Introduction
Diapause is a seasonal, programmed state of developmental and/or metabolic arrest, which can occur at various stages of an insect's life, including egg, larval, pupa, and adult (Tauber et al., Reference Tauber, Tauber and Masaki1986; Denlinger, Reference Denlinger, Lee and Denlinger1991, Reference Denlinger2002). Insect diapause consists of several phases: induction, preparation, initiation, maintenance, termination, and post-diapause quiescence (Koštál, Reference Koštál2006). Each diapause phase may be influenced by environmental factors as well as insect genetics (Han and Denlinger, Reference Han and Denlinger2009). Changes in the photoperiod provide reliable seasonal signals for insects and other organisms, thereby allowing them to ensure that their life history is synchronized with seasonal changes; thus, the photoperiod is a key factor related to the regulation of seasonal development and life history in temperate species (Tauber et al., Reference Tauber, Tauber and Masaki1986; Tanaka et al., Reference Tanaka, Arai and Tanaka1999). For example, Velarifictorus parvus Chopard (Orthoptera: Gryllidae) larvae develop faster under long photoperiod conditions, whereas larval development is inhibited under short photoperiod conditions, and the diapause status of larvae ends rapidly after moving from short photoperiod conditions to long-day sunshine conditions (Tanaka et al., Reference Tanaka, Arai and Tanaka1999).
In addition to controlling diapause induction, photoperiod also influences diapause incidence and diapause intensity in insects. For example, in Ostrinia furnacalis Guenée (Lepidoptera: Crambidae), the diapause incidence increases significantly as the photoperiod becomes shorter, and a short photoperiod below the critical day length can induce entry into deeper diapause than did the relatively short photoperiod below the critical day length (Yang et al., Reference Yang, Tu, Xia, He, Chen and Xue2014). In order to adapt to local seasonal changes, the diapause characteristics of widespread insects have also changed significantly during long-term evolution; thus, the critical photoperiod for inducing diapause will be shorter for low-latitude populations compared with high-latitude populations, and the diapause intensity is deeper (Danks, Reference Danks1987; Chen et al., Reference Chen, Chen, He, Xia and Xue2013). The deeper diapause intensity plays an important role in avoiding an untimely exit from diapause due to high temperatures before winter for low-latitude populations (Tanaka and Zhu, Reference Tanaka and Zhu2008).
In the diapause state, most insects are unable to move and feed, so the energy reserves stored during the diapause preparation phase must meet the needs for both basic metabolism during the diapause period and for their development after diapause (Hahn and Denlinger, Reference Hahn and Denlinger2011; Sinclair, Reference Sinclair2015). During diapause induction phase, token stimuli initiate physiological changes that lead to increased energy storage and preparation for diapause (Koštál, Reference Koštál2006). For example, in Colaphellus bowringi Baly (Coleoptera: Chrysomelidae), the diapause-destined adults will store more lipids in response to short photoperiod; by contrast, C. bowringi will store more carbohydrates and protein when the adult senses the signal of long photoperiod (Tan et al., Reference Tan, Feng, Liu, Zhu, Lei and Wang2016).
The fall webworm, Hyphantria cunea (Drury) (Lepidoptera: Arctiinae), is native to North America and was introduced into Japan around 1970 and was subsequently introduced into China (Ji et al., Reference Ji, Xi, Li, Gao and Li2003). Due to its wide range of food plants and high adaptability, fall webworm has caused severe forestry production losses in China. Fall webworm completes one generation each year in the North American region, but two generations each year in the Tokyo area of Japan, and three generations or more can occur each year toward the south of Japan (Gomi, Reference Gomi1997). Fall webworm was introduced into China in 1979 in Dandong city (about 40oN), Liaoning province and it spread to areas such as Nanjing city (about 32oN), Jiangsu Province (Liu et al., Reference Liu, Ye, Cheng, Xiong and Chen2016). When fall webworm was first introduced into China, it completed two generations each year, but three generations now occur each year in most parts of China (Ji et al., Reference Ji, Xi, Li, Gao and Li2003; Wei et al., Reference Wei, Xiao, Yang, Wu and Xue2006).
Fall webworm overwinters as diapausing pupae from October to May of next year in Shanghe County of Shandong Province. Photoperiodic cues during early larval stage determine whether diapause is induced (Masaki et al., Reference Masaki, Umeya, Sekiguchi and Kawasaki1968). The critical photoperiod for inducing diapause is 14 h 30 min to 14 h 45 min at 25°C (Gomi, Reference Gomi1997). The critical photoperiod is shorter as temperature increases during the induction period, and the diapause-inducing short day lengths of 8, 10, and 12 h evoke greater intensities of diapause than did 13 and 14 h (Chen et al., Reference Chen, Wei, Xiao, He, Xia and Xue2014). We know that energy reserve patterns differ in the diapause pupae and non-diapause pupae of the fall webworm and that diapause pupae reserve more lipid and carbohydrate content relative to the non-diapause pupae (Zhao et al., Reference Zhao, Wang, Qiu and Torsonunpublished). However, it is still unclear whether there is variation in energy accumulation in fall webworm when it experiences a shortened photoperiod during diapause induction phase.
The present study aimed to determine whether the energy reserve patterns change during the diapause preparation phase when fall webworm is exposed to different photoperiods below the critical photoperiod during the diapause induction phase. We tested the hypothesis that photoperiod influences energy reserve accumulation during diapause preparation in fall webworm. We predicted that fall webworm larvae that experience a short photoperiod will increase their feeding efficiency or food intake, or both, to increase their energy reserve during the diapause preparation period. We also predicted that body size, dry mass, and energy reserves would be significantly greater in diapausing pupae exposed to a shorter photoperiod during the diapause induction phase as larvae than relatively short photoperiod diapausing pupae. Furthermore, we expected that, if short-photoperiod diapausing pupae store more energy, the short photoperiod would promote increased energy storage in larvae during diapause preparation via increased feeding efficiency, food intake, or both. As a result, we expected that a short photoperiod would induce the higher lipase, amylase, and protease activities in the midgut of diapause-destined larvae in order to increase the storage of lipid, carbohydrates, and protein.
Materials and methods
Insect rearing
Fall webworm larvae were obtained from poplar trees (Populus deltoids) in Shanghe County (37.35oN, 117.34oE) of Shandong Province in May 2018 and reared in the laboratory for three generations under non-diapause-inducing conditions (25°C, L:D 16:8) by the methods described by Zhao et al. (Reference Zhao, Wang, Qiu and Torsonunpublished). The newly laid eggs were moved to a Petri dish (7 cm diameter) containing absorbent cotton and moist qualitative filter paper at the bottom to maintain humidity. We checked the eggs every day after the initiation of larvae hatching. The newly hatched larvae were divided into two groups after hatching. The first group was transferred into a chamber at 25°C with a photoperiod of L:D 13:11, and the second group was kept at 25°C with a photoperiod of L:D 10:14. All the larvae were raised in aggregate during the 1st to 4th instars, subsequently they were raised separately after entering the 5th instar with artificial diet consisting of wheat germ (12%), casein (4.5%), sugar (2%), agar (1.5%), and other dietary ingredients (Cao et al., Reference Cao, Yang, Tang and Chen2014). Given that fall webworms were exposed to two groups of photoperiods below the critical photoperiod during the diapause induction phase, these larvae were destined to enter the diapause state after pupation.
Body size and dry mass
On the day of pupation, we transferred pupae to Petri dishes containing absorbent cotton and moist filter paper at the bottom. The Petri dishes with pupae were transferred to chambers at a constant temperature of 25 ± 1°C with a photoperiod of L:D 13:11 and L:D 10:14. The sex of each pupa was determined by the presence (female) or absence (male) of a line intersecting the first abdominal sternite (Loewy et al., Reference Loewy, Flansburg, Grenis and Murphy2013; Williams et al., Reference Williams, Chick and Sinclair2015). To investigate the effect of photoperiod variation on body size and dry mass of diapausing pupae, on the 2nd day after pupation, we measured the lengths and widths of the pupae with a micrometer (0.1 cm) and dry mass with a balance (AL104 Mettler-Toledo; Mettler-Toledo, d = 0.0001 g) after drying on a slide glass at 110°C for 24 h according to the method of Tanaka (Reference Tanaka1991).
Body composition
To examine the effect of photoperiod variation below the critical photoperiod on the energy reserve during the diapause preparation phase, we extracted the lipid, protein, and glycogen contents from pupae on the 1st, 3rd, and 5th days after pupation according to the method described by Lorenz (Reference Lorenz2003). The lipid contents were determined using the sulfophosphovanillin method (adapted from Lorenz, Reference Lorenz2003). Samples (20 μl) were added to 980 μl sulfuric acid, and then the mixtures were incubated at 100°C for 10 min. After cooling to room temperature, 5 ml of 0.2% phosphovanillin in 57% ortho-phosphoric acid was added to the mixtures. Samples were measured at 530 nm and concentrations were determined against cholesterol standards. The protein was quantified by Coomassie blue staining. Samples (25 μl) were added to 125 μl phosphate-buffered saline, then mixed with 2.85 μl Coomassie blue fast staining solution. Absorbance was measured against bovine serum albumin standards at 595 nm. Carbohydrate contents were quantified using the anthrone method (Carroll et al., Reference Carroll, Longley and Roe1956). We mixed the sample (100 μl) with 900 μl distilled water, then added 4 ml of the 0.1% (1 g anthrone in 1000 ml 80% sulfuric acid) anthrone to the mixture, and heated the mixture to 100°C for 10 min. Absorbance was measured at 620 nm using the known glucose concentration as standards.
Feeding experiment
Since early-instar larvae were aggregated (Rehnberg, Reference Rehnberg2006), we only measured the food utilization in the 5th to 6th instar in the short-photoperiod diapause-destined and relatively short-photoperiod diapause-destined larvae. After molting into the 5th instar, we transferred larvae to a new container to rear independently. On day 2 after entering the 5th instar, we fed larvae ad libitum with a pre-weighed diet that was replaced every 2 days. The remaining diet and frass were weighed after each diet change. The dry mass of the food and larvae was regressed against the wet mass of food and larvae to calculate the wet-to-dry conversion equation of linear regression according to the method described by Zhao et al. (Reference Zhao, Liao, Zeng, Wu and Zhu2017) (the diet: dry mass = 0.313 × wet mass−0.003, r 2 = 0.99; short-photoperiod diapause-destined larvae: dry mass = 0.148 × wet mass + 0.00004, r 2 = 0.99; relatively short-photoperiod diapause-destined larvae: dry mass = 0.157 × wet mass + 0.00003, r 2 = 0.99). We used growth, relative growth, consumption, approximate digestibility (AD), the efficiency of converting ingested food into body matter (ECI), and the efficiency of conversion of digested food into body matter (ECD) to analyze the consumption and feeding efficiency. The formulae used to calculate these nutritional indices are as follows (adapted from Mole and Zera, Reference Mole and Zera1993): growth = [dry body weight at the end day−initial dry body weight]; relative growth = [growth/initial dry body weight]; consumption = dry weight of food ingested during the feeding trial; AD = [(dry weight of food ingested−dry weight of feces)/dry weight of food ingested] × 100%; ECI = (dry weight gain/dry feed ingested); and ECD = growth/(dry weight of food ingested−dry weight of feces).
Enzyme activities
Two hours after the beginning of the photophase on the 3rd day after molting, the 5th and 6th instar larvae were transferred to an empty plastic container without food. At the beginning of the photophase on the 4th day after molting, the larvae, which had been starved for 22 h, were fed ad libitum for 6 h. Groups of larvae from both instars were then transferred to −40°C. To dissect the midgut, frozen larvae were removed from the freezer, thawed on ice, and pinned ventral slide up on a silicon surface and cut longitudinally. The digestive tract was then transferred to a glass slide. The midgut was isolated and placed in a 1.5 ml microcentrifuge tube containing 200 μl of 0.15 mol l−1 NaCl. The midgut tissue was disrupted by sonification and centrifuged at 10,500 g for 15 min at 4°C. The supernatant was used in the enzyme assays. Amylase activity was measured by the dinitrosalicylic acid (DNS) method of Bernfeld (Reference Bernfeld, Colowick and Kaplan1955). Enzyme stock solution (20 μl) was added to 2 ml of phosphate buffer (pH 7.2) containing 250 μg of soluble starch (Sigma-Aldrich, Louis), and 2 ml of the DNS reagent was then added to the solution. The solution was heated to 100°C for 10 min. After cooling in water, absorbance was measured at 530 nm. To assay the lipase activity, the enzyme stock solution (20 μl) was mixed with 1.5 ml of phosphate buffer (pH 7.2) containing 80 nmol triglyceride. The concentration of free fatty acids released was measured using a test kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) and measuring the absorbance at 550 nm. The protease activities were assayed using a test kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions and measuring the absorbance at 660 nm.
Statistical analysis
The experimental data were analyzed in SPSS 22.0 (IBM Inc., New York, USA). General linear model (GLM) was used to analyze the body size and dry mass between short-photoperiod diapause and relatively short-photoperiod diapause pupae. Student's t-test was used to compare lipase, amylase, and protease activity of short-photoperiod diapause-destined larvae and relatively short-photoperiod diapause-destined larvae within the same instars. The lipid and glycogen between short-photoperiod diapause and relatively short-photoperiod diapause pupae were analyzed using an analysis of covariance (ANCOVA) with total protein (mg protein per individual) as a covariate (Williams et al., Reference Williams, Marshall, MacMillan, Dzurisin and Hellmann2012) and followed by Tukey's multiple range test. The content of protein between short-photoperiod diapause and relatively short-photoperiod diapause pupae was analyzed using analysis of variance with post-hoc Tukey's multiple range test. Growth, relative growth, consumption, ECI, ECD, and AD were analyzed with ANCOVA with the numerator of the ratio as the independent variable and the denominator as the covariate (Raubenheimer and Simpson, Reference Raubenheimer and Simpson1992; Mole and Zera, Reference Mole and Zera1993).
Results
Body size and dry mass
The result of GLM analysis revealed that photoperiod and sex had a significant effect on body size and dry mass (width: F 1,171 = 47.2 and 124.5 for photoperiod and sex; length: F 1,171 = 25.2 and 90.34 for photoperiod and sex; body mass: F 1,171 = 53.7 and 180.3 for photoperiod and sex; P < 0.001 for all treatments, fig. 1), and body size and dry mass of short-photoperiod diapause pupae were significantly greater than those of the relatively short-photoperiod diapause pupae (P < 0.001 for all treatments, fig. 1).
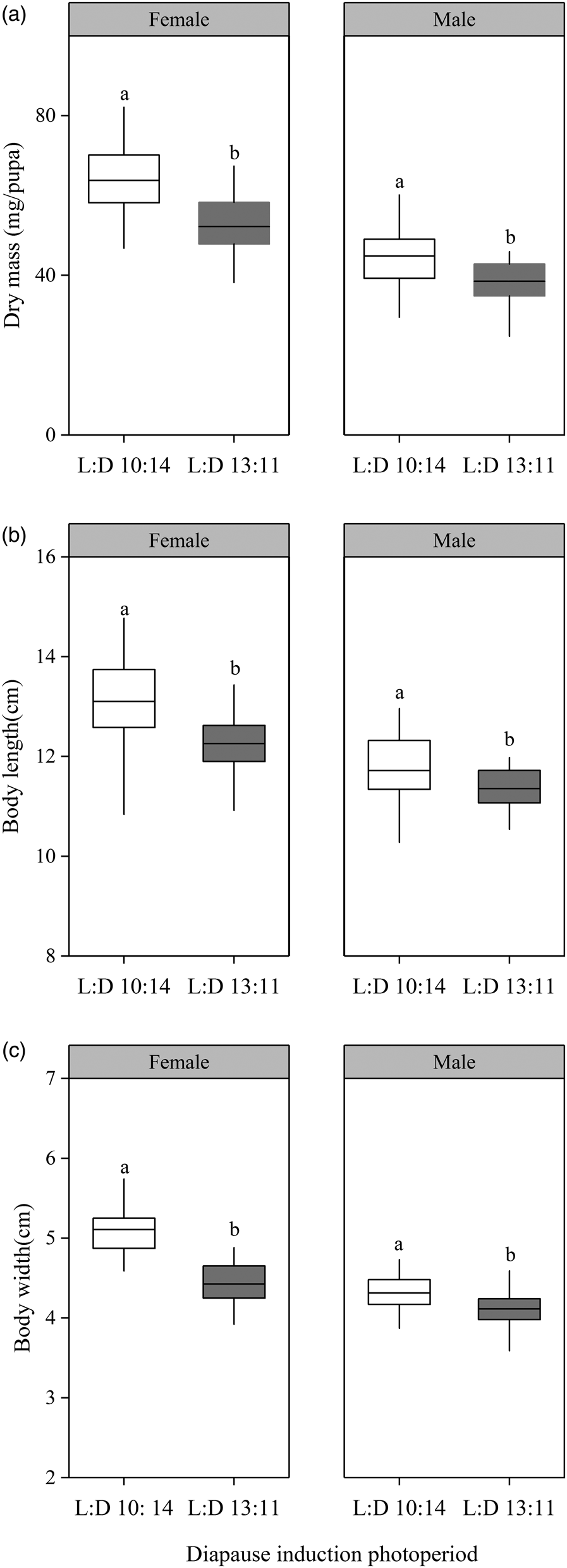
Figure 1. Comparison of the body size and dry mass of relatively short photoperiod and short photoperiod diapause pupae. (a) Dry mass (in mg). (b) Body length (cm). (c) Body width (cm). We measured the width of the pupae at the basal margin of the abdomen. White: short photoperiod diapause pupae; gray: relatively short photoperiod diapause pupae. Different letters indicate significant differences between relatively short photoperiod diapause and short photoperiod diapause pupae at P < 0.05 according to t-tests (n = 40 for short photoperiod female diapause pupae; n = 45 for relatively short photoperiod female diapause pupae, relatively short and short photoperiod male diapause, respectively).
Body composition
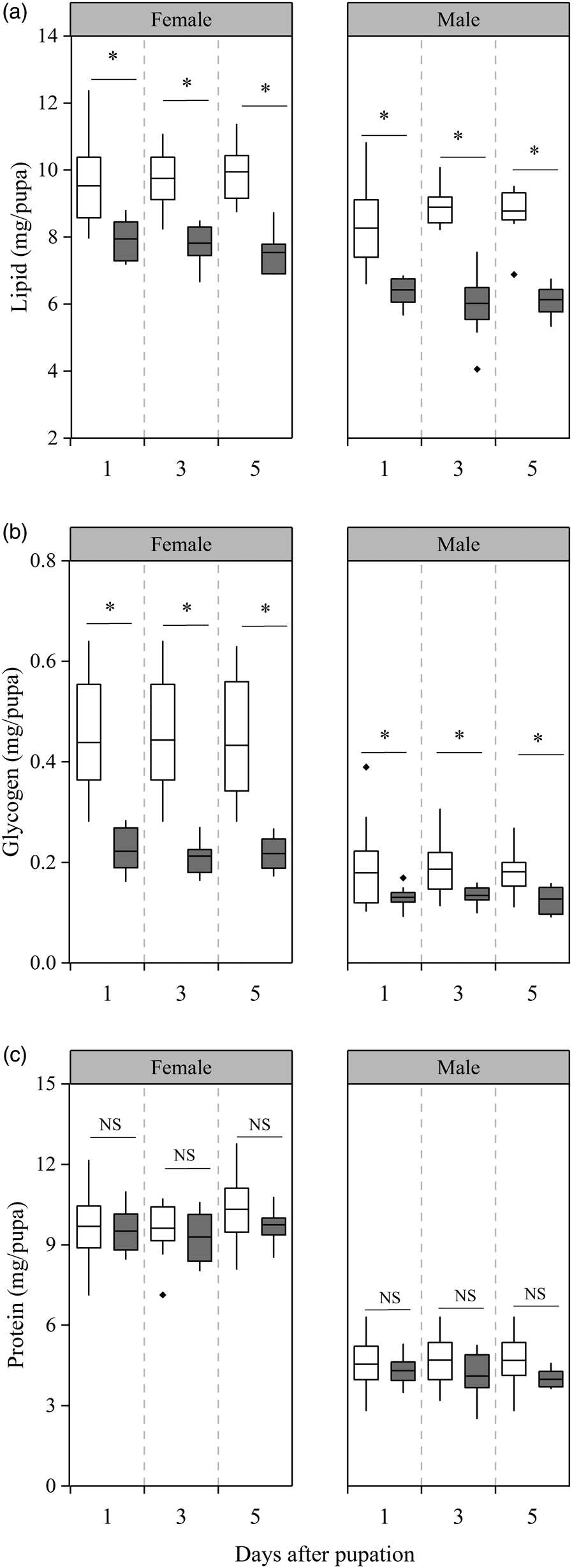
We observed significant differences in lipid content between short-photoperiod diapause pupae and relatively short-photoperiod diapause pupae (female: F 1,64 = 74.12, P < 0.001; male: F 1,74 = 92.34, P < 0.001, fig. 2a), time (days after pupation) had no effect on the content of lipid (female: F 2,70 = 0.016, P = 0.985; male: F 2,80 = 0.11, P = 0.89, fig. 2a), and there was no interaction between pupae development status and time (female: F 2,69 = 1.11, P = 0.34; male: F 2,79 = 2.71, P = 0.073, fig. 2a). The lipid content within treatments for female diapause pupae did not change significantly within 5 days after pupation, but lipid content of short-photoperiod diapause pupae was, on average, 26% higher than the relatively short-photoperiod group (P < 0.001 for all days, fig. 2a). Similar to the female diapause pupae, the lipid contents of males in the two groups of diapause pupae remained stable within 5 days after pupation, but the lipid content of short-photoperiod diapause pupae was, on average, 39% higher than the relatively short-photoperiod group (P < 0.001 in all days, fig. 2a).
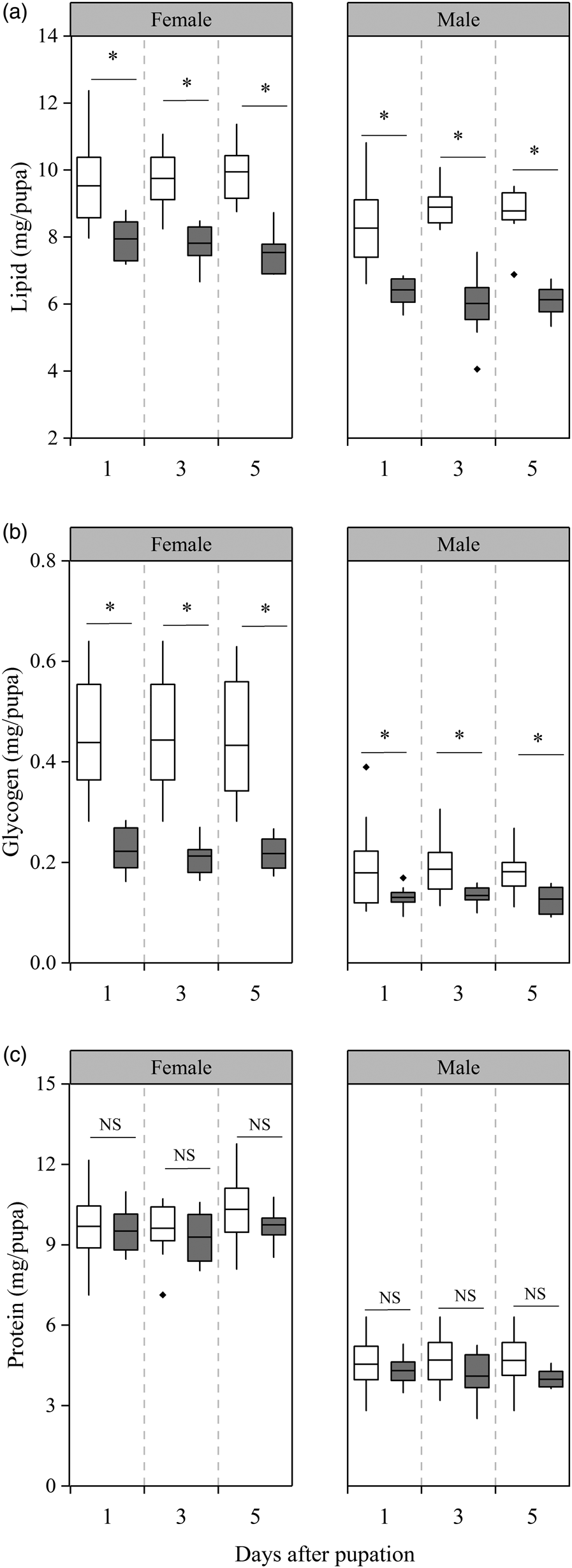
Figure 2. Lipid (a), carbohydrate (b), and protein (c) contents on the 1st, 3rd, and 5th days after pupation in relatively short photoperiod and short photoperiod diapause pupae. White: short photoperiod diapause pupae; gray: relatively short photoperiod diapause pupae. Asterisks indicate significant differences between relatively short photoperiod diapause and short photoperiod diapause pupae of the same age (P < 0.05), with NS indicating no significant difference (P > 0.05) (n = 10–30).
We observed significantly higher glycogen content in short-photoperiod diapause pupae relative to relatively short-photoperiod diapause pupae (female: F 1,69 = 100.87, P < 0.001; male: F 1,94 = 22.86, P < 0.001, fig. 2b), time (days after pupation) had no effect on the content of glycogen (female: F 2,75 = 0.075, P = 0.93; male: F 2,100 = 0.084, P = 0.92, fig. 2b), and there was no interaction between pupae development status and time (female: F 2,74 = 0.04, P = 0.96; male: F 2,99 = 0.049, P = 0.95, fig. 2b). The glycogen content of two groups of female diapause pupae did not change significantly and was maintained at a relatively high and low level, respectively, but a significant difference was detected between them at 1st, 3rd, and 5th days after pupation (P < 0.001 in all days, fig. 2b). Similar to the female diapause pupae, the glycogen content of two groups of male diapause pupae did not change significantly and was maintained at relatively high and low levels, respectively, with significantly higher glycogen levels observed in the short-photoperiod male diapause pupae compared to the relatively short-photoperiod male diapause pupae on the 1st, 3rd, and 5th days after pupation (day 1: P = 0.010; day 3: P = 0.015; day 5: P = 0.027, fig. 2b).
The protein contents of two groups of female diapause pupae, as well as the two groups of male diapause pupae, remained stable within 5 days after pupation. There is no significant difference between short-photoperiod diapause pupae and relatively short-photoperiod diapause pupae (female: F 1,73 = 2.19, P = 0.143; male: F 1,93 = 1.29, P = 0.28, fig. 3c), time (days after pupation) had no effect on the content of protein (female: F 2,79 = 1.98, P = 0.144; male: F 2,99 = 0.089, P = 0.92, fig. 3c), and there was no interaction between pupae development status and time (female: F 2,78 = 0.23, P = 0.79; male: F 2,98 = 0.56, P = 0.57, fig. 2c).

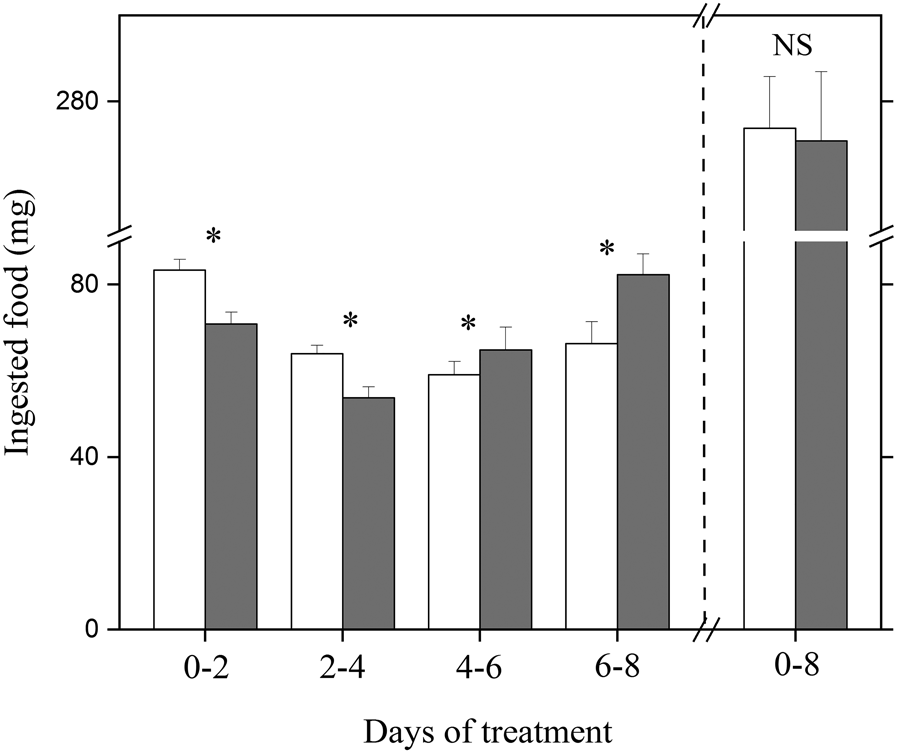
Figure 3. Ingested food during 8th day treatment in relatively short-photoperiod diapause-destined and short-photoperiod diapause-destined larvae. White: short-photoperiod diapause-destined larvae; gray: relatively short-photoperiod diapause-destined larvae. Asterisks indicate significant differences between relatively short-photoperiod diapause-destined and short-photoperiod diapause-destined larvae (P < 0.05), with NS indicating no significant difference (P > 0.05) (relatively short-photoperiod diapause-destined larval: n = 26; short-photoperiod diapause-destined larval: n = 25).
Food utilization
During the 8th day experimental treatment, the growth of relatively short-photoperiod diapause-destined larvae was only 81.7% of that of short-photoperiod diapause-destined larvae (F 1,48 = 128.24, P < 0.001, table 1). Relative growth of short-photoperiod diapause-destined larvae was also higher than that of the relatively short-photoperiod diapause-destined larvae (F 1,48 = 246.52, P < 0.001, table 1). Similar to the growth and relative growth, the ECI and ECD were significantly higher in the short-photoperiod diapause-destined larvae than the relatively short-photoperiod diapause-destined larvae (ECI: F 1,48 = 6.46, P = 0.0087; ECD: F 1,48 = 9.82, P = 0.0054, table 1). However, AD did not differ between relatively short-photoperiod diapause-destined larvae and short-photoperiod diapause-destined larvae (F 1,48 = 1.76, P = 0.55, table 1).
Table 1. Mean nutritional indices in relatively short-photoperiod diapause-destined larvae and short-photoperiod diapause-destined larvae of fall webworm

SPDD, short-photoperiod diapause-destined; RPDD, relatively short-photoperiod diapause-destined. Different letters after means within a column indicate significant differences between the two treatments (relatively short-photoperiod diapause-destined larval: n = 26; short-photoperiod diapause-destined larval: n = 25).
The total food consumption of short-photoperiod diapause-destined larvae was similar to that of the relatively short-photoperiod diapause-destined larvae (F 1,48 = 0.27, P = 0.31, fig. 3), but there was an obvious difference in the pattern of food intake between the relatively short-photoperiod diapause-destined larvae and short-photoperiod diapause-destined larvae. The consumption by relatively short-photoperiod diapause-destined larvae and short-photoperiod diapause-destined larvae declined from 0 to 4 days, and then increased during days 4–8. During 0–2 and 2–4 days, the consumption by short-photoperiod diapause-destined larvae was significantly higher than that of relatively short-photoperiod diapause-destined larvae (0–2 days: F 1,48 = 12.46, P = 0.0041; 2–4 days: F 1,48 = 6.52, P = 0.022, fig. 3); by contrast, consumption was significantly greater in relatively short-photoperiod diapause-destined larvae than the short-photoperiod diapause-destined larvae during 4–6 and 6–8 days, respectively (4–6 days: F 1,48 = 5.54, P = 0.036; 6–8 days: F 1,48 = 11.44, P = 0.0052, fig. 3).
Digestive enzyme activities
Both the lipase and amylase activities in the midguts of the short-photoperiod diapause-destined larvae and the relatively short-photoperiod diapause-destined larvae increased after the 5th instar larvae molted to the 6th instar larvae. However, the lipase and amylase activities in the midguts of the 5th and 6th instar of short-photoperiod diapause-destined larvae were significantly higher than those of the relatively short-photoperiod diapause-destined larvae (5th instar: t 45 = 2.43, P = 0.019; 6th instar: t 44 = 3.46, P = 0.0017 for lipase activity; 5th instar: t 45 = 2.16, P = 0.036; 6th instar: t 44 = 2.11, P = 0.023 for amylase activity, figs 4a, b). The protease activities in the midguts of the short-photoperiod diapause-destined and relatively short-photoperiod diapause-destined 5th instar larvae underwent a slight decline after the 5th instar larvae molted to the 6th instar larvae but the protease activities did not differ significantly between the two groups of larvae at either instar (5th instar: t 45 = 0.77, P = 0.44; 6th instar: t 44 = 1.24, P = 0.22, fig. 4c).
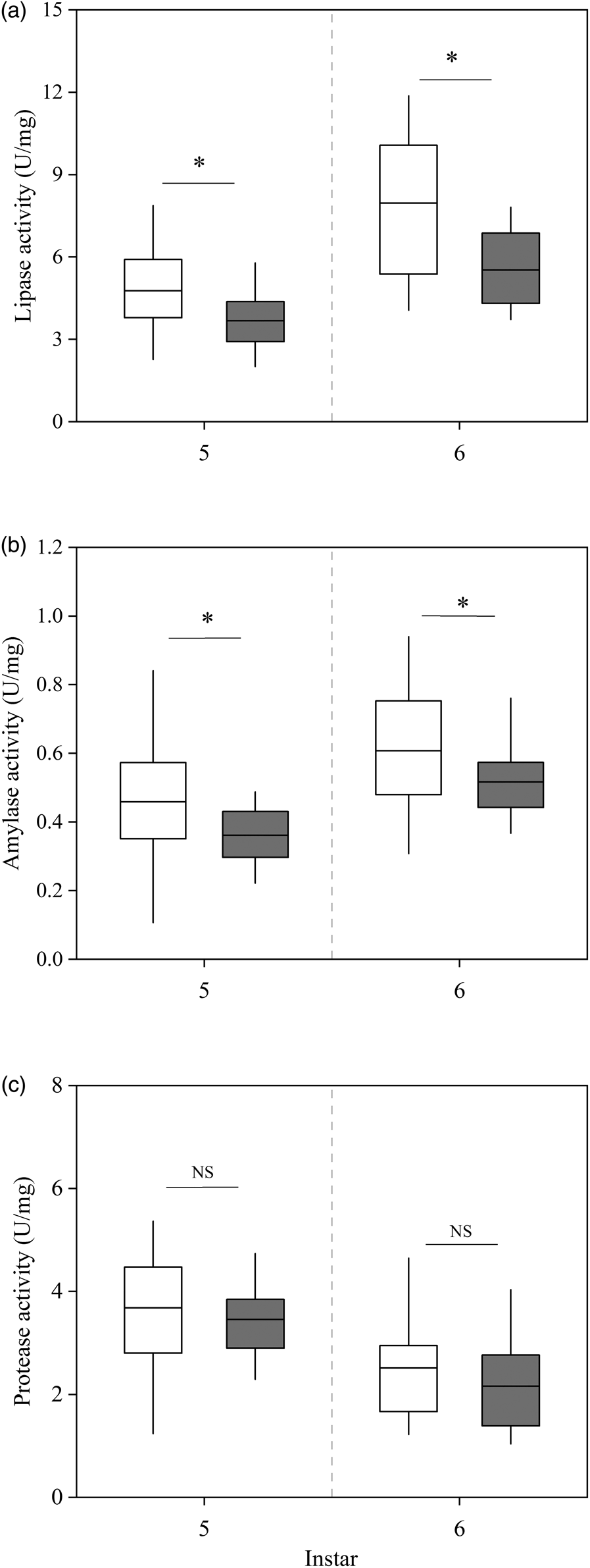
Figure 4. Comparison of digestive enzyme activities in the midguts of relatively short-photoperiod diapause-destined and short-photoperiod diapause-destined 5th and 6th instar larvae. (a) Amylase activity (in U mg−1). (b) Lipase activity (in U mg−1). (c) Protease activity (in U mg−1). White: short-photoperiod diapause-destined larvae; gray: relatively short-photoperiod diapause-destined larvae. Asterisks indicate significant differences between relatively short-photoperiod diapause-destined and short-photoperiod diapause-destined larvae of the same age (P < 0.05), with NS indicating no significant difference (P > 0.05) (n = 24 for relatively short-photoperiod diapause-destined 5th instar larvae; n = 23 for short-photoperiod diapause-destined 5th instar larvae; n = 23 for relatively short-photoperiod and short-photoperiod diapause-destined 6th instar larvae, respectively).
Discussion
Consistent with our predictions, the diapausing pupae exposed to a short photoperiod during larval development had significantly higher body size and dry mass, as well as higher lipid and carbohydrate content, relative to those exposed to a longer photoperiod, suggesting that the short-photoperiod diapausing pupae reserved more energy. These results indicate that the energy reserve pattern of diapausing fall webworm pupae is plastic in response to photoperiod during diapause induction phase.
Diapause is promoted by environmental factors during the diapause induction phase. Insects that are destined for diapause undergo a suite of physiological changes, such as changes in body size and energy reserves, during the diapause preparation phase (Tauber et al., Reference Tauber, Tauber and Masaki1986; Danks, Reference Danks1987; Denlinger et al., Reference Denlinger, Yocum, Rinehart, Gilbert, Iatrou and Gill2005). Our results showed that body size and dry mass were significantly higher for both female and male fall webworm diapause pupae subjected to a short photoperiod compared to those that had a relatively short photoperiod during the diapause induction phase. The larger size of the diapause individuals may provide more energy stores to meet the requirements for surviving under harsh environmental conditions and completing metamorphosis in the spring (Hahn and Denlinger, Reference Hahn and Denlinger2007; Shin et al., Reference Shin, Akram and Lee2012). We found that the lipid and carbohydrate contents were significantly greater in female diapause pupae exposed to a short photoperiod during the diapause induction phase than female diapause pupae exposed to a relatively short photoperiod. We observed a similar trend in male diapause pupae. These results indicate that the energy storage mode in fall webworm diapause pupae is influenced by photoperiod during the diapause induction phase, where a short photoperiod below the critical day length can promote increased storage of energy for diapause.
Insects use two strategies in order to meet energy consumption requirements during diapause: (1) storing energy reserves during the diapause preparation phase, and (2) inhibiting metabolism during diapause (Hahn and Denlinger, Reference Hahn and Denlinger2011). Diapause can reduce the energy consumed by insects, but an increase in the temperature during diapause accelerates the metabolic rate and increases the energy consumed while wintering, thereby decreasing the survival rate of insects during wintering or lowering the fitness of the population post-diapause (Irwin and Lee, Reference Irwin and Lee2003; Schulte et al., Reference Schulte, Healy and Fangue2011; Williams et al., Reference Williams, Marshall, MacMillan, Dzurisin and Hellmann2012). Therefore, the accumulation of nutrients in the diapause preparation phase is crucial because any lack of nutrition will reduce the likelihood of insects completing diapause, thereby decreasing their chances of survival and reproduction (Denlinger, Reference Denlinger2002). The energy reserves stored before diapause mainly comprise lipid and carbohydrates (Hahn and Denlinger, Reference Hahn and Denlinger2007; Sinclair, Reference Sinclair2015; Sinclair and Marshall, Reference Sinclair and Marshall2018).
Fall webworm was introduced into Dandong city (about 40oN), China during 1979 and it spread to Nanjing city (about 32oN) in Jiangsu Province. The winter temperature exhibits a clear geographical latitudinal gradient from Dandong to Nanjing (Liu et al., Reference Liu, Ye, Cheng, Xiong and Chen2016). Therefore, after fall webworm spread from the north to south following its introduction into China, the southern population likely experienced a strong selective pressure due to an increased metabolic rate and higher energy consumption caused by warmer winters. We consider that the short photoperiod induces fall webworm pupae to store more lipid and carbohydrates in order to increase their energy stores before overwintering and this change will increase the fitness of the population after overwintering following the spread from north to south.
In order to store more energy, diapause individuals must ingest more food, increase their food utilization efficiency, divert nutrients from supporting somatic cell growth to storage, or a combination of these processes (Hahn and Denlinger, Reference Hahn and Denlinger2007). Our results showed that the short-photoperiod diapause-destined larvae stored more lipid and carbohydrates compared with the relatively short-photoperiod diapause-destined larvae. The growth rate, relative growth rate, ECI, and ECD were significantly higher for the short-photoperiod diapause-destined larvae, but there was no significant difference in the food intake between the two larval treatments. These results indicate that the short photoperiod under the critical photoperiod stimulated the diapause-destined larvae to enhance their energy reserves by increasing ECI and ECD rather than increasing their food intake during diapause preparation. This is possibly an adaptive strategy in response to changes in the quantity and quality of food that is likely to occur in late autumn as plants begin to senesce. Thus, if the final generation of fall webworm (i.e. third generation of trivoltine populations) could not enhance its energy reserves by increasing the intake of food, then improving the efficiency of food utilization could also provide sufficient resources to successfully overwinter. In addition, variation in the pattern of food intake between the relatively short-photoperiod diapause-destined larvae and short-photoperiod diapause-destined larvae reflected the different feeding strategies employed by the two groups of larvae. The use of artificial diet in our experiment did not reflect the natural changes in the food quality over time or the gradual fading of autumn leaves under natural environmental conditions, but the increased early feed intake by the diapause-destined larvae with a short photoperiod was a better strategy that allowed them to cope with the declining leaf quality in the autumn.
The short-photoperiod diapause pupae stored more lipid and carbohydrates, and the ECI and ECD values were significantly higher for the short-photoperiod diapause-destined larvae than the relatively short-photoperiod larvae. Therefore, we suggest that the different energy reserve patterns of the short-photoperiod and relatively short-photoperiod diapause pupae may have been related to differences in the activities of digestive enzyme in the midgut. In insects, digestive enzyme activities are affected by the type of food consumed, larval density, larval stage, and photoperiod (Woodring et al., Reference Woodring, Hoffmann and Lorenz2007; Kong et al., Reference Kong, Luo, Jiang, Zhang, Yang and Hu2012; Weidlich et al., Reference Weidlich, Müller, Hoffmann and Woodring2012, Reference Kong, Luo, Jiang, Zhang, Yang and Hu2013; Zhao et al., Reference Zhao, Wang, Qiu and Torsonunpublished). The elevated midgut lipase and amylase activities that we observed in short-photoperiod diapause-destined larvae are consistent with the higher lipid and carbohydrate contents in diapausing pupae. Thus, diapause-destined larvae exposed to a short photoperiod increased the lipase and amylase activities in their midgut in order to store more lipid and carbohydrates.
Our results support the hypothesis that photoperiod influences energy reserve accumulation during diapause preparation in fall webworm. Consistent with our expectations, the body size and dry mass were significantly larger in the short photoperiod diapause pupae than the relatively short photoperiod pupae. Moreover, the lipid and carbohydrate contents of the short photoperiod diapause pupae were significantly higher than those of the relatively short photoperiod diapause pupae. The increased energy reserves were due to changes in the lipase and protease activities in the midgut of short photoperiod diapause-destined larval. The results obtained in this study indicate that the photoperiod can regulate energy accumulation in fall webworm. A shorter photoperiod, below the critical photoperiod, promoted larvae to store more lipid and carbohydrates during diapause preparation. These increased energy reserves will likely help this species to overcome increased energetic demands in locations with warmer winters.
Acknowledgements
We thank Jia-heYan for his assistance on the collection of the fall webworm. This study was supported by the Natural Science Foundation of Jiangsu Province (BK20181399) and the Highly Educated Talents Foundation in Nanjing Forestry University (GXL2017001).
Author contributions
L.Z. designed the experimental protocol. W.W. and Y.Q. carried out the experimental work. L.Z. analyzed the data. L.Z. and A.S.T. wrote the manuscript.