Introduction
An essential feature of fertilization is the activation of the oocyte, the process by which it can be transformed into an embryo. The earliest signalling event in the activation of an egg by a spermatozoon is a large transient increase in intracellular free calcium ion concentration (Runft et al., Reference Runft, Jaffe and Mehlmann2002; Dale et al., Reference Dale, Wilding, Coppola and Tosti2010). In many non-mammalian species, such as sea urchin and amphibian, the observed Ca2+ increase in the egg comprises a single transient rise but in mammals there is a series of repetitive Ca2+ oscillations (Swann et al., Reference Swann, Larman, Saunders and Lai2004, Miyazaki & Ito, Reference Miyazaki and Ito2006).
In amphibians, this Ca2+ signal is sufficient to trigger the events associated with egg activation, including a depolarization of the egg membrane and the exocytosis of cortical granules (Talevi et al., Reference Talevi, Dale and Campanella1985; Bement & Capco, Reference Bement and Capco1989; Oterino et al., Reference Oterino, Sánchez Toranzo, Zelarayán, Valz-Gianinet and Bühler2001; Runft et al., Reference Runft, Carroll, Gillett, Giusti, O'Neill and Foltz2004). This signal takes the form of a single wave, which passes across the egg from the site of sperm fusion (Jones, Reference Jones1998).
In echinoderms, ascidians and some nematodes, such as Caenorhabditis elegans, egg activation is governed by similar mechanisms (Runft et al., Reference Runft, Carroll, Gillett, Giusti, O'Neill and Foltz2004; Bembenek et al., Reference Bembenek, Richie, Squirrell, Campbell, Eliceiri, Poteryaev, Spang, Golden and White2007).
The Ca2+ increase at fertilization in frog eggs is thought to be mediated by inositol triphosphate (IP3) receptors in the egg endoplasmic reticulum (Nuccitelli et al., Reference Nuccitelli, Yim and Smart1993; Stith et al., Reference Stith, Goalstone, Silva and Jaynes1993; Snow et al., Reference Snow, Yim, Leibow, Saini and Nuccitelli1996). This Ca2+ release is necessary and sufficient for the re-entry of the egg into the meiotic cell cycle (Douglas & Kline, Reference Douglas and Kline1992) and the start of embryonic development (Whitaker & Swann, Reference Whitaker and Swann1993; Stricker, Reference Stricker1999).
The mechanism whereby the sperm triggers the release of Ca2+ in the egg has been a subject of controversy with opinions divided as to whether egg activation is initiated by a surface interaction between a sperm ligand and an egg receptor (Gould & Stephano, Reference Gould and Stephano1991; Mizote et al., Reference Mizote, Okamoto and Iwao1999; Dale & DeFelice, Reference Dale and DeFelice2011) or by a cytosolic sperm factor that is released into the egg during gamete fusion (Dale et al., Reference Dale, DeFelice and Ehrenstein1985; Wilding et al., Reference Wilding, Kyozuka, Ruso, Tosti and Dale1997; Evans & Kopf, Reference Evans and Kopf1998; Wilding & Dale, Reference Wilding and Dale1998; Wolny et al., Reference Wolny, Fissore, Wu, Reis, Colombero, Ergün, Rosenwaks and Palermo1999; Machaty et al., Reference Machaty, Bonk, Kuhholzer and Prather2000, Saunders et al., Reference Saunders, Larman, Parrington, Cox, Royse, Blayney, Swann and Lai2002, Kurokawa et al., Reference Kurokawa, Sato and Fissore2004). In mammals, injection of the sperm extract into the unfertilized eggs causes intracellular Ca2+ release and Ca2+ oscillations (Parrington et al., Reference Parrington, Swann, Shevchenko, Sesay and Lai1996, Reference Parrington, Jones, Lai and Swann1999). In intact eggs and egg homogenates, mammalian sperm extract triggers Ca2+ release by stimulating IP3 production (Jones, Reference Jones1998; Jones et al., Reference Jones, Cruttwell, Parrington and Swann1998, Reference Jones, Matsuda, Parrington, Katan and Swann2000; Rice et al., Reference Rice, Parrington, Jones and Swann2000; Wu et al., Reference Wu, Smyth, Luzzi, Fukami, Takenawa, Black, Allbritton and Fissore2001; Saunders et al., Reference Saunders, Larman, Parrington, Cox, Royse, Blayney, Swann and Lai2002), indicating the involvement of a phospholipase C (PLC) in the signal transduction mechanism (Sato et al., Reference Sato, Tokmakov, Iwasaki and Fukami2000; Mehlmann et al., Reference Mehlmann, Chattopadhyay, Carpenter and Jaffe2001).
Different isoenzymes of PLC are capable of catalyzing the hydrolysis of phosphatidylinositol 2,5-biphosphate (PIP2), generating IP3 and diacylglycerol (DAG). IP3 regulates the release of Ca2+ from the endoplasmic reticulum (Singer et al., Reference Singer, Brown and Sternweis1997; Dale & DeFelice, Reference Dale and DeFelice2011), while DAG is the physiological activator of PKC (Nishizuka, Reference Nishizuka1984; Bement, Reference Bement1992). As the natural substrate used by PLC are phosphatidylinositol (PI) and phosphatidylcholine (PC), they can be classified into PI-PLC (specific for PI) and PC-PLC (specific for PC) (Hinkovska-Galchev & Srivastava, Reference Hinkovska-Galchev and Srivastava1992; Szumiło & Rahden-Staroń, Reference Szumiło and Rahden-Staroń2008). At present there is abundant knowledge of the properties and activation of PI-PLC, while there is scarce information concerning PC-PLC (Ramoni et al., Reference Ramoni, Spadaro, Menegon and Podo2001, Reference Ramoni, Spadaro, Barletta, Dupuis and Podo2004).
Among the phospholipases that hydrolyze phosphatidylinositol we find PLCγ, which is activated by tyrosine phosphorylation (Runft & Jaffe, Reference Runft and Jaffe2000) and PLCζ, which has been identified in mice, humans, monkeys, bovine and porcine (Cox et al., Reference Cox, Larman, Saunders, Hashimoto, Swann and Lai2002; Saunders et al., Reference Saunders, Larman, Parrington, Cox, Royse, Blayney, Swann and Lai2002; Fujimoto et al., Reference Fujimoto, Yoshida, Fukui, Amanai, Isobe, Itagaki, Izumi and Perry2004; Coward et al., Reference Coward, Ponting, Zhang, Young, Huang, Chou, Kashir, Fissore and Parrington2011)
Microinjection with PLCζ recombinant or its RNA in mouse, bovine and human oocytes was capable of releasing Ca2+ from its reservoirs with pronucleus formation (Cox et al., Reference Cox, Larman, Saunders, Hashimoto, Swann and Lai2002; Saunders et al., Reference Saunders, Larman, Parrington, Cox, Royse, Blayney, Swann and Lai2002; Fujimoto et al., Reference Fujimoto, Yoshida, Fukui, Amanai, Isobe, Itagaki, Izumi and Perry2004; Larman et al., Reference Larman, Saunders, Carroll, Lai and Swann2004; Rogers et al., Reference Rogers, Hobson, Pickering, Lai, Braude and Swann2004; Malcuit et al., Reference Malcuit, Knott, He, Wainwright, Parys, Robl and Fissore2005).
In Xenopus oocytes, PLCγ activation induced cortical granule exocytosis (Sato et al., Reference Sato, Tokmakov, Iwasaki and Fukami2000, Reference Sato, Fukami and Stith2006; Carroll et al., Reference Carroll, Ramarao, Mehlmann, Roche, Terasaki and Jaffe1997).
The species-specific ryanodine receptor (RyRc), which takes part in Ca2+-induced Ca2+ release (CICR), is involved in the Ca2+ release from intracellular stores. Functional RyR/channel complexes seem to be present in the eggs of some species such as sea urchin, mice, bovine and humans (Ayabe et al., Reference Ayabe, Kopf and Schultz1995; Galione & Summerhill, Reference Galione, Summerhill and Sorrentino1995; Yue et al., Reference Yue, White, Reed and Bunch1995), even if their role in Ca2+ signalling at fertilization is unclear (Galione et al., Reference Galione, McDougall, Busa, Willmott, Gillot and Whitaker1993; Ayabe et al., Reference Ayabe, Kopf and Schultz1995; Berridge, Reference Berridge1997). RyRc agonists include exogenous ryanodine and caffeine, and endogenous cyclic ADP ribose (c-ADP), a CICR modulator that has been proposed as second messenger (Galione et al., Reference Galione, McDougall, Busa, Willmott, Gillot and Whitaker1993). In this sense another Ca2+ mobilizing messenger, cADPR, has also been shown to play a role during egg activation (Parrington et al., Reference Parrington, Davis, Galione and Wessel2007).
The participation of inositol trisphosphate and RyRs in Rhinella arenarum oocyte activation was demonstrated previously by Ajmat et al. (Reference Ajmat, Bonilla, Zelarayán and Bühler2011, Reference Ajmat, Bonilla, Hermosilla, Zelarayán and Bühler2013).
When sperm extract from chicken or Xenopus laevis is injected into mouse oocytes, it triggers Ca2+ oscillations similar to those found during fertilization (Busa et al., Reference Busa, Ferguson, Joseph, Williamson and Nuccitelli1985; Dong et al., Reference Dong, Tang and Sun2000). However, to date, there have been no reports that sperm extract from amphibians such as Rhinella arenarum can trigger Ca2+ release in homologous oocytes. In addition, it remains far from clear whether such sperm factor activities are functions of a PLC or are caused by a different signalling protein.
In Rhinella arenarum, the mechanism by which the oocytes are activated has not been elucidated yet. However, Bonilla et al. (Reference Bonilla, Ajmat, Sánchez Toranzo, Zelarayán, Oterino and Bühler2008) demonstrated the presence in sperm extract of a 24 kDa protein able to induce activation when it was microinjected into the oocytes.
The purpose of this work was to isolate and characterize the components of Rhinella arenarum sperm extract that have the capacity to induce activation when microinjected into homologous oocytes, the participation of IP3R, RyR and PKC in the process and the location of the active factor in the sperm.
Materials and methods
Animals
Sexually mature Rhinella arenarum males and females were collected in the northwestern area of Argentina and kept at 15 °C until use, which generally took place 15 days after collection.
Sperm extract preparation
Sperm suspensions were obtained by gently disrupting the testes in 4 ml Ringer solution (AR) and centrifuging at 1085 g for 10 min; then, the pellet was resuspended in AR. A swim-up procedure was used for the selection of motile sperm. The high quality sperm suspension was centrifuged at 1085 g for 10 min. Sperm were resuspended in calcium-free Tris-saline buffer (7.59 g NaCl/l, 2.40 g Tris/l–HCl, pH 7.4). The number of sperm was adjusted to 1 × 109 sperm/ml. The sample was separated into two aliquots. The first was processed immediately. The second was placed in 10% AR, incubated for 4 h to induce the acrosome reaction (Raisman et al., Reference Raisman, de Cunio, Cabada, del Pino and Mariano1980; Martinez & Cabada, Reference Martinez and Cabada1996; Krapf et al. Reference Krapf, O'Brien, Cabada, Visconti and Arranz2009) and washed in Tris-saline solution.
Both samples were lysed by three cycles of freezing (–196 °C) and thawing (25 °C). The lysates were centrifuged for 30 min at 16,000 g at 6 °C and the supernatant was collected as crude sperm extract. The extracts were observed under a microscope to check that intact sperm or sperm heads were not present. The protein concentration of the samples was adjusted to 9.0 μg/μl.
The sperm extract obtained from intact and reacted sperm was run through size exclusion chromatography using a Bio-Gel P-60 chromatography column. The column was washed with calcium-free Tris-saline buffer and the 27 fractions obtained were stored at –20 °C until use.
The biological activity of all fractions was assayed according to Bonilla et al. (Reference Bonilla, Ajmat, Sánchez Toranzo, Zelarayán, Oterino and Bühler2008).
Fast protein liquid chromatography (FPLC)-mono Q column
The fraction with biological activity (fraction 18 from size exclusion chromatography) was submitted to a FPLC–Mono Q column. The chromatographic columns were washed with Ca2+-free buffer. The proteins were eluted with elution buffer (Tris 20 mM, NaCl 1 M and HCl pH 7) at a flow rate of 0.1 ml/min and detected at OD280.
The sample obtained from FPLC was dialyzed and concentrated by evaporation of solvents in SAVANT SpeedVac® Concentrators. The protein concentration of the samples was adjusted to 0.1 μg/μl with Tris-saline and tested for biological activity.
Oocyte isolation and maturation
Denuded oocytes were obtained according to Lin & Schuetz (Reference Lin and Schuetz1985). Follicle cells were removed by incubation of defolliculated oocytes in AR (6.6 g NaCl/l, 0.15 g CaCl2/l, 0.15 g KCl/l) containing penicillin G-sodium (30 mg/l) and streptomycin sulphate (50 mg/l), pH 7.4, for 5 min with gentle shaking (100 oscillations/min). Denuded oocytes were kept in AR until use.
Maturation was induced by progesterone treatment (2.5 μM) and scored for the presence of a transient white spot in the animal pole.
In vitro cultures were carried out at room temperature (22–25 °C) using plastic multiwell culture dishes (Costar 3524, Cambridge, MA, USA). Randomized samples of 20 freshly denuded oocytes were distributed into separate wells containing 2 ml of AR; the reagents were added (5 μl) directly to the culture medium. Two-well duplicates were routinely run in each experimental group.
Microinjection
The different fractions from the sperm extract were microinjected into in vitro matured oocytes to test for biological activity using intracytoplasmic sperm injection (ICSI) micropipettes (Humagen™ Fertility Diagnostics) attached to a micromanipulator (Leitz). The injection volume was 30 nl. The microinjections were carried out in calcium-free Tris-buffered saline (7.59 g NaCl/l, 2.40 g Tris/l–HCl, pH 7.4) at 20 °C. Following injection, the oocytes were kept in Tris-buffered saline. Injection of Tris-buffered saline alone was used as control.
The injection volume was approximately 30 nl corresponding to the 0.10% of the oocyte volume. The amount of protein microinjected per oocyte was 3.0 μg. In all the experiments, the oocytes were injected into the animal hemisphere. Activation was scored 20 min after injection.
We considered as activation parameters the disappearance of the white spot, the elevation of the vitelline envelope and the exocytosis of the cortical granules.
For pronuclear observation, oocytes were fixed 120 min after injection.
Cytological preparations
The oocytes were fixed in Ancel and Vintemberger's solution (10% formol; 0.5% acetic acid and 0.5% NaCl), embedded in paraffin and sliced into 7-μm thick sections that were then stained with Ehrlich hematoxylin and eosin. This method allowed us easily to observe the pronucleus (Bühler et al., Reference Bühler, Petrino, Zelarayán and Legname1994).
Determination of enzymatic activity of F1
Phospholipase activity was determined by the effect of F1 (0.1 μg/μl) on PC (0.30 μM) and phosphatidylinositol (PI) (0.50 μM).
PC and PI were incubated with F1 for 60 min at 37 °C. The lipids obtained were extracted and subjected to thin layer chromatography (TLC).
Thin layer chromatography (TLC)
In total, 20 μl of the fraction with biological activity obtained with the FPLC (Fraction F1) was incubated in 300 μl Dulbecco's phosphate-buffered saline (PBS; Irvine Scientific®) with PC (0.26 μmol) and PI (0.50 μmol) at 37 °C for 60 min. The products generated during the reaction were studied using TLC. Extractions of lipids from the products were performed with the solvent combination chloroform:methanol (2:1) using the Folch technique (Folch et al., Reference Folch, Lees and Stanley1957). The sample was shaken and equilibrated. The mixture partitioned into two layers, the lower one containing virtually all the lipids. This layer was treated with nitrogen vapors to dryness and resuspended with chloroform.
The chloroform fraction was put on silica gel plates that were then placed in a TLC tank. The phospholipids were separated by TLC. The plate for polar lipids was developed in one dimension with chloroform/methanol/acetic acid. Lipids patterns are chromatographed in adjacent lanes.
The presence of lipids was revealed using iodine vapours (Minahk et al., Reference Minahk, Kim, Nelson, Trigatti, Lehner and Vance2008).
Electrophoresis
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) of sperm fractions was performed according to Laemmli (Reference Laemmli1970) with 7.5% running gels. Proteins were stained with silver staining (Richert et al., Reference Richert, Luche, Chevallet, Van Dorsselaer, Leize-Wagner and Rabilloud2004).
Hydrolysis of egg yolk
In order to test the phospholipase activity of Fraction F1, 20 μl of different dilutions of F1 were seeded in 2 mm thick wells on an egg yolk agar plate at 1% (2 mm thick). Serial dilutions were made from the F1 fraction (protein concentration 0.1 μg/μl). The Petri plate was kept at 37 °C for 60 min. At the end of this period, the halos caused by the hydrolysis were measured. As a positive control we used PC-PLC from Bacillus cereus (final concentration 0.1 μg/μl) and Tris-saline as a negative control.
Effect of phospholipase inhibitors on F1 activity
Fraction F1 was pre-treated with different phospholipase inhibitors for 30 min and then incubated with comercial PC for 60 min. The lipids produced were extracted and TLC for neutral lipids was performed. The following were used as inhibitors: n-butanol (50 μg/ml), neomycin (5 mM) and D-609 (50 μg/ml).
Participation of IP3R, RyR and PKC on F1 activity
The assays were carried out by microinjecting the inhibitors into the mature oocyte. Ten min after the injection of the inhibitor the activation of the oocytes was induced by injecting the biologically active fraction (F1) at a concentration of 0.01 mg/ml. Activation was assessed after 20 min.
Immunocytochemical studies
The cellular location of phospholipase with biological activity was studied using for this purpose a polyclonal antibody specific for phosphatidylcholine-specific PLC. An aliquot of the reacted sperm suspension was extended for processing glass slides and another aliquot was kept overnight in methanol to facilitate permeabilization of the sperm (Sheikhnejad & Srivastava, Reference Sheikhnejad and Srivastava1986).
Smears from both samples were washed in Dulbecco's PBS and then incubated in blocking solution (PBS–2% BSA) for 30 min. The first incubation was performed with the primary antibody anti-PC-PLC obtained from Bacillus cereus at a 1:100 dilution of serum from immunized rabbits (New Zealand variety). Antibodies (IgG) were isolated and purified from antiserum by column chromatography on an Affi-Prep Protein A matrix.
As the negative control we incubated 12-h smears processed in the same way but without the primary antibody. Mouse embryo fibroblasts were used as the positive control (data not shown). Smears were washed with PBS. The second incubation was performed with a secondary antibody (anti-rabbit IgG) at 1:2000 dilution.
After washing with PBS the samples were incubated with streptavidin–alkaline phosphatase for 30 min. at 25 °C. We performed a final wash with PBS and nitroblue tetrazolium (NBT) was added prepared from 0.023 g/ml. The reaction was stopped with the addition of distilled water.
Reagent
Progesterone, n-butanol, neomycin, phospholipids, ruthenium red, heparin, 1,5-isoquinolinesulfonyl-2-methylpiperazine (H7) and PC-PLC from Bacillus cereus were provided by Sigma and D-609 by Calbiochem.
Anti-PC-PLC antibody was obtained from rabbits immunized with PC-PLC from Bacillus cereus kindly provided by Dr H. Borsetti, University of Houston, USA.
Results
Size exclusion chromatography of sperm extract
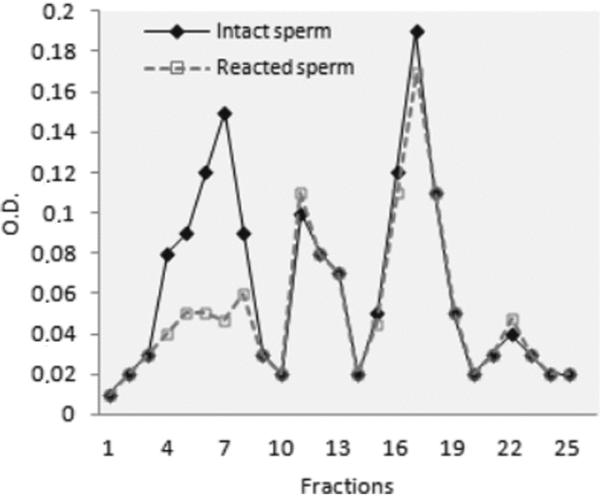
Crude sperm extract of intact sperm and acrosome-reacted sperm, obtained according to Materials and Methods, were subjected to chromatography. Twenty-seven fractions were obtained. As shown in Fig. 1, the chromatogram exhibited differences in peak I in the extract obtained from intact sperm or from acrosome-reacted sperm, the other peaks being similar. All fractions were microinjected into mature oocytes to test for biological activity.

Figure 1 Size exclusion chromatography of intact sperm extract and acrosome-reacted sperm extract. The solid line indicates intact sperm extract (–) and the broken line indicates acrosome-reacted sperm extract (– –). The protein was detected at OD280. Aliquots of all the fractions were injected into oocytes matured in vitro. Only fraction 18, corresponding to peak III of the reacted and non-reacted sperm, was able to induce activation when it was microinjected into the oocytes matured in vitro (+).
Only fraction 18, corresponding to peak III of reacted and non-reacted sperm, was able to induce activation when injected into mature oocytes.
FPLC of fraction 18
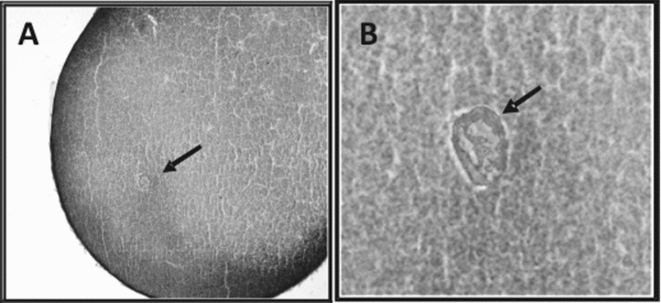
Fraction 18 (with biological activity) obtained from size exclusion chromatography was subjected to a Mono Q column using an FPLC system. As shown in Fig. 2, three peaks (F1, F2, F3) were obtained with this procedure. The first peak corresponds to washing the column. The fractions belonging to the three peaks were injected to determine their ability to activate mature oocytes. Activation was scored 20 min after injection and pronuclear development was observed after 120 min of culture (Fig. 3). Only the first peak (F1) of the Mono Q column using FPLC showed biological activity when it was microinjected. The activity of F1 disappeared when it was heated for 3 min at 90 °C. Microinjections with Tris-saline had no effect on oocyte activation.

Figure 2 Mono Q column using an fast protein liquid chromatography (FPLC) system of fraction 18 from size exclusion chromatography of sperm extract. Fractions (0.30 ml) from each peak were collected (F1, F2, F3) and concentrated to give a final protein concentration of 0.1 μg/μl.

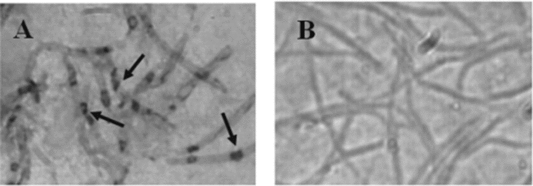
Figure 3 Cytological preparations. (A) Formation of female pronucleus 120 min post-microinjection of F1 (magnificatioin ×400). (B) Hematoxylin–eosin (magnification ×1000). Arrows show the pronucleus.
Biochemical characterization of F1
Electrophoretic analysis of the active sperm fractions F1
F1 from FPLC, which exhibited highest biological activity, were analyzed by SDS-PAGE.
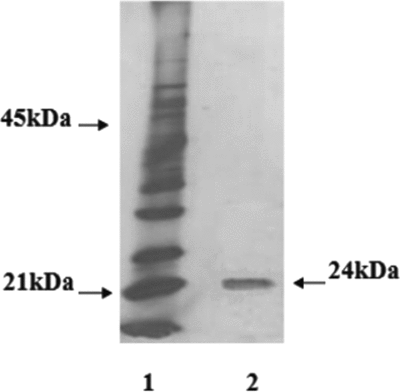
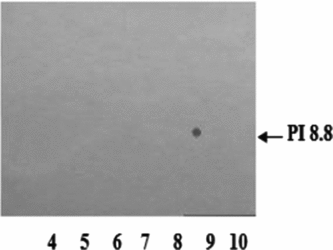
Electrophoretic profiles obtained by SDS-PAGE (Fig. 4) indicate that the fraction with biological activity (F1) is a protein of approximately 24 kDa and isoelectric point (IEP) of 8.8 (Fig. 5).

Figure 4 Electrophoresis of F1. Lane 1, Molecular weight markers. Lane 2, F1 from the Mono Q column.

Figure 5 Isoelectric point of F1. F1 was subjected to bidimensional electrophoresis. Silver staining.
Determination of phospholipase activity of F1
In order to determine if F1 had phospholipase activity, different concentrations of F1 were seeded in a Petri dish with egg yolk agar at 1%.
As shown in Fig. 6, the halo around the wells indicates hydrolysis on the phospholipids present in the egg yolk in a dose-dependent manner, suggesting the presence in F1 of a protein with phospholipase activity.

Figure 6 Effect of different F1 concentrations on egg lipids. F1 was seeded on egg yolk agar. The halo produced by F1 was measured at 60 min.
Effect of F1 on different phospholipids
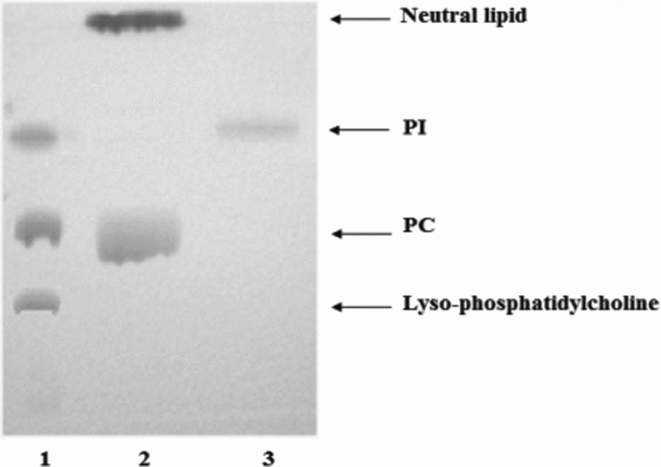
TLC from hydrolytic products from F1 on PC and PI are shown in Fig. 7. Results indicated that F1 hydrolyzed only phosphatidylcholine with neutral lipids formation without generating lyso-PC. In addition, F1 was unable to hydrolyze PI. These results indicate that F1 would not have PLA2 activity.

Figure 7 Thin layer chromotography (TLC) of hydrolysis products from phosphatidylcholine (PC) and phosphatidylinositol (PI). PC and PI were exposed to F1 for 60 min at 37 °C. The lipids obtained were recovered in chloroform/methanol and processed with thin layer chromatography. Lane 1, markers (lyso-PC, PC and PI). Lane 2, product of hydrolysis from PC. Lane 3, product of hydrolysis from PI.
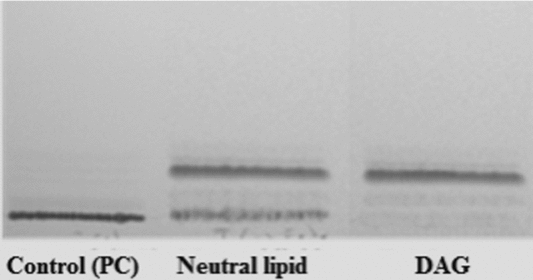
The analysis of the neutral lipids generated by the effect of F1 on PC (Fig. 8) showed that F1 was able to hydrolyze PC with DAG production, which would indicate that it has PLC activity.

Figure 8 Thin layer chromotography (TLC) of neutral lipids formed in the incubation of phosphatidylcholine (PC) with F1. The neutral lipids formed in the incubation of PC with F1 were subjected to thin layer chromatography. TLC revealed the presence of 1,2 diacylglycerol. Commercial diacylglycerol (DAG; Sigma) was used as a control.
Treatment of fraction F1 with phospholipase inhibitors
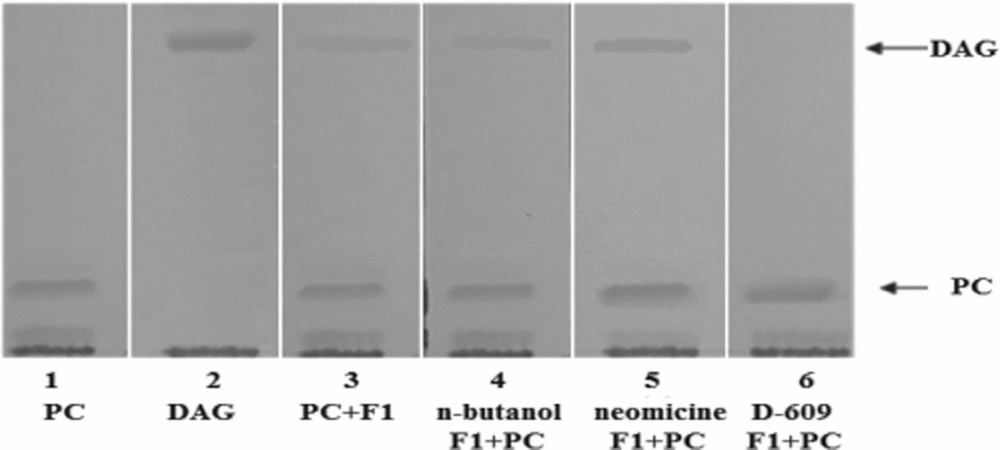
The ability of F1 to hydrolyze PC in the presence of different inhibitors was analyzed. Fraction F1 was pretreated for 30 min with different phospholipase inhibitors: neomycin (5 mM), a specific inhibitor of PI-PLC, D-609 (50 μg/ml), a specific inhibitor of PC-PLC, and n-butanol (50 μg/ml), a specific inhibitor of phospholipase D (PLD).
The results in Fig. 9 show that the activity of F1 was inhibited by D-609 but not by neomycin or n-butanol.

Figure 9 Thin layer chromotography (TLC) of the products resulting from the effect of F1 on PC in the presence of inhibitors. 1, phosphatidylcholine; 2, diacylglycerol (DAG) as control; 3, hydrolysis products of phosphatidylcholine by F1; 4, phosphatidylcholine, n-butanol and F1; 5, phosphatidylcholine, neomycin and F1; 6, phosphatidylcholine, D-609 and F1.
The lack of inhibition by n-butanol or neomycin suggests that F1 is not a PLD or a PLC specific for PI. The fact that the action of F1 was inhibited only by D-609 suggests that it would be a PLC specific for PC.
Effect of PC-PLC from Bacillus cereus on oocytes matured in vitro
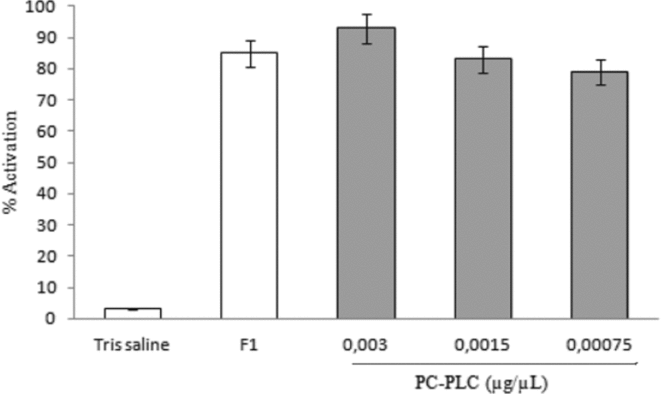
In order to determine whether a commercial PC-PLC has the same effect as the F1 fraction, the oocytes matured in vitro were injected with different concentrations of PC-PLC (0.003; 0.0015, 0.00075 μg/μl) and activation was scored 20 min after injection.
The results obtained (Fig. 10) indicated that the injection of this enzyme induced activation at percentages similar to the ones obtained by injection of the F1 fraction.

Figure 10 Effect of microinjection of phospholipase C (PLC) that is specific for phosphatidylcholine (PC-PLC) from Bacillus cereus. Oocytes matured in vitro were injected with 30 nl of PC-PLC from Bacillus cereus (0.003, 0.0015, 0.00075 μg/μl). Values are the mean ± standard error of the mean (SEM) of three experiments. Each experiment was performed on a different animal.
Immunolocation of PC-PLC
The cellular location of the PC-PLC in sperm was studied using antibodies obtained by immunization of rabbits with PC-PLC from Bacillus cereus. The assays were performed with sperm obtained by swim-up method and permeabilized according to Materials and methods. The images reveal the presence of PC-PLC distributed throughout the sperm head showing a discontinuous pattern (Fig. 11). The acrosomal status was confirmed by scanning electron microscopy from aliquots of sperm suspensions (Fig. 12).

Figure 11 (A) Immunolocation of phospholipase C (PLC) that is specific for phosphatidylcholine (PC-PLC) in Rhinella arenarum spermatozoa (magnification ×1600). (B) Negative control (magnification ×1600). Arrows indicate the presence of PC-PLC in sperm heads.

Figure 12 Scanning micrograph of Rhinella arenarum sperm. Magnification ×3600. AC, acrosome.
Participation of IP3R and RyR on oocytes activation induced by F1
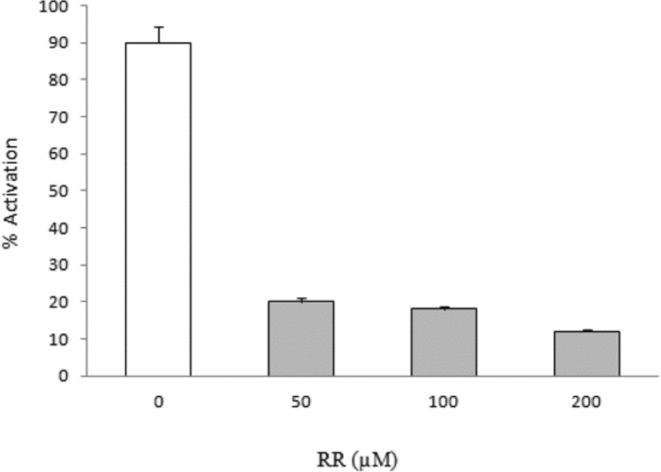
Oocytes matured in vitro were microinjected with different heparin doses (0–1 μM), antagonist of the IP3 receptor or ruthenium red (50–200 μM) antagonist of RyR for 15 min. Then they were microinjected with F1 (0.01 mg/ml). The apparition of signs of activation was assessed after 20 min. The results in Fig. 13 indicate the inhibition of the RyR significantly blocks the activation induced by F1 in a dose-dependent manner, suggesting the participation of this receptor in the process.

Figure 13 Inhibition of the activation produced by F1 with ruthenium red (RR). The oocytes were preinhibited with RR and subsequently microinjected with fraction F1. Activation was measured at 20 min.
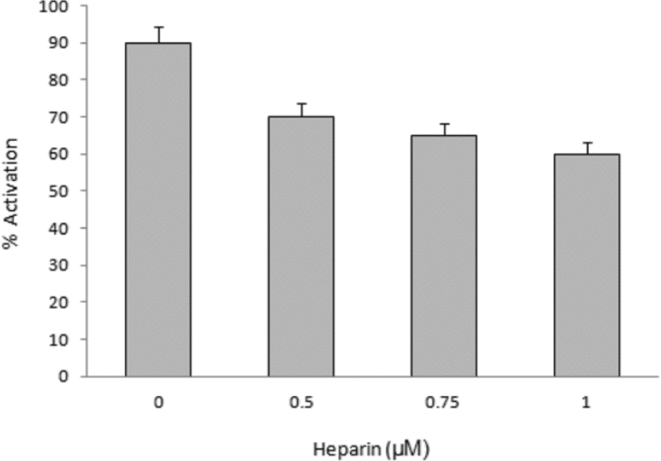
In the case of the inhibition of the IP3/IP3 receptor, a decrease was observed in the percentage of activation induced by F1 (30%) after treatment with heparin, but activation was not blocked, suggesting that the participation of this receptor would not be critical in the process (Fig. 14).

Figure 14 Inhibition of the inositol triphosphate (IP3) receptor on the activation induced by microinjection of F1. The oocytes were preinhibited with heparin and subsequently microinjected with fraction F1. Activation was measured at 20 min.
Effect of inhibition of PKC on oocytes activation induced by F1
Taking into account that PC-PLC produces DAG, a PKC activator, we studied the effect of the inhibition of this kinase on the activation induced by F1 injection.
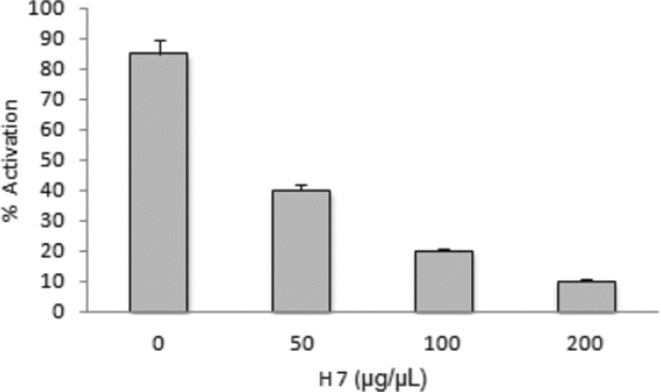
We used 1,5-isoquinolinesulfonyl-2-methylpiperazine (H7) as PKC inhibitor. The oocytes pretreated with H7 for 15 min were microinjected with F1 (0.01 mg/ml). The signs of activation were assessed 20 min after F1 injection. The results (Fig. 15) showed that H7 inhibited the activation induced by F1 in a dose-dependent manner.

Figure 15 Effect of inhibition of protein kinase C (PKC) on the activation induced by microinjection of F1. H7 was microinjected (0, 50, 100 or 200 μg/μl) and 20 min later activation was induced with F1.
Discussion
During fertilization, sperm fusion triggers an increase in egg cytosolic Ca2+ that traverses the egg in a wave-like fashion (Nuccitelli et al., Reference Nuccitelli, Yim and Smart1993; Horner & Wolfner, Reference Horner and Wolfner2008). These transient increases in [Ca2+]i are essential for cortical granule exocytosis, meiosis resumption and pronuclear formation (Bement & Capco, Reference Bement and Capco1989; Oterino et al., Reference Oterino, Sánchez Toranzo, Zelarayán and Bühler1997; Ducibella et al., Reference Ducibella, Huneau, Angelichio, Xu, Schultz, Kopf, Fissore, Madoux and Ozil2002). The rise in IP3 concentration is responsible for inducing Ca2+ release from the endoplasmic reticulum (ER) by binding to the IP3 receptor (Jaffe et al., Reference Jaffe, Giusti, Carroll and Foltz2001). The increase in IP3 is caused mainly by the hydrolysis of phosphatidyl 4,5-bisphosphate (PIP2) by a family of specific phospholipases.
Our results indicated the presence of a 24 kDa protein with PLC activity in the biologically active sperm fraction of Rhinella arenarum.
The experiments in vitro using phospholipid inhibitors indicated the presence of PLC activity specific for phosphatidylcholine (PC-PLC) in the biologically active fraction of the sperm extract of Rhinella arenarum. It is interesting to note that the biological activity is similar in the whole sperm extract and in the reacted sperm extract, which would indicate that this enzyme would not be an acrosomal enzyme.
This type of phospholipase (PC-PLC) has been identified in preparations from bull sperm (Sheikhnejad & Srivastava, Reference Sheikhnejad and Srivastava1986; Hinkovska-Galchev & Srivastava, Reference Hinkovska-Galchev and Srivastava1992).
The concentration of phospholipids present in the mature oocytes of Rhinella arenarum is as follows: phosphatidylcholine 186.23 nmol/mg of protein, phosphatidylethanolamine 41.2 nmol/mg of protein, phosphatidylinositol 11.3 nmol/mg of protein, sphingomyelin 8.5 nmol/mg of protein, phosphatidylserine 4.0 nmol/mg of protein (Sánchez Toranzo et al., Reference Sánchez Toranzo, Oterino, Zelarayán, Bonilla and Bühler2007). Phosphatidylcholine, the most abundant phospholipid in this species, plays a major role in intracellular signalling methods and is a source of lipidic second messengers such as DAG, phosphatidic acid (PA) and arachidonic acid (AA) (Sánchez Toranzo et al., Reference Sánchez Toranzo, Oterino, Zelarayán, Bonilla and Bühler2007; Mateos et al., Reference Mateos, Uranga, Salvador and Giusto2008).
Stith et al. (Reference Stith, Goalstone, Silva and Jaynes1993) showed that DAG and IP3 levels increase during Xenopus egg activation (Berridge, Reference Berridge1984). Their work indicates that Xenopus fertilization triggers a large increase in egg DAG (two- to four-fold) and IP3 (up to seven-fold). DAG increase is rapid, occurring within a minute of sperm addition, as would be expected if PKC activation controlled events such as cortical granule (CG) exocytosis and cortical contraction (Bement & Capco, Reference Bement and Capco1989; Bement, Reference Bement1992; Douglas & Kline, Reference Douglas and Kline1992, Wu X et al., Reference Wu, Zhang, Li, Cheng, Kuai, Wang and Guo2006). They also found that approximately 99% of the DAG increase was derived from non-PIP2 sources. DAG may also act as a fusogen during cortical granule–plasma membrane fusion (Whitaker & Zimmerberg, Reference Whitaker and Zimmerberg1987; Petcoff et al., Reference Petcoff, Holland and Stith2008). The DAG mass increases from 48 pmol to 110 pmol/cell post fertilization (Stith et al., Reference Stith, Woronoff, Espinoza and Smart1997).
These previous data support our results, in which we describe the presence in the Rhinella arenarum sperm extract of a protein with PC-PLC activity. This enzyme, which presents PLC activity in vitro, is capable of forming DAG and is inhibited by D-609 but not by neomycin or n-butanol. D-609 is a potent inhibitor of PC-PLC, while neomycin and n-butanol are specific inhibitors of PI-PLC and of PLD respectively. This result suggests that the biologically active protein in the sperm extract would be a PC-PLC. DAG production by the effect of the PC-PLC present in the sperm extract would be responsible for the activation of the homologous oocytes.
Our results of the injection of a commercial PC-PLC into mature oocytes, which induced activation at percentages similar to the ones obtained by the injection of the F1 fraction, confirm the presence of a PC-PLC in the active fraction of sperm extract in this species. However, the molecular weight of the protein isolated in our active fraction is 24 kDa, quite small if we compare it with the 74 kDa of the PLCζ described in mammals (Kurokawa et al., Reference Kurokawa, Yoon, Alfandari, Fukami, Sato and Fissore2007), or with the 66 kDa of the PC-PLC of mouse fibroblasts (Ramoni et al., Reference Ramoni, Spadaro, Barletta, Dupuis and Podo2004, Spadaro et al., Reference Spadaro, Cecchetti, Sanchez, Ausiello, Podo and Ramoni2006) or with the 40 kDa of human PC-PLC (Ramoni et al., Reference Ramoni, Spadaro, Menegon and Podo2001; Roldan & Shi, Reference Roldan and Shi2007; Szumiło & Rahden-Staroń, Reference Szumiło and Rahden-Staroń2008). It is possible that PC-PLC may have undergone physiological proteolysis due to the extraction methodology. In this sense, Kurokawa et al. (Reference Kurokawa, Yoon, Alfandari, Fukami, Sato and Fissore2007) demonstrated that, in mammals, PLC fragments (27 kDa) in sperm factor retain activity, suggesting that spontaneous proteolysis of PLCs may play a physiological role in cellular function.
The finding that PLC fragments consistent with enzyme cleavage can be observed in fresh intact sperm and in sperm extracts (Kurokawa et al., Reference Kurokawa, Sato, Wu, He, Malcuit, Black, Fukami and Fissore2005, Reference Kurokawa, Yoon, Alfandari, Fukami, Sato and Fissore2007) suggests that the 24 kDa protein with PC-PLC activity identified in the Rhinella arenarum sperm extract could also be a product of the proteolysis of the enzyme.
In sea urchin eggs, in vivo studies have demonstrated that PKC phosphorylates and solubilizes the sperm nuclear lamina in a Ca2+-dependent manner (Stephens et al., Reference Stephens, Beyer, Balthazar-Stablein, Duncan, Kostacos, Lukoma, Green and Poccia2002), and that PKC is required for the eggs respiratory burst after activation (Heinecke & Shapiro, Reference Heinecke and Shapiro1992).
Other reports on mammalian eggs have attributed a role to PKC in the regulation of cortical granule exocytosis, resumption of the cell cycle, and second polar body formation at fertilization (Halet et al., Reference Halet, Tunwell and Parkinson2004). In sea urchins, DAG may also contribute to Ca2+ release from the ER by stimulating a pathway leading to the production of cyclic ADP ribose, which mobilizes Ca2+ from RyRs on ER (Horner & Wolfner, Reference Horner and Wolfner2008; Dale & DeFelice, Reference Dale and DeFelice2011).
Our results indicated that the inhibition of PKC by treatment with H-7 inhibits the activation produced by the sperm fraction, suggesting that this second messenger is implicated in the signalling route (Fig. 15), which would support the idea that in the biologically active fraction of sperm extract there would be a protein with PC-PLC activity that would be responsible for inducing the activation in the mature oocytes of Rhinella arenarum.
In the mature oocytes of Rhinella arenarum the presence of RyRs has been described, their participation in the activation process having also been demonstrated (Ajmat et al., Reference Ajmat, Bonilla, Hermosilla, Zelarayán and Bühler2013). Our results show that if the RyRs is blocked, F1 is unable to induce activation, thus confirming the importance of this receptor in the activation of the oocytes of this species. There is data from other species that support this pathway, since the microinjection of cyclic ADP ribose or ryanodine in mouse oocytes is capable of inducing oocyte activation (Ayabe et al., Reference Ayabe, Kopf and Schultz1995).
The immunomarkation of Rhinella arenarum sperm with anti-PC-PLC antibodies shows that the PC-PLC is distributed in the whole sperm head and that it is intracytoplasmic, since in the assays performed with non-permeabilized sperm no signalling was observed (Hinkovska-Galchev & Srivastava, Reference Hinkovska-Galchev and Srivastava1992).
The PLCδ was found in the lipid rafts present in the plasma membrane, as reported previously by Yamaga et al. (Reference Yamaga, Kawai, Kiyota, Homma and Yagisawa2007).
The heterogeneous distribution of immunostaining in the head of Rhinella arenarum sperm (Fig. 11) could be attributed to the presence of PC-PLC related to lipid rafts (Kawano et al., Reference Kawano, Yoshida, Miyado and Yoshida2011).
In conclusion, in this study we demonstrated that a fraction of the extract of reacted sperm of Rhinella arenarum purified by FPLC was able to induce activation when it was microinjected into homologous oocytes matured in vitro and has a 24 kDa protein with PLC activity specific for phosphatidylcholine. Immunolocation revealed that the enzyme is found throughout the sperm head. The transduction pathway of this enzyme involves de activation of PKC and the mayor participation of RyRc in the release mechanisms of Ca2+.
Although the amount of proteins microinjected into the oocytes is greater that the amount present in a single spermatozoon, the present work proposes a mechanism of oocyte activation with a future biotechnological application that directly involves a phospholipase specific for PC.
Acknowledgements
This work was supported by a grant from the Science Council of the National University of Tucumán (CIUNT) and the National Agency for the Promotion of Science and Technology (FONCYT). We are grateful to Dr Hugo Borsetti for providing antibodies.