INTRODUCTION
The number of non-indigenous species in aquatic habitats has increased rapidly with increasing international trading globally (Ruiz et al., Reference Ruiz, Carlton, Grosholz and Hines1997; Galil et al., Reference Galil, Marchini, Occhipinti-Ambrogi, Minchin, Narščius, Ojaveer and Olenin2014). Non-indigenous species affect local communities and the magnitude and direction of impacts is mostly determined by their role in the ecosystem. Often the strongest impacts are due to novel predators both in aquatic and terrestrial ecosystems (Thomsen et al., Reference Thomsen, Byers, Schiel, Bruno, Olden, Wernberg and Silliman2014a; Doherty et al., Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016).
The majority of studies targeting non-indigenous species are focused on impacts on specific species and fail to describe impacts to the whole community (Parker et al., Reference Parker, Simberloff, Lonsdale, Goodell, Wonham, Kareiva, Williamson, Von Holle, Moyle, Byers and Goldwasser1999; Thomsen et al., Reference Thomsen, Byers, Schiel, Bruno, Olden, Wernberg and Silliman2014a, Reference Thomsen, Wernberg, Olden, Byers, Bruno, Silliman and Schielb). Similarly, assessments of impacts of multiple co-occurring invaders are rare, even though invasion rates are accelerating worldwide and in many ecosystems invasive species are interacting with each other (Simberloff & Von Holle, Reference Simberloff and Von Holle1999; Jackson, Reference Jackson2015). These interactions can either amplify or mitigate their impacts (Jackson, Reference Jackson2015 and references therein). Information collected from one system (e.g. terrestrial) cannot be directly translated to another system (e.g. aquatic) due to differences in system properties such as the stability and dynamics of ecosystems and variables of an external nature that influence the state of an ecosystem (Carr et al., Reference Carr, Neigel, Estes, Andelman, Warner and Largier2003).
To date, there are only a few laboratory and field studies in marine ecosystems where the effects of co-occurring non-indigenous species have been assessed in the same framework, and these studies have been mostly conducted in fully marine environments (Lohrer & Whitlatch, Reference Lohrer and Whitlatch2002; Wonham et al., Reference Wonham, O'Connor and Harley2005; Griffen & Williamson, Reference Griffen and Williamson2008; Griffen & Byers, Reference Griffen and Byers2009; Collin & Johnson, Reference Collin and Johnson2014; Newsom & Williams, Reference Newsom and Williams2014). These studies predict that in marine environments ecological interactions between invasive species are mostly antagonistic, compared with freshwater or terrestrial environments, where mainly neutral interactions are reported (Jackson, Reference Jackson2015). Thus, such negative interspecific interactions among invaders may reduce their overall community impact as expected from their separate effects (Burlakova et al., Reference Burlakova, Tulumello, Karatayev, Krebs, Schloesser, Paterson, Griffith, Scott, Crail and Zanatta2014) and ultimately may lead to the replacement of one non-native species by another (Lohrer & Whitlatch, Reference Lohrer and Whitlatch2002).
The brackish Baltic Sea has recently witnessed the arrival of many non-native species (Orlova et al., Reference Orlova, Telesh, Berezina, Antsulevich, Maximov and Litvinchuk2006; Ojaveer & Kotta, Reference Ojaveer and Kotta2015) and as such can be regarded as an excellent model system to assess how an addition of non-indigenous species separately and/or together affects native communities. Two non-native species, the Ponto-Caspian round goby, Neogobius melanostomus (Pallas, 1814) and the North American mud crab, Rhithropanopeus harrisii (Gould, 1841), arrived in the north-eastern Baltic Sea in the early 2000s, either via ballast water or hull fouling (Ojaveer, Reference Ojaveer2006; Kotta & Ojaveer, Reference Kotta and Ojaveer2012). The round goby represents a secondary invasion from the southern Baltic Sea (Kotta et al., Reference Kotta, Nurkse, Puntila and Ojaveer2016) whereas the mud crab possibly represents a primary invasion as evidenced from a clear genetic separation of the Estonian population from the rest of the Baltic Sea (Forsström et al., Reference Forsström, Ahmad and Vasemägi2017). To date, the round goby has achieved a pan-Baltic distribution (Kotta et al., Reference Kotta, Nurkse, Puntila and Ojaveer2016) and the mud crab is rapidly expanding its population in the area. Both species tolerate a large range of salinity and temperature conditions as they have been reported from fresh water to full oceanic conditions and from freezing point to over 30°C. Moreover, they can inhabit various types of benthic habitats (Turoboyski, Reference Turoboyski1973; Karsiotis et al., Reference Karsiotis, Pierce, Brown and Stepien2012; Nurkse et al., Reference Nurkse, Kotta, Orav-Kotta, Pärnoja and Kuprijanov2015). As a result of this broad tolerance of environmental conditions they will likely become dominant components of benthic communities all over the Baltic Sea. The two species are benthic predators and in the coastal ecosystems of the Baltic Sea functions involving epibenthic predation are highly underrepresented or at some basins completely novel. Based on earlier laboratory experiments round goby and mud crab feed on benthic invertebrate species, mostly on dominant mussels, clams and amphipods (Forsström et al., Reference Forsström, Fowler, Manninen and Vesakoski2015; Nurkse et al., Reference Nurkse, Kotta, Orav-Kotta and Ojaveer2016). Round goby has higher mobility and is mainly a visual predator, whereas mud crab is slower and relies more on chemical cues to detect its prey; however, based on their predatory impacts the two species represent the same function for the local benthic communities (Diggins et al., Reference Diggins, Kaur, Chakraborti and DePinto2002; Kidawa et al., Reference Kidawa, Markowska and Rakusa-Suszczewski2004). Together the two species have substantially increased the importance of benthic predation in the local ecosystem. Still, the nature of their joint effect is unclear. Moreover, the larger goby could prey on the mud crabs, with first signs observed in range overlap areas (the database of the Estonian Marine Institute). The presence of two predators may thus modify each other's feeding behaviour as well as reduce their overall predation pressure (Marentette & Balshine, Reference Marentette and Balshine2012).
The Baltic Sea is a seasonally variable ecosystem. Water temperature affects species' metabolic demands and predation rates (Lee & Johnson, Reference Lee and Johnson2005) with maximum feeding rates coinciding with the species' thermal optimum (Iacarella et al., Reference Iacarella, Dick, Alexander and Ricciardi2015). Temperature optima for the more southern round goby and the more northern mud crab are recorded at 26 and 20°C, respectively (Lee & Johnson, Reference Lee and Johnson2005; Hegele-Drywa & Normant, Reference Hegele-Drywa and Normant2014). Thus, temperature may set interaction strength among invasive predators, modify predation rates through species-specific temperature-consumption responses and thereby define overall impacts of novel predators on local benthic invertebrate communities (Oyugi et al., Reference Oyugi, Cucherousset and Britton2012).
The main goal of this study was to experimentally assess the separate and interactive predation rates of round goby and mud crab on benthic invertebrates inhabiting a shallow coastal ecosystem of the Gulf of Riga, the Baltic Sea. The feeding rates were evaluated at two temperature conditions. Our hypotheses were: (1) Owing to its large size, round goby is expected to prey on mud crab or reduce its overall feeding activity. Consequently, the impacts of the studied predators on invertebrate communities are smaller when they co-occur compared with communities including either the round goby or the mud crab. (2) Temperature is expected to affect their feeding rates differentially. This is because the round goby has a more southerly origin and therefore its thermal optimum is higher as compared with the mud crab (Lee & Johnson, Reference Lee and Johnson2005; Hegele-Drywa & Normant, Reference Hegele-Drywa and Normant2014). Consequently, the round goby is likely to be more affected by temperature change than the mud crab.
MATERIALS AND METHODS
The indoor laboratory experiment was conducted at Kõiguste field station, located in the north-eastern Baltic Sea on the northern shore of the Gulf of Riga (58.37′′N 22.98″E) from 1–3 November 2015. In the experiment, habitat characteristics as well as prey and predator densities were kept similar to the field conditions in the area (Kotta et al., Reference Kotta, Lauringson, Martin, Simm, Kotta, Herkül, Ojaveer and Schiewer2008; Estonian national projects ‘Surveillance monitoring of Estonian coastal sea in 2016’ and ‘Round goby in Estonian coastal waters: applied research for developing further action plan’). The experiment was conducted in 50 l aquaria (bottom area 0.11 m2). Tanks were filled with gently aerated seawater collected from Kõiguste Bay (7°C, salinity 6.0), 4 cm layer of silted sand and a medium sized boulder (with largest dimension of 20 cm) covered with macroalgae for shelter and habitat. Over 95% of boulder substrate was covered by the red seaweed Furcellaria lumbricalis (Hudson) (J.V. Lamouroux, 1813) and marginal amounts of other red algae species occurred. Prior to the placement all rocks were gently shaken in tap water to remove associated invertebrates. Macroalgal biomass did not differ between treatments (P = 0.54) and on average each aquarium contained 1.23 ± SE 0.23 g dry weight of F. lumbricalis. Prey communities in each aquarium consisted of local dominant invertebrates at natural densities: the mussel Mytilus trossulus (Gould, 1850) (50 ind., average size 20 mm); the clam Macoma balthica (Linnaeus, 1758) (46 ind., average size 12 mm); the gastropod Theodoxus fluviatilis (Linnaeus, 1758) (45 ind., average size 5 mm); and gammarid amphipods (45 ind., average size 12 mm) (mainly Gammarus tigrinus Sexton, 1939, Gammarus duebeni Lilljeborg, 1852, Gammarus salinus Spooner, 1947, Gammarus zaddachi Sexton, 1912 and Gammarus oceanicus Segerstråle, 1947).
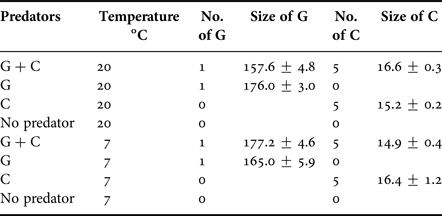
The experiment included altogether the following treatments and treatment levels: predator community (no predators, round goby, mud crab, both round goby and mud crab) and temperature (7 and 20°C). Such experimental design resulted in eight combinations of treatments each replicated five times (Table 1). The number of predators used in the predator community resembled species densities in the field conditions (the database of the Estonian Marine Institute; Estonian national projects ‘Surveillance monitoring of Estonian coastal sea in 2016’ and ‘Round goby in Estonian coastal waters: applied research for developing further action plan’). The levels of water temperature treatment represented spring/autumn and summer conditions. The experiment was run for 48 h. Photoperiod (9:15 h L:D) and light intensity were kept similar to the local ambient environment in October–November.
Table 1. The number and size (mean ± SE mm) of predators in different treatments. G denotes round goby and C denotes mud crab.
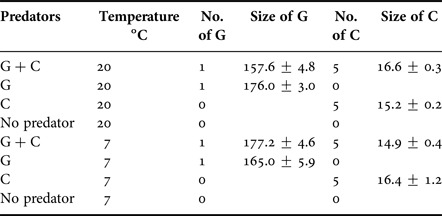
All prey animals were collected adjacent to the field station by a landing net or by a scuba diver. Round goby were collected from a fisherman's basket trap and mud crabs were caught using special live traps in Pärnu Bay. All animals were acclimated in 15 l aquaria for 24 h prior to the experiment. All acclimation aquaria were equipped with stones and sand for shelter and gently aerated but no food was provided. Due to specific bottom topography and extensive shallow areas water temperature often naturally fluctuates on an hourly/daily scale between 5 and 20 degrees in the north-eastern shallows of the Gulf of Riga. Such high variability in water temperature is mostly due to storms and/or currents that irregularly bring deep and cold water to the near-coastal areas. Therefore, a 24 h acclimation period is considered satisfactory for such a dynamic coastal area. A longer acclimation period would result in an abnormal feeding pattern of the studied predators we wanted to avoid. All round goby and mud crabs were adults, with ecologically irrelevant size differences (Table 1). As male individuals dominate in populations of both species, only male individuals were included.
After the experiment, the total length of round goby and the carapace width of mud crabs were measured to the nearest 0.01 mm. Sediment with invertebrates was analysed for the remaining prey animals under a binocular microscope. Amphipods were determined to species level. All invertebrates were counted.
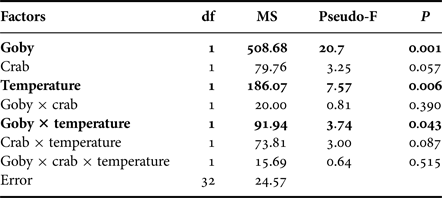
PERMANOVA in the PRIMER 7 environment was used to assess statistical significance of the studied factors (goby, crab, temperature) and their interactions on prey abundances of the post-experimental communities. PERMANOVA does not assume the data to have any specific distributions (Anderson, Reference Anderson2005). The assumption of homogeneity of spread was checked using the non-parametric Fligner–Killeen test (P-value > 0.05). The Bray–Curtis similarity measure was used to construct the resemblance matrix. Significance tests were done using F-tests (with type III errors) based on sums of squares from permutation of raw data, as this technique had the best power with relatively small sample sizes (Anderson & Ter Braak, Reference Anderson and Ter Braak2003). SIMPER tests were used to assess which invertebrate species contributed most to the differences in predation rates among factor levels (Clarke & Warwick, Reference Clarke and Warwick2001).
The univariate prey species abundance data are naturally modelled using a Poisson distribution. Poisson regression models were used to assess the significance of the effects of the above factors (goby, crab, temperature) and their interactions separately on the abundance of each prey species. We fitted the Poisson regression models with over-dispersion to account for possible over-/under-dispersion of the count data (R Stats package, R Core Team, 2016). Multiple comparisons were carried out using the multcomp package (Hothorn et al., Reference Hothorn, Bretz and Westfall2008).
RESULTS
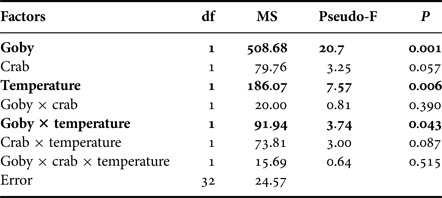
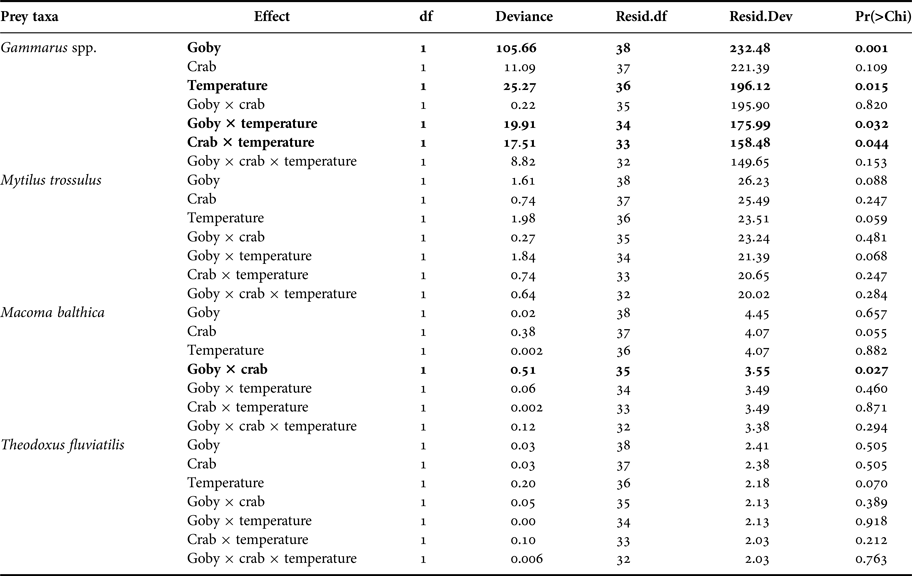
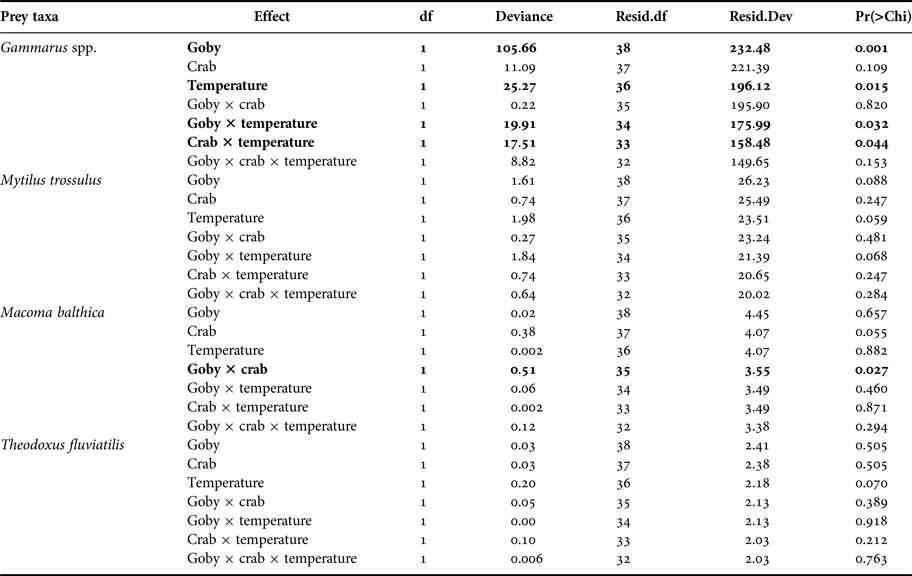
At the community level, the presence of goby, water temperature and their interactive effects all significantly impacted species abundances (Table 2). SIMPER test indicated that the round goby mostly reduced the abundance of Gammarus spp. at both temperature treatments. Moreover, higher temperature induced different predation pressure. The SIMPER test showed that predation rates increased in the order mud crab < round goby < mud crab + round goby.
Table 2. Community specific PERMANOVA analyses on the effects of goby, crab, temperature and their interactions on prey abundance. Significant effects and interactions are marked in bold.
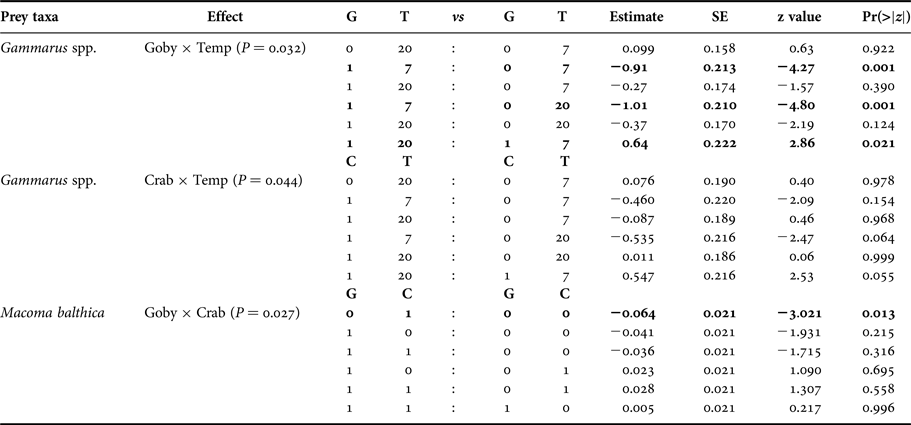
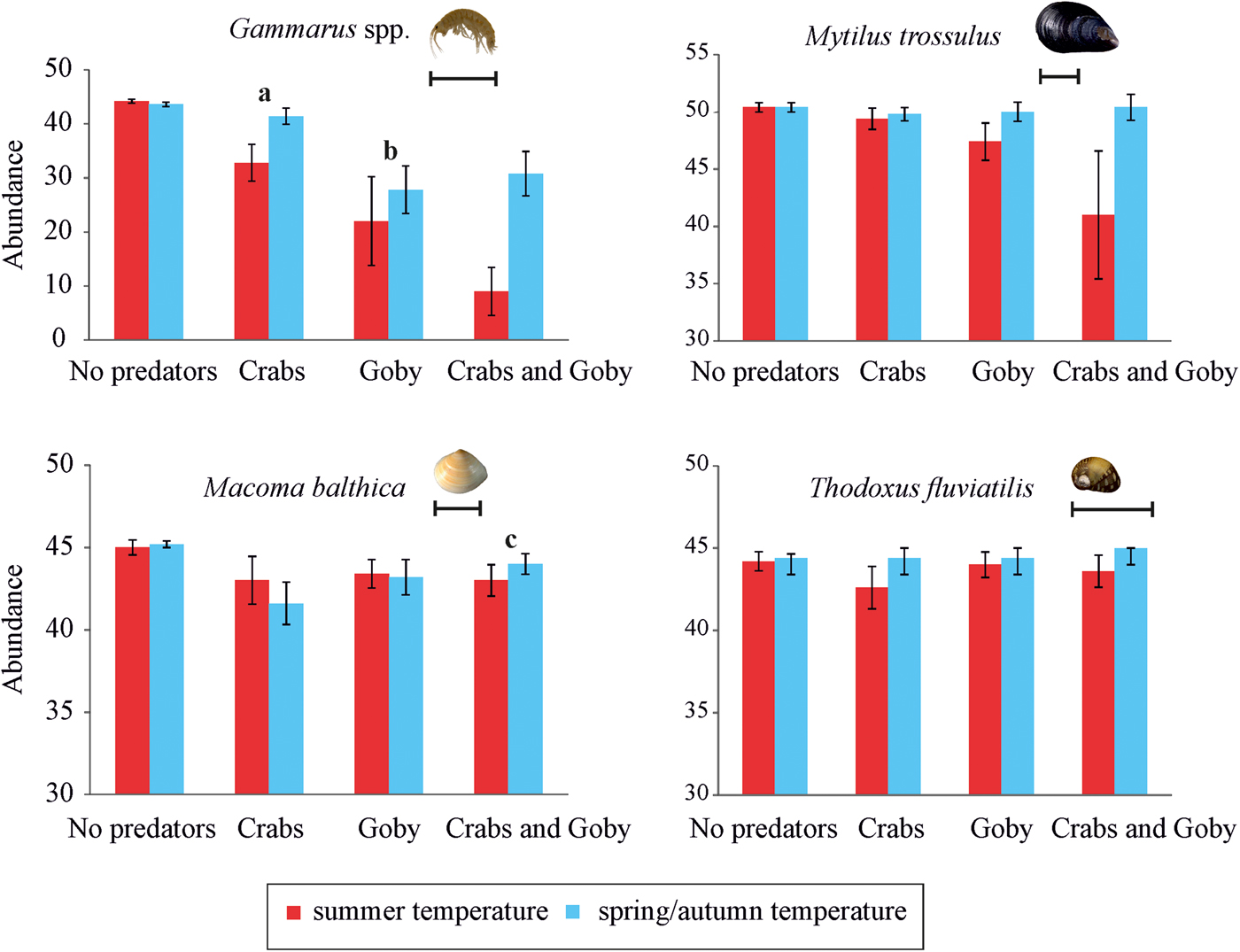
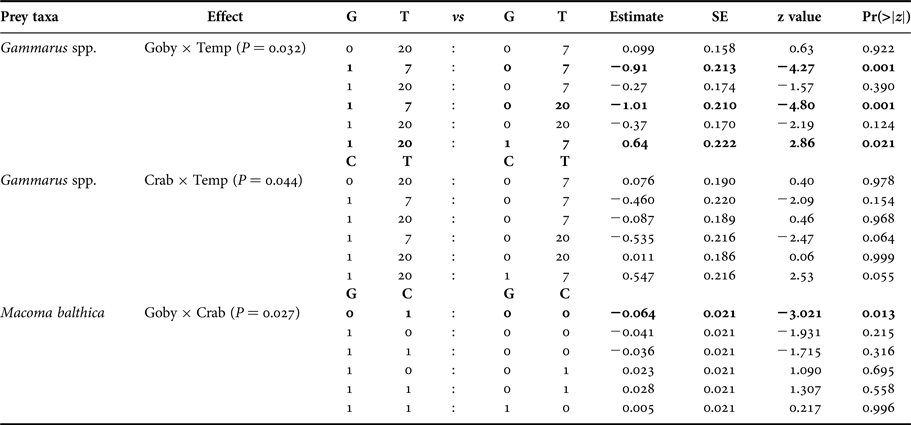
At species level, the presence of goby, and the presence of goby and mud crab in interaction with temperature significantly affected the density of Gammarus spp. (Figure 1, Table 3). Round goby preyed on higher amounts of gammarid amphipods than mud crab and elevated temperature increased the predation pressure (P = 0.021) (Table A1). In addition, the presence of goby and mud crab interactively affected the density of M. balthica (Figure 1, Table 3). Here, the predation of mud crab became significant only in the absence of round goby (P = 0.013), suggesting the presence of interference competition among the predator species (Table A1). During the experiment, the round goby did not prey on the mud crabs.
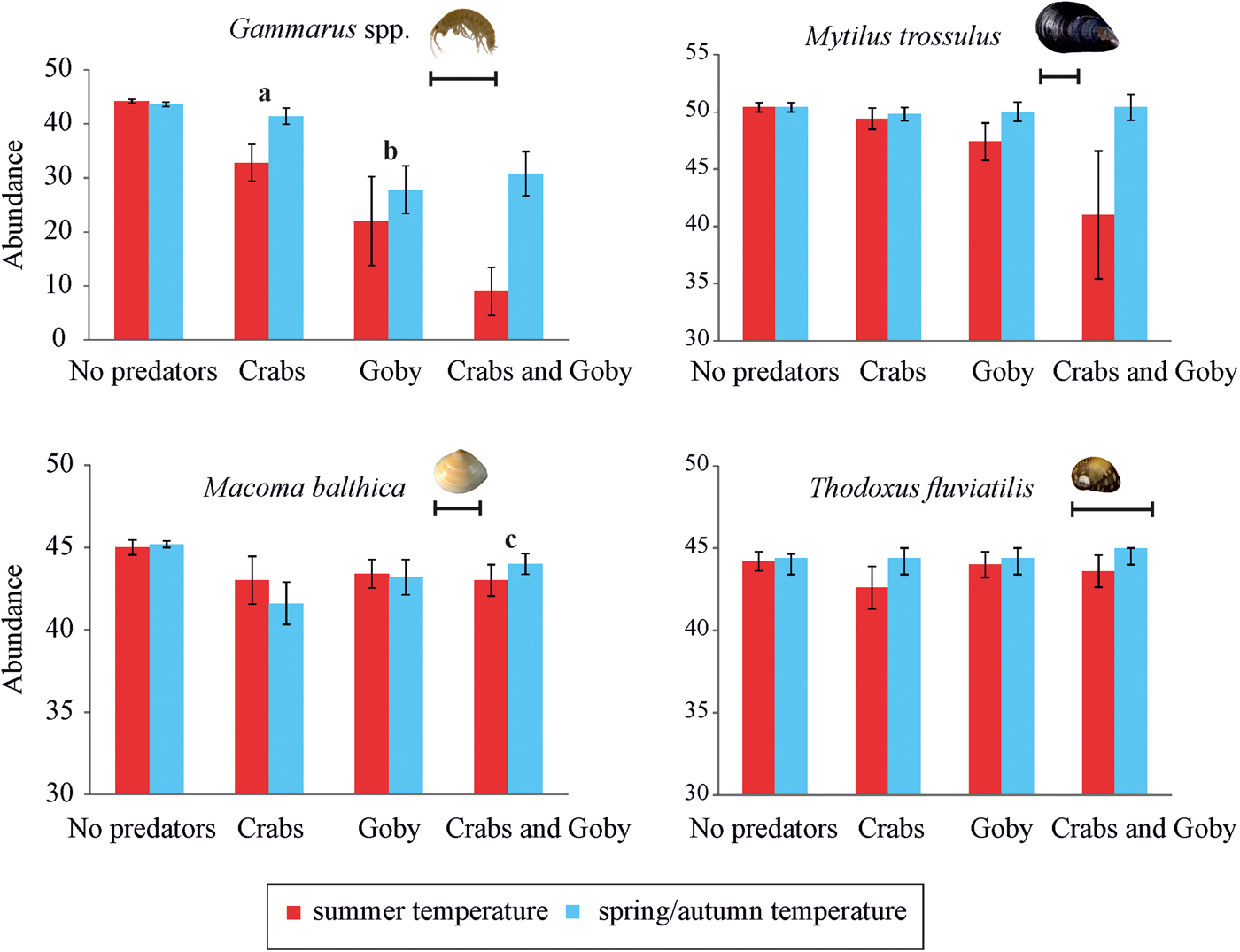
Fig. 1. Post-experiment abundances (mean ± SE; N = 5 for all treatments) of each prey taxa in all treatment levels (No. predators; 5 crabs; 1 goby; 5 crabs and 1 goby) at summer temperature (20°C) and spring/autumn temperature (7°C) in 40 l aquaria (0.11 m2 bottom area). The scale bars equal 10 mm. Statistically distinguished groups are labelled with letters (a, b, c; for statistical significances see also Table 3).
Table 3. Separate prey-specific Poisson regression analyses on the effects of predator community, temperature and their interaction on prey abundance. Significant effects and interactions are marked in bold. Three-way interaction term denotes the deviance for error of each model (e.g. the Gammarus spp. model has a residual deviance of 149.65 with 32 degrees of freedom).
DISCUSSION
Our experimental study demonstrated that the studied predators induced a significant mortality to the gammarid amphipods and partly to the clams but not for the hard-shelled mussels and snails. As for the mussel M. trossulus the effects of predators were close to being statistically significant (P goby = 0.088, P goby×temperature = 0.068) suggesting that a longer duration of the experiment (or perhaps even higher number of replicates) may result in stronger effects and more significant differences. In addition to significant separate effects, the experiment also showed that predators affected experimental invertebrate communities interactively with temperature.
We assessed the predation pressure of round goby and mud crab on benthic invertebrates under typical predator densities. Although extrapolation of laboratory trials to field conditions should be done with great caution, the predation rates quantified under the current experimental condition would probably translate to significant predation impact of benthic invertebrate communities in the shallow-water environments of the Gulf of Riga.
We also showed that the two predators had largely independent effects on the invertebrate prey. As the only exception, round goby reduced the predation rate of mud crab on the clam M. balthica. As such, our results are in the line of a recent meta-analysis that, across a global scale, invaders often have independent effects on native communities (Jackson, Reference Jackson2015). The same meta-analysis also stresses that although several studies indicate a prevalence of additive effects, invaders still tend to reduce one another's performance when analyses involve the whole invaders' community. In our experiment, however, round goby did not prey on the mud crab and neither did we observe any aggressive behaviour among the predators in the experimental conditions. In field conditions under inadequate food supply, the novel fish may occasionally prey on mud crabs (‘Estonian Environmental Investment Centre project on round goby in Estonian coastal waters: applied research for developing further action plan').
Our experiment also showed that water temperature regulates predation rates of these novel invaders but the effects were not consistent and many species-specific patterns emerged. If the mud crab preyed less on the gammarids at lower temperature, then the predation rate on the clam M. balthica was independent of temperature. On the other hand, the round goby retained most of its amphipod feeding activity in a colder environment. Such temperature-induced differences in feeding may reflect the more southerly origin of round goby compared with mud crab (Lee & Johnson, Reference Lee and Johnson2005; Hegele-Drywa & Normant, Reference Hegele-Drywa and Normant2014).
In conclusion, although the current study showed that mobile amphipods are consumed more than other species, the results of feeding experiments should always be interpreted as context specific, where local communities determine what is consumed (Foley et al., Reference Foley, Henebry, Happel, Bootsma, Czesny, Janssen, Jude, Rinchard and Höök2017). Nevertheless, the experimentally quantified feeding rates suggest that the establishment of round goby and mud crab significantly intensified a function of benthic predation in the shallow-water environments of the Gulf of Riga that previously lacked benthic predators or their densities were low. Contrary to our expectations, the round goby did not prey on mud crab or reduced its overall feeding activity. Water temperature significantly affected feeding rates of the studied predators but often the effects were very context-specific.
Invasive species are among the large-scale stressors interacting to impact marine ecosystems and requiring understanding for management of essential ecosystem services. In the Baltic Sea, invasions are escalating rapidly and predicted to cause severe changes. Many of these recent invaders of the Baltic Sea bring novel functions to the ecosystem and are situated at high trophic levels. The elevated predation pressure is expected to proliferate through lower levels and result in positive and negative feedbacks (e.g. Heath et al., Reference Heath, Speirs and Steele2014). However, the rise of communities with no past analogues, characterized by new species interactions and novel ecological functions makes it very challenging to predict outcomes of invasion events. These interactions will only be understood using rigorous, logical and well-planned experimental investigations targeting multiple habitats as well as multiple trophic levels along key environmental gradients.
ACKNOWLEDGEMENTS
We thank M. Teeveer for help with conducting the experiment.
FINANCIAL SUPPORT
This work was supported by the Estonian Research Council (Institutional research funding, IUT02-20); the European Union's Seventh Programme for research, technological development and demonstration jointly with the Estonian Research Council (BONUS project BIO-C3); and the European Union INTERREG Baltic Sea region programme (Baltic Blue Growth).
APPENDIX
Table A1. Species-specific pair-wise comparisons on significant interactions (P > 0.05). G denotes round goby presence, C denotes mud crab presence and T denotes temperature in degrees.