INTRODUCTION
Synthesis and study of new salts of dicarboxylic acids with metals can lead to new types of structures, which are of interest for the crystal engineering and practical applications. Compounds of this type can form layered materials, three-dimensional micro- and macroporous systems. Properly selected dicarboxylic acids may be substrates used in the synthesis of complex three-dimensional hierarchical materials. The poor solubilities of such salts of certain metals, e.g., calcium, may lead to practical applications in chemical analysis, ecology, and industry. In crystallographic databases CSD (Version 5.31, Nov. 2009), there are 28 entries describing uranium compounds of dicarboxylic or polycarboxylic acids (Borkowski and Cahill, Reference Borkowski and Cahill2006); similar salts of other heavy metal elements (e.g., Np) are also quite numerous.
The compounds obtained by us are similar to metal organic framework (MOF) materials, created by dicarboxylic acids and transition metals. Increasing interest in MOF materials is connected with their possible use similar to zeolites, including sorption and separation of gases, ion exchange, and catalytic properties. This paper presents synthesis and diffraction data for six new salts of barium and selected dicarboxylic acids with the number of carbon atoms from 5 to 12. The obtained salts were examined at room and elevated temperatures by powder diffraction methods.
EXPERIMENTAL
Synthesis
To synthesize six new compounds of barium—glutaric, adipic, pimelic, suberic, sebacic, and dodecanedioic acids—0.005 mol of barium carbonate and 0.005 mol of appropriate dicarboxylic acid were dissolved in 50 ml of water. In each synthesis, the solution was first boiled for 1 h. The hot solution was then filtered and left for crystallization. After about 24 h, white precipitates crystallized from each solution. The precipitates were filtered off, washed with warm mixture of water and propanol (1:1), and finally dried in air.
Diffraction measurements
The powder diffraction data were collected at room temperature using a Panalytical X’pert PRO MPD diffractometer working in the Bragg-Brentano geometry in the 2θ range from 4.0 (or 5.0) to 60°2θ with a step size of 0.02°2θ and 2.15 to 80 s/step. Radiation types Cu K α (1.541 87 Å) for compounds {1,3,4} and Cu K a 1 (1.540 56 Å) for compounds {2,5,6} were used. Other experimental details are generator conditions of 40 kV, 30 mA, fixed divergence slit of 1/4°, secondary monochromator, and a PIXCEL detector. A rotated back-loaded sample was used during an XRPD measurement. Peak positions were determined with the use of the second derivative method using the program written by Sonneveld and Visser (Reference Sonneveld and Visser1975). The unit-cell parameters were determined using indexing programs of the PROSZKI package (Łasocha and Lewiński, Reference Łasocha and Lewiński1994).
In the case of barium glutarate {1}, pimelate {3}, suberate {4}, and sebacate {5}, additional in situ X-ray diffraction investigations of thermal decomposition were performed. The in situ measurements were carried out in air at a temperature range from 27 to 600 °C in the 2θ range from 4.0 to 60°2θ, with a step size of 0.025°2θ and count time of 1.35 s/step. The X-ray radiation source was a Cu tube. The XRPD vs temperature investigations were carried out using an X’pert PRO diffractometer equipped with an XRK 900 (Anton Paar) reaction chamber.
RESULTS AND DISCUSSION
Room-temperature XRPD analysis
Figure 1 shows X-ray diffraction patterns for all six investigated compounds and XRPD data for the compounds
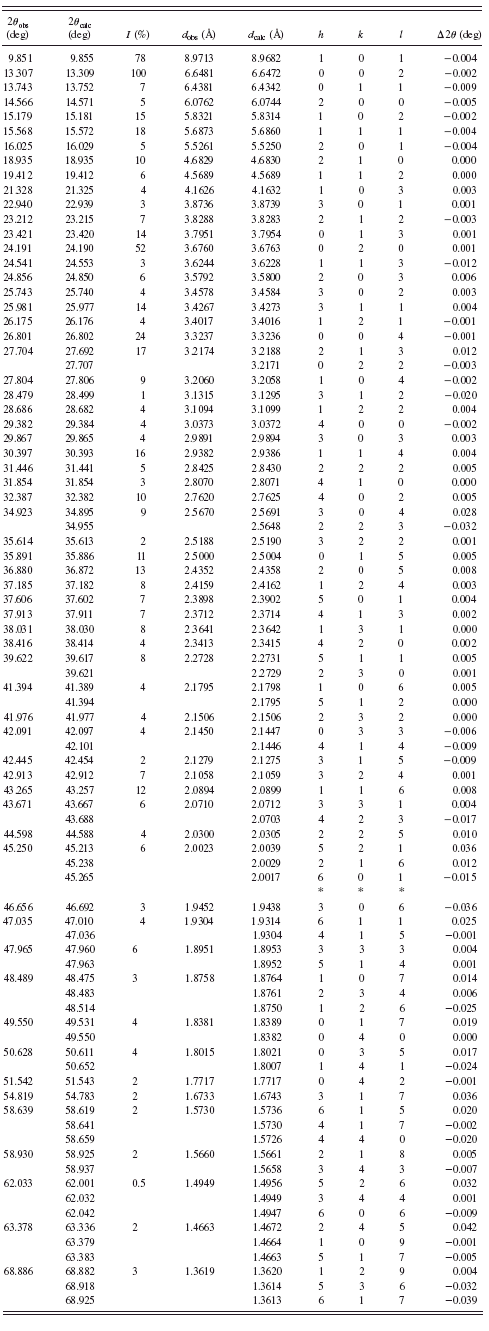
TABLE I. Powder diffraction data for BaC5H6O4·6H2O {1}.
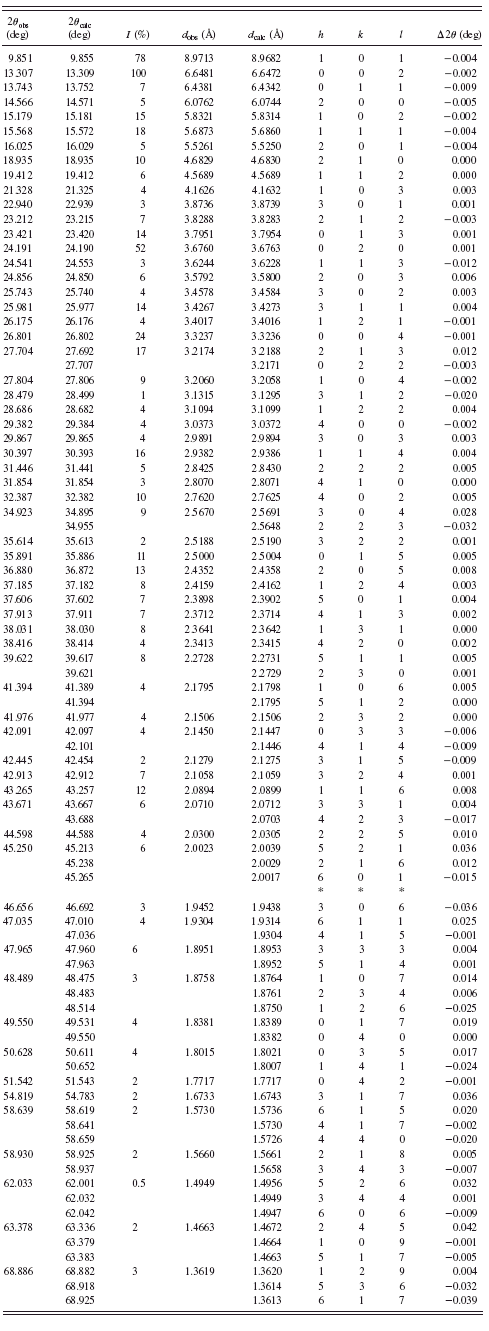
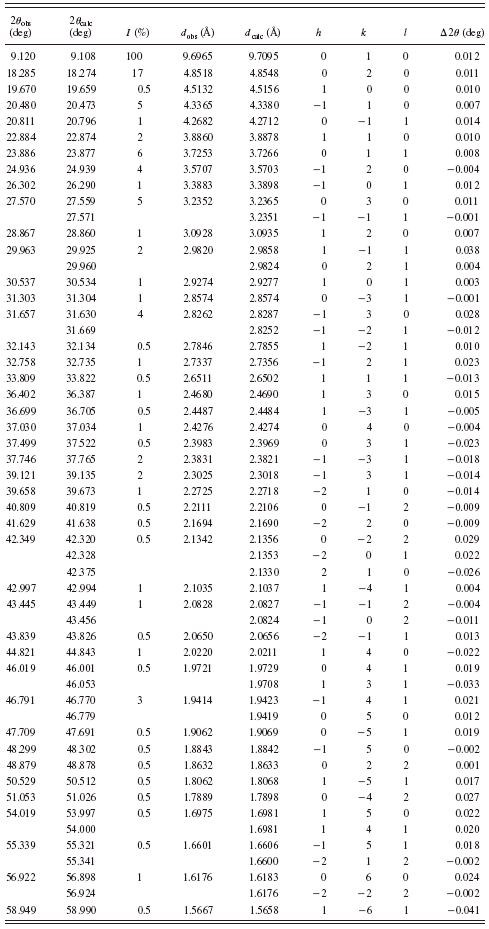
TABLE II. Powder diffraction data for BaC6H8O4 {2}.
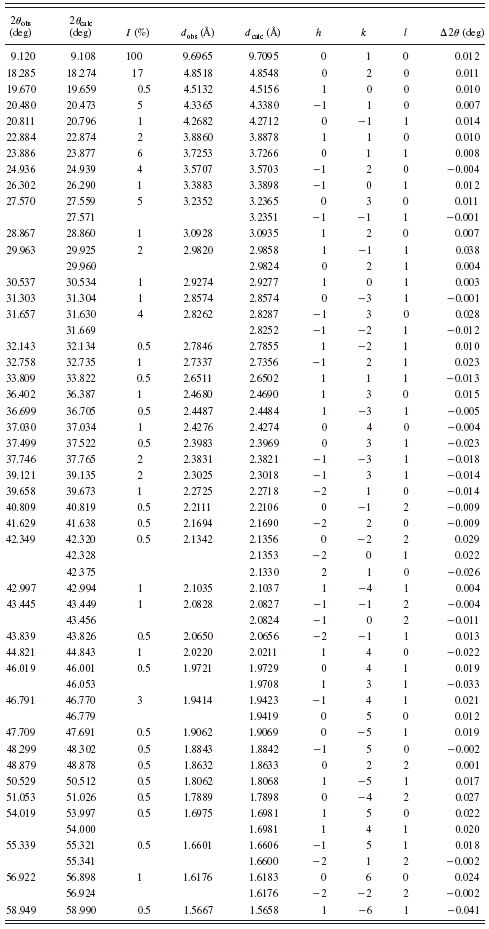
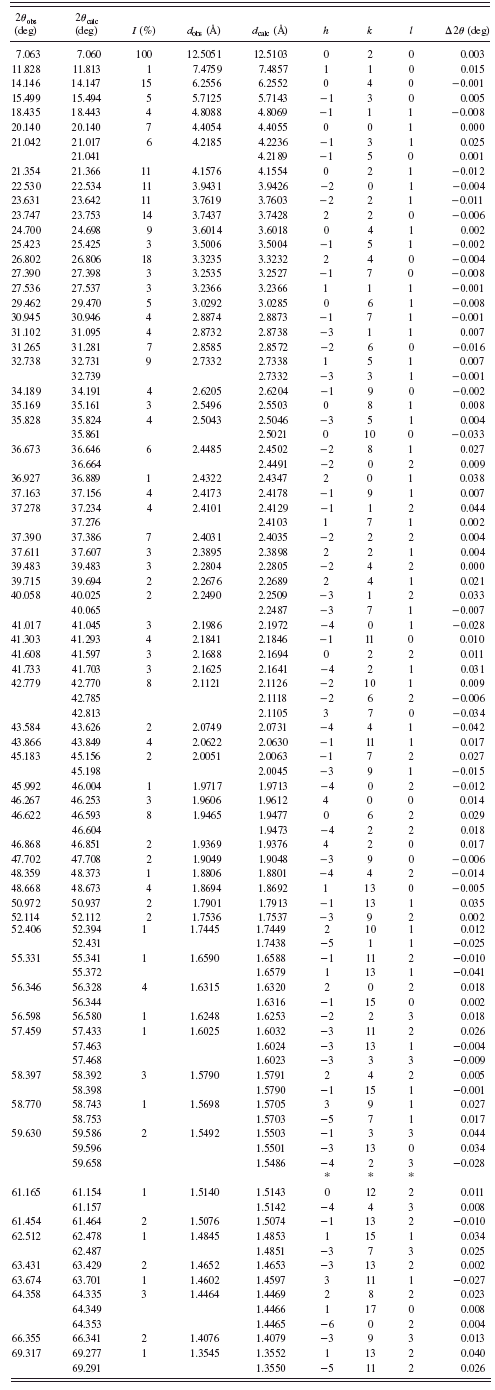
TABLE III. Powder diffraction data for BaC7H10O4 {3}.
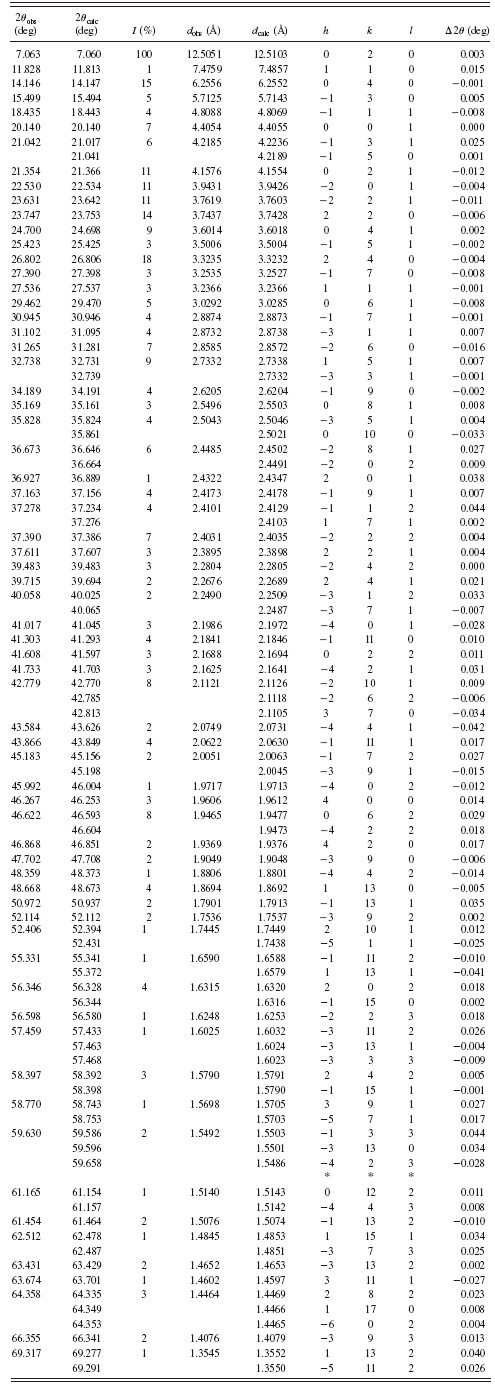

Figure 1. Diffraction patterns for barium dicarboxylates.

Figure 2. High-temperature XRPD patterns for BaC5H6O4·6H2O {1}.

Figure 3. High-temperature XRPD patterns for BaC7H10O4 {3}.
are given in Tables I–VI. Basic crystallographic data such as unit-cell parameters, V, Z, space groups, and densities are presented in Table VII. The unit-cell parameters for compounds {1} and {4} were confirmed by single-crystal studies (Grzesiak et al., Reference Grzesiak, Nitek and Łasocha2010).
In barium glutarate {1}, infinite hybrid inorganic-organic layers are present. They are built of infinite barium-oxygen slabs connected by dicarboxylic linkers. Thanks to the single-crystal analysis, it was found that using subaric acid, barium disuberate with formula Ba(COOC6H12COOH)2 was obtained. For simplicity its formula is written as Ba(C8H13O4)2 and it is named barium suberate {4}. This compound can be considered as tridimensional coordination polymer in which barium-oxygen nodes are connected by dicarboxylic linkers. Complete structural data for these compounds will be the subject of our future publications.
Most of the obtained salts crystallize as anhydrous compounds, only barium glutarate BaC5H6O4·6H2O {1} contains six water molecules. Barium suberate Ba(C8H13O4)2 {4} and barium dodecanediate BaC12H20O4 {6} crystallize in the tetragonal system, barium glutarate hexahydrate in orthorhombic {1}, and barium pimelate BaC7H10O4 {3} in monoclinic. Barium adipinate BaC6H8O4 {2} and barium sebacate BaC10H16O4 {5} crystallize in the triclinic system.
High-temperature XRPD analysis
Evolution of the diffraction patterns obtained during thermal decompositions in air is presented in Figures 2–5. The thermal decomposition study by XRPD methods of barium glutarate {1} was limited to low temperatures because during heating in higher temperatures the sample bulged, which made high-temperature XRPD measurements impossible (see Figure 2). Please note that we used the flat-sample Bragg-Brentano geometry. Diffraction data obtained for barium glutarate {1}, reloaded after thermal decomposition, show that the compound decomposed to barium carbonate.
As shown in Figure 3 for the case of barium pimelate (BaC7H10O4) {3}, the thermal decomposition clearly shows that the phase of barium pimelate disappeared because of heating and is replaced by a new compound stable at a temperature range from 200 to 300 °C. At 400 °C, this compound disappears and at 500 °C barium carbonate is the only phase present in the sample. In the case of barium subarate Ba(C8H13O4)2 {4} similar observations as for barium pimelate can be made (see Figure 4).
TABLE IV. Powder diffraction data for BaC16H26O8 {4}.

TABLE V. Powder diffraction data for BaC10H16O4 {5}.


Figure 4. High-temperature XRPD for Ba(C8H13O4)2 {4}.
As shown in Figure 5, barium sebacate (BaC10H16O4) {5} is stable at a temperature range from 27 to 100 °C. It is replaced by a new unknown compound, which is stable at a temperature range from 200 to 400 °C. This compound can be tentatively indexed with another triclinic cell very similar to the original one shown in Table VII: a=9.1385, b=14.9888, c=4.6877 Å, α=99.2394, β=100.8070, γ=79.5253°, V=614.86 Å3, and F(20)=18.0. This phase,
TABLE VI. Powder diffraction data for BaC12H20O4 {6}.

however, will be the subject of our further investigations. At 500 °C, we observed the disappearance of diffraction pattern of this phase due to an increase in the sample volume. A control experiment performed after the end of heating (the sample was reloaded into a sample holder) showed that the sample decomposed to BaCO3.
CONCLUSION
Six new salts of barium and dicarboxylic acids were synthesized and characterized by X-ray powder diffraction methods. Most of the obtained salts crystallize as anhydrous compounds, and only barium glutarate {1} contains six water molecules. Barium suberate and dodecanedioate {4 and 6}
TABLE VII. Unit-cell parameters for barium dicarboxylates.

a Smith and Snyder, Reference Smith and Snyder1979.
b de Wolff, Reference de Wolff1968.

Figure 5. High-temperature XRPD for BaC10H16O4 {5}.
crystallize in the tetragonal system, barium glutarate hexahydrate {1} in the orthorhombic system, barium pimelate {3} in monoclinic, and barium adipinate and sebacate {2 and 5} in the triclinic system.
During the thermal treatment all the compounds decompose to barium carbonate at temperatures between 400 and 500 °C. Investigations of thermal behavior of the investigated salts were hampered by the formation of copious products, which caused attenuation of the X-ray beam in the Bragg-Brentano geometry.
ACKNOWLEDGMENT
M. Grzesiak has been partially supported by the EU Human Capital Operation Programme, Polish Project No. POKL 04.0101-00-43/08-00 The research was carried out with the equipment purchased, thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08).