0.1%) and M. marshalli (11.7 ± 0.07%). In Scimitar-horned Oryx, two parasite species were identified; N. helvetianus (73 ± 0.1%) and N. sphatiger (26 ± 0.1%). In Barbary sheep, and Barbary red deer only N. helvetianus was identified. In Slender-horned gazelle only N. sphatiger was identified.
Geographic distribution of nematode species
In the humid zone (National Park of El Feidja), only N. helvetianus was identified, in the semi-arid zone (National Park of Orbata) and arid zone (National Park of Bouhedma and Haddaj) N. helvetianus, N. sphatiger and M. marshalli were identified. In the Saharan zone (National Parks: Dghoumes, Djbil, Sidi Toui and the Nature Reserve of Oued Dekouk) four parasite species were identified; C. mentulatus, C. ovina, N. helvetianus and N. sphatiger.
Camelostrongylus mentulatus and C. ovina were identified only in the samples collected from Djbil National Park and M. marshalli only in Bouhedma and Orbata National Parks. Nematodirus sphatiger was absent in Dghoumes, El Feidja National Parks and Oued Dekouk Nature Reserve.
Intraspecific variation
The genetic analysis of 66 N. helvetianus amplicons from different hosts and geographic regions in Tunisia showed 100% of similarity. Ninety per cent (19/21) of N. spathiger isolated from different hosts and geographic regions were identical. Two out of 21 N. spathiger amplicons showed a low intraspecific variation (0·34%) by single point mutation that causes a nucleotide base substitution (A199C) for sequence KY930439 in Slender-horned gazelle (Sidi Toui National Park) and (A211G) for sequence KY930440 in goats (Bouhedma National Park) (Table 4). All eggs isolated from Slender-horned gazelle faecal samples were N. spathiger. The majority (4/5) of the isolates were genetically identical to N. spathiger isolated from various wild and domestic ruminants in the different locations, only one (1/5) amplicon showed an intraspecific variation. All the M. marshalli amplicons were 100% identical. The comparison of C. mentulatus amplicon from addax (KY930444) with an Australian amplicon from dromedary (Camelus dromedarius) (Y14819) showed 99.57% of similarity and a low intraspecific variation (0.42%) represented by a single point mutation (T47A) (Table 4). The comparison of C. ovina amplicon from addax (KY930445) with other sequences available in GenBank showed an intraspecific variation varying from 0.3 to 0.9%. It showed a high (99.65%) intraspecific similarity with C. ovina isolated from goat in China (KF913466). This genetic variation is a single nucleotide insertion (279insA) (Table 4).
Table 4. Fixed differences distinguishing on the ITS2 region of the rDNA sequences of Chabertia ovina (KY930445), Nematodirus spathiger (KY930434, KY930435, KY930436, KY930437, KY930439 and KY930440) and Camelostrongylus mentulatus (KY930444) extracted from wild and domestic ruminants’ faecal samples in Tunisia.
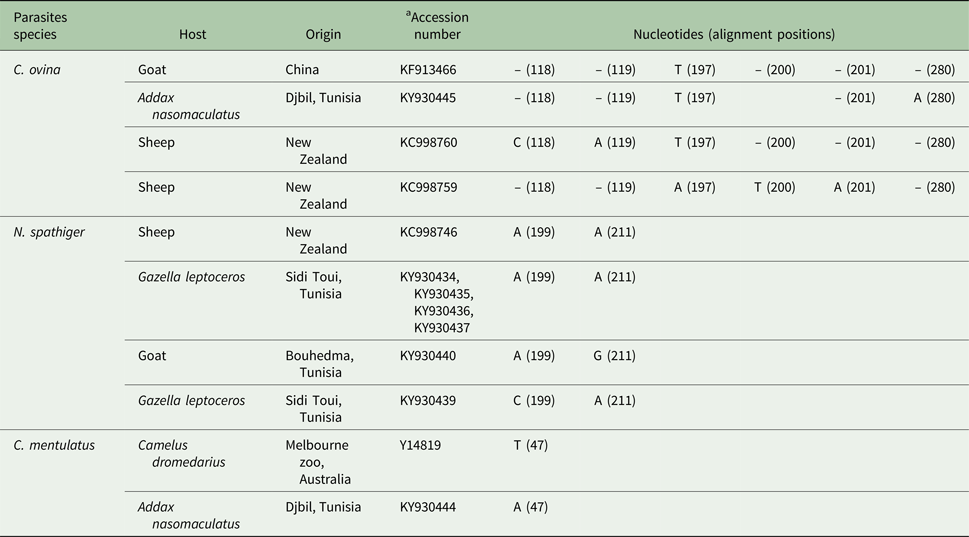
Nucleotides: T, Thymine; C, Cytosine; G, Guanine; A, Adenine.
a GenBank Accession number.
(–): Gap in the sequence alignment.
Phylogenetic analyses
NJ tree of N. helvetianus isolated from Tunisian wild and domestic ruminants showed that they clustered in the same clade with isolates from China (sheep) (KC580751 and KC580752) (bootstrap value = 78%) (Fig. 2). A phylogenetic tree of N. spathiger showed that all the isolates from various Tunisian ruminant species clustered in the same clade with the New Zealander (KC998746, KC998748) and American (AF194143) variants (bootstrap value = 87%). One isolate from goats (Bouhedma National Park) (KY930440) formed an innermost clade with two other amplicons from New Zealand (KC998747) and USA (AF194144) (bootstrap value = 66%) (Fig. 3).
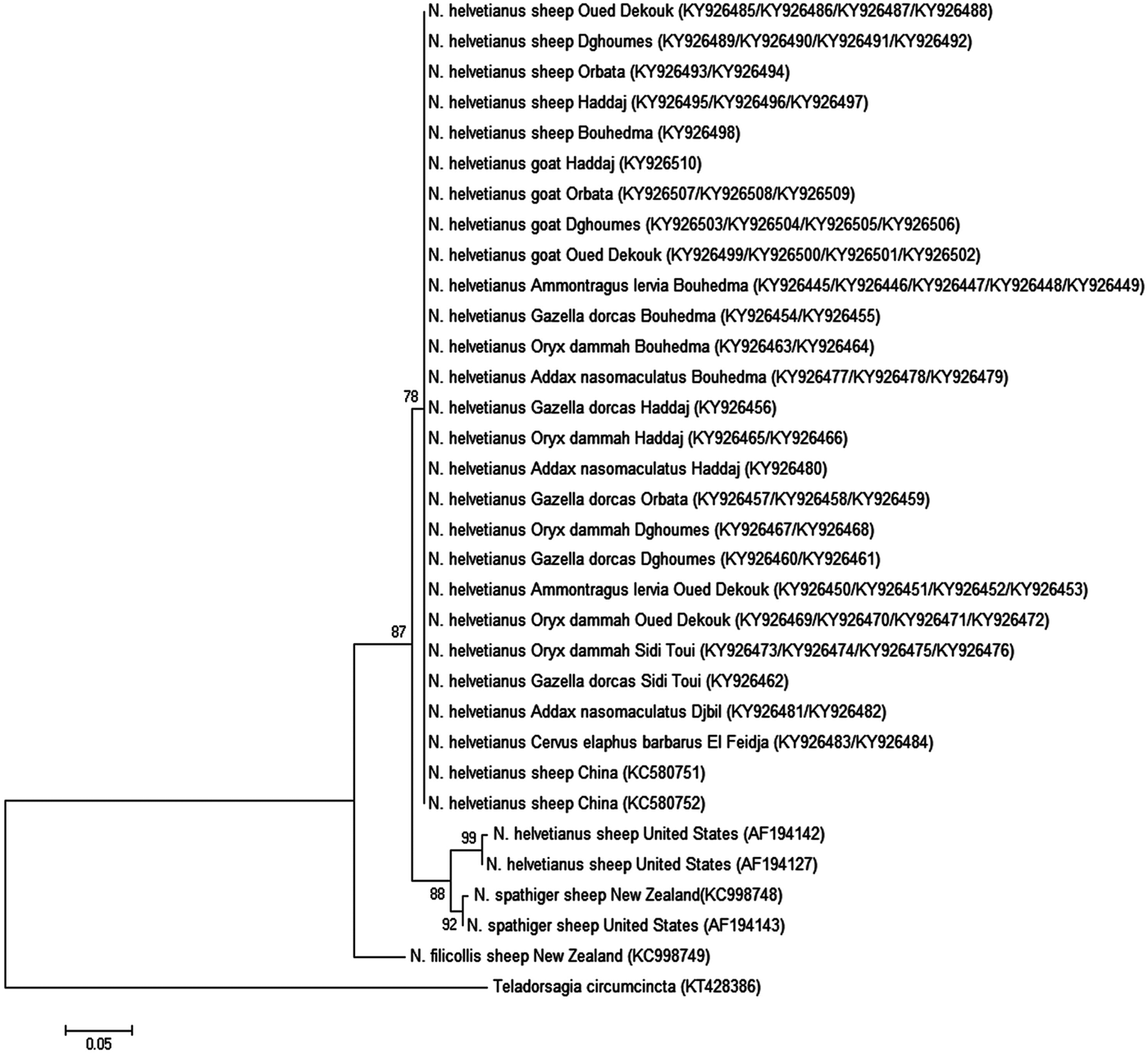
Fig. 2. Phylogenetic tree of Nematodirus helvetianus based on ITS2 sequences of the rDNA. The ITS2 sequences were aligned with Mega 6. Neighbour-joining (NJ) tree represent phylogenetic relationships for 66 sequences from wild and domestic ruminants from Tunisian's national parks and nature reserves and 8 sequences from Genbank database. The scale bar represents 0.05 substitution per site. Numbers associated with nodes represent the percentage of 1000 bootstrap supporting the nodes (only percentages higher than 70% were represented).

Fig. 3. Phylogenetic tree of Nematodirus spathiger based on ITS2 sequences of the rDNA. The ITS2 sequences were aligned with Mega 6. Neighbour-joining (NJ) tree represent phylogenetic relationships for 21 sequences from wild and domestic ruminants from Tunisian's national parks and nature reserve and 9 sequences from Genbank database. The scale bar represents 0.1 substitution per site. Numbers associated with nodes represent the percentage of 1000 bootstrap supporting the nodes (only percentages higher than 65% were represented).
Phylogenetic analysis of the three identical isolates of M. marshalli from two Dorcas gazelle (KY930442 and KY930443) and one addax (KY930441) in Tunisia clustered with M. marshalli from Iranian sheep (HQ389231) and M. marshalli from Russian sheep (JQ863403). Two M. marshalli amplicons from Uzbekistan (KT428384) and Russia (JQ839283) form a separate innermost clade in the first one. M arshallagia marshalli from France (AJ577469) and M. marshalli from Svalbard (Norway) (AH010211) clustered separately in a third clade (Fig. 4). Camelostrongylus mentulatus isolated from addax (KY930444) clustered in the same clade with the Australian amplicon from one dromedary (Y14819) with a high bootstrap support value (100%) (Fig. 4). C habertia ovina (KY930445) clustered with amplicons from New Zealander sheep (KC998759 and KC998760) and goats from China (KF913466, KF913467 and KF913470).

Fig. 4. Neighbour-joining (NJ) tree, based on ITS2 sequences of the rDNA, represents phylogenetic relationships of Marshallagia marshalli (3 sequences), Camelostrongylus mentulatus (1 sequence) isolated from Tunisian Wild ruminants and 12 sequences from the trichostrongylidae family from GenBank database (Marshallagia occidentalis, Marshallagia marshalli, Ostertagia ostertagi, Ostertagia leptospicularis, Teladorsagia circumcincta and Teladorsagia trifurcata). The scale bar represents 0.01 substitution per site. Numbers associated with nodes represent the percentage of 1000 bootstrap supporting the nodes (only percentages higher than 70% were represented).
Discussion
This study revealed a diversity in nematode species infecting the Tunisian wild ruminants. The addax nematode fauna was the most varied with five parasite species; N. helvetianus, N. sphatiger, M. marshalli, C. mentulatus and C. ovina. From all identified nematodes only N. sphatiger and C. ovina have been found or reported previously from domestic sheep and goat in Tunisia, which reflects the loss of species specific of these nematodes. On a worldwide basis, a comparison of the helminth fauna in a number of wild ruminants species indicated that cross-transmission with domestic hosts is common and achieved by a multi-host parasites (Dunn, Reference Dunn1969; Sharhuu and Sharkhuu, Reference Sharhuu and Sharkhuu2004; Walker and Morgan, Reference Walker and Morgan2014).
In Scimitar-horned Oryx only two parasite species (N. helvetianus and N. sphatiger) were identified in Tunisia. Despite the two antelopes, Scimitar-horned Oryx and addax, shared the same grazing areas in Bouhedma National Park, M. marshalli was found only in the addax. The absence of this generalist nematode species in Scimitar-horned Oryx can be probably due (i) to the small number of collected samples compared with the number of the estimated population (6/38) or (ii) to a low-level parasitic burden of M. marshalli in the grazing areas especially since this parasite has not been reported previously in Tunisia. Pauling et al. (Reference Pauling, Oller and Jackson2016) revealed the presence of more diversified parasite fauna in two captive herds of Scimitar-horned Oryx, in Fossil Rim Wildlife Center (FRWC) and Kansas City Zoo (KCZ), in the USA.
Three parasite species were found in Dorcas gazelles (N. helvetianus, N. sphatiger and M. marshalli), and only a single species (N. helvetianus) from Barbary sheep and Barbary red deer. All parasites isolated from Slender-horned gazelle fecal samples were N. spathiger, which is in agreement with previous studies showing that N. spathiger was the most frequent species in Arabian sand gazelle (Gazella subgutturosa marica) and Arabian mountain gazelle (Gazella gazella) in Saudi Arabia (Mohammed et al. Reference Mohammed, Omer and Sandouka2007).
The report of few nematode species per host can be related to the number of samples and to the low density of wild population. The estimated population of the studied wild ruminants is about only 1000 animals or the host–parasite relationship are ineluctably linked to host-population density and some localized parasite loss can occur when a host population density falls below a minimal threshold for parasite transmission (Lyles and Dobson, Reference Lyles and Dobson1993).
Regarding the different bioclimatic zones, a richer parasite diversity was found in the Saharan zone, which may be related to the presence of four protected areas in this zone and to a higher sampling. In fact, our results support that certain parasite species have evolved and acquired a high capacity of adaptation to resist to desiccation, and to low and very high temperature characterizing this area.
Marshallagia marshalli, parasite of domestic and wild ruminants, was isolated from addax and Dorcas gazelle living in two national parks (Bouhedma and Orbata) located in semi-arid and arid zones of Tunisia, which is in agreement with the study of Meradi et al. (Reference Meradi, Bentounsi, Zouyed and Cabaret2011) showing that this species is more commonly found in the arid zones (Steele et al. Reference Steele, Orsel, Cuyler, Hoberg, Schmidt and Kutz2013). In addition, the occurrence of this nematode species in addax and Dorcas gazelle was not reported previously in the literature in spite of its occurrence in different other wild Bovidae species.
Nematodirus spathiger has not been isolated in humid (El Feidja National Park) and Saharan zones (Djbil and Dgoumes National Parks and Oued Dekouk Nature Reserve). A previous study on goats from the northwest of Tunisia (humid zone) revealed the presence of N. spathiger and N. filicollis in this region (Azizi, Reference Azizi1985). Therefore, the absence of this species in our study can be probably due to the small number of examined samples and the regular anthelmintic treatments administered to sheep and goats.
Among the five isolated parasites species, N. helvetianus was the most prevalent in domestic sheep and goat and wild ruminants, present in all studied bioclimatic zones and not reported previously in Tunisia. This result is in agreement with the observations of Meradi et al. (Reference Meradi, Bentounsi, Zouyed and Cabaret2011) who reported a very high prevalence of N. helvetianus in sheep in Algeria. This nematode is a common parasite species of cattle (Anderson, Reference Anderson2000) or its large distribution in different bioclimatic areas and different host shows that the co-evolution of parasites with their hosts and host switching can have profound effects on host specificity.
Previous studies on Tunisian domestic ruminants reported the presence of only N. spathiger and N. filicollis (Hmidi, Reference Hmidi1982; Azizi, Reference Azizi1985). Thus, it can be concluded that there are at least 3 species of Nematodirus in Tunisian ruminants as in Algeria (Meradi et al. Reference Meradi, Bentounsi, Zouyed and Cabaret2011).
In this study, the occurrence of C. mentulatus is reported for the first time in the addax and in a Tunisian wild host. Currently, this pathogenic nematode is not considered as a common parasite but it was reported in various domestic and wild hosts in several regions of the world, such as sheep, goats, camels, Arabian oryx (Oryx leucoryx), Slender-horned gazelle and giraffes (Lichtenfels and Hoberg, Reference Lichtenfels and Hoberg1993; Fukumoto et al. Reference Fukumoto, Uchida, Ohbayashi, Ikebe and Sasano1996; Ruiz de Ybáñez et al. Reference Ruiz de Ybáñez, Garijo, Carpintero, Martínez-Carrasco and Ortiz2003; Goossens et al. Reference Goossens, Dorny, Boomker, Vercammen and Vercruysse2005). In Tunisia, C. mentulatus was reported only in dromedary by Mabrouk (Reference Mabrouk1988). The presence of C. mentulatus in addax living in Djbil National Park may be related to the presence of dromedary herds living near this park and to the fact that this protect area was previously a grazing area of this animal species before the reintroduction of addax. In fact, this explanation support that parasite transmission could have occurred from environment, water, or grazing areas even when there is no direct contact between hosts. The identification of this parasite species in a wild critically endangered species is very important and requires vigilance to ensure their survival. In the literature this nematode species seemed to cause lethal infection in wild hosts such as in Blackbuck, Dorcas gazelle and Thomson's Gazelle (Eudorcas thomsonii) (Flach and Sewell, Reference Flach and Sewell1987; Ortiz et al. Reference Ortiz, Ruiz de Ybáñez, Abaigar, Goyena, Garijo, Espeso and Cano2006) and in domestic hosts only cattle were apparently refractory to infection (Thornton et al. Reference Thornton, Galvin, Bell and Ramsey1973a,Reference Thornton, Galvin, Bell and Ramseyb).
All identified nematodes in this study, except C. mentulatus, are multi-host species and two of them (N. helvetianus and N. sphatiger) were confirmed to be concurrently found in domestic hosts living around the protected areas. The results of this study are in agreement with the results of Walker and Morgan (Reference Walker and Morgan2014) that reported that generalist parasites contribute more than specialists to the diversity of parasites in both wild and domestic species and that cross-transmissions between domestic and wild hosts are variable and depending on the regions.
Fifty positive samples by PCR were negative with the flotation technique. PCR method detected animals with very low infection levels because of the very high PCR sensitivity. PCR is not used as a routine screening laboratory tool but is suitable to detect asymptomatic infections or animals with low infection levels. This status is frequent in wild animals since they are more resistant and express low or no clinical signs. Due to the low parasite loads in the fecal samples only the association of three PCRs allowed to get a higher concentration of PCR products.
No intraspecific variation has been recorded in N. helvetianus isolated from various animal species and geographic localities as reported previously by Heise et al. (Reference Heise, Epe and Schnieder1999). Only 10% of isolated N. spathiger represented a low intraspecific variation by a single nucleotide difference (0·34%). The observed number of variation site is in agreement with the proportion of polymorphic ITS2 sites (0–0·9%) in Nematodirus (Audebert et al. Reference Audebert, Durette-Desset and Chilton2000) but less than that reported by Nadler et al. (Reference Nadler, Hoberg, Hudspeth and Rickard2000) within N. spathiger, N. filicollis and N. battus (12; 15 and 19 sites, respectively).
Even though only five nematode species were reported in this study, this parasite biodiversity have a great value and could serve as a baseline to understand the multi-host/multi-parasite systems and parasite distribution in wild hosts. To the best of our knowledge, this is the first molecular identification of five gastrointestinal nematode species (C. ovina, C. mentulatus, M. marshalli, N. helvetianus and N. spathiger) of North African wild ruminants and the first report of C. mentulatus and M. marshalli in addax and of M. marshalli in Dorcas gazelle. Also two new records of nematode species in Tunisia, N. helvetianus and M. marshalli were reported in this study, which extends the knowledge on the biodiversity of nematode parasites of wild ruminants in Tunisia.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182017001895.
Acknowledgements
We thank Mr Rjeibi M.R. postdoc in the Parasitology Laboratory of the National School of Veterinary Medicine Sidi Thabet for his help in molecular analysis and the General Direction of Forest of Tunisia (D.G.F) for providing us with unlimited access to the National Parks and Nature reserves.
Financial support
The study was supported financially by PARAVAC European project (ID: 265862FP7-KBBE) and Laboratoire d’épidémiologie des infections enzootiques des herbivores en Tunisie: application à la lutte (Ministère de l'enseignement supérieur et de la recherche scientifique et des technologies de l'information et de la communication, Tunisia).
Conflict of interest statement
None of the authors of this paper has a conflict of interest.











