Introduction
Dopamine (DA) is a key brain neurotransmitter that contributes to the control of different behaviors, including locomotion, cognition, reward, and motivation.Reference Biesdorf, Wang and Topic1–Reference Kegeles, Abi-Dargham and Frankle4 Its activity is mediated by two receptor families: the “D1-like” family includes D1 and D5 receptors that stimulate adenylyl cyclase activity, while the “D2-like” receptors (D2, D3, and D4) inhibit the production of cAMP and also regulate other systems, including K+ channels, AKT (AKT serine/threonine kinase)–GSK3 (glycogen synthase kinase 3 beta)–βarrestin as well as intracellular calcium levels.Reference Gingrich and Caron5–Reference Beaulieu, Gainetdinov and Caron7
Alterations of dopaminergic functions have been associated with the pathophysiology of different brain disorders, including Parkinson’s disease, attention-deficit hyperactivity disorder (ADHD), bipolar and mood disorders, schizophrenia, and drug addiction. The association between DA and schizophrenia is particularly complex since a dopaminergic hyperactivity in the mesolimbic regions appears to contribute to psychotic symptoms, such as hallucinations and delusions, as opposed to a dopaminergic hypoactivity in cortical regions, which underlies the negative symptoms and cognitive deficits of the disease. The management of these “opposite” dopaminergic dysfunctions may represent a major challenge for pharmacological intervention. With this respect, currently available antipsychotic drugs (APDs) are quite effective in managing the positive symptoms of schizophrenia, but they are less effective in addressing the negative symptoms and cognitive deficitsReference Leucht and Davis8 even if a partial agonist like aripiprazole has been shown to improve quality of life in schizophrenic patients compared to paliperidone.Reference Potkin, Loze and Forray9 On these bases, a better control of these core symptoms, which often persist during periods of clinical stability and can be severe enough to impair the daily functional activities of patients,Reference Nemeth, Laszlovszky and Czobor10, Reference Tsapakis, Dimopoulou and Tarazi11 represents a critical aspect for the improvement of the clinical outcome.
Considering that DA-related dysfunction represents a hallmark in schizophrenia, there is always a great deal of interest in developing novel strategies to modulate the “dopaminergic” function with the aim to address clinical “unmet needs.” Among different potential targets, there has been an increasing interest in DA D3 receptors (D3Rs), whose modulation may improve the outcome of schizophrenia treatment. In this review, we discuss the contribution of these receptors in the etiology and pathophysiology of schizophrenia and the potential benefits that may be associated with a more selective targeting of DA D3Rs by APDs, as compared to other dopaminergic and non-dopaminergic receptor subtypes.
From D2 to D3 Receptor
DA D3Rs, which were identified in 1990,Reference Sokoloff, Giros, Martres, Bouthenet and Schwartz12 show higher affinity for endogenous DA, as compared to D2, and their distribution is restricted to limbic regions, including the islands of Calleja, the shell of nucleus accumbens (NAc), and the olfactory tubercles, with much lower levels of expression in basal ganglia or other brain structures. Although restricted, the distribution of D3Rs appears to be critically involved in the regulation of important functions, such as motivation, emotion, and reward as well as cognition,Reference Sokoloff, Giros, Martres, Bouthenet and Schwartz12–Reference Sokoloff and Le Foll14 which represent key pathologic domains for several psychiatric disorders, including schizophrenia.Reference Sokoloff, Diaz and Le Foll15
DA D3Rs are scarcely found in the majority of DA symmetric synapses, while they are detected at the levels of asymmetric synapses at the head of dendritic spines, a localization that is in sharp contrast with DA D1Rs and DA D2Rs, which are either pre-synaptic or spread all over dendrites and dendritic spines in neurons of the caudate putamen and NAc.Reference Sokoloff and Le Foll14, Reference Stahl16 Since asymmetrical synapses are typically glutamatergic (Glu) and may be located at some distance from DA terminals, it is expected that DA D3Rs may play a peculiar role in the modulation of neurotransmitter circuitry. Indeed, on the basis of the higher affinity of endogenous DA for D3Rs, over other DA receptors, it has been hypothesized that DA D3Rs would be less sensitive to rapid (phasic release) than slower (tonic release) changes in DA concentration. Moreover, considering that phasic release in mesolimbic areas mediates the responses to salient stimuli (such as reward-relevant event or potential threat), while tonic release mediates the amplitude of the response,Reference Grace17 it is feasible that enhanced DA D3Rs sensitivity would result in the aberrant assignment of salience to elements one’s experiences as it may occur in schizophrenia.Reference Kapur18 Moreover, D3Rs may exert a tonic inhibition of DA neurons in the ventral tegmental area (VTA) projecting to the NAc by stimulating GABA release, whereas D3Rs expressed on dopaminergic neurons of the VTA inhibit DA synthesis and release. Taken together, these observations support a negative control of D3-mediated signaling on DA neurons, either by acting directly on its autoreceptors (located both at nerve terminals and in the cell bodies) or by modulating GABA release, which eventually leads to a downregulation of DA release in PFC.Reference Sokoloff, Diaz and Le Foll15, Reference Stahl16
Similar to the majority of GPCR, D3Rs may form homo- and heterodimersReference Maggio, Scarselli, Capannolo and Millan19 with D2RsReference Scarselli, Novi and Schallmach20 and D1Rs,Reference Fiorentini, Busi, Spano and Missale21, Reference Marcellino, Ferre and Casado22 as well as with the adenosine A2 receptors,Reference Torvinen, Marcellino and Canals23 and they may also interact with nicotinic acetylcholine receptors (nAChRs),Reference Collo, Bono and Cavalleri24 a property that could increase their functional heterogeneity. Moreover, it has been recently shown that D3Rs positively regulate several intracellular pathways such as Erk1/2 and Akt through G protein-dependent as well as independent mechanisms,Reference Cussac, Newman-Tancredi, Pasteau and Millan25, Reference Collo, Zanetti, Missale and Spano26 suggesting that their functional activity may be different depending on the interactions with other membrane receptors or transduction proteins, a concept known as bias agonism.
The high density of the D3Rs in the ventral striatum, as compared to the dorsal part, increased the expectation that selective D3 antagonists would exert antipsychotic activity with minimal or no side effects including extrapyramidal side effects (EPS)Reference Schwartz, Diaz, Pilon and Sokoloff27 and catalepsy.Reference Sokoloff and Le Foll14, Reference Gyertyan and Saghy28 Moreover, antagonists at D3Rs can increase cognitive performance and may reverse cognitive deficits in rodents and monkeys.Reference Nakajima, Gerretsen and Takeuchi29–Reference Watson, Loiseau, Ingallinesi, Millan, Marsden and Fone33 Additionally, D3Rs are implicated in executive functions that are often disrupted in schizophrenia.Reference Lumme, Aalto, Ilonen, Nagren and Hietala34 Interestingly, overexpression of D3Rs specifically in the ventral striatum is sufficient to decrease motivation, an important component of the negative symptoms in schizophrenia, and this may be due to secondary effects on DA D1Rs.Reference Simpson, Winiger, Biezonski, Haq, Kandel and Kellendonk35 While it can be inferred that D3R antagonism represents a relevant strategy to ameliorate the negative symptoms, a major unmet need in the treatment of schizophrenia,Reference Sokoloff, Diaz and Le Foll15, Reference Gross, Wicke and Drescher36, Reference Joyce and Millan37 it must be pointed out that DA D3R stimulation may also be neurotrophic and neuroprotective on DA neurons during development.Reference Collo, Zanetti, Missale and Spano26, Reference Van Kampen and Eckman38
On these bases, partial agonism at D2Rs and D3Rs may represent a promising approach, according to the concept of “dopamine stabilization,” since a single compound may increase or decrease dopaminergic activity according to the state of a given circuit.Reference Mailman and Murthy39, Reference Pich and Collo40 Specifically, in patients with schizophrenia, this strategy reduces the hyperactivity of the dopaminergic tone in the mesolimbic regions while increasing dopaminergic hypoactivity in the frontal cortex. The first partial D2/D3 agonist approved for the treatment of schizophrenia was aripiprazole, and there are now two drugs that share a similar mechanism of action: brexpiprazole and cariprazine.Reference Frankel and Schwartz41 We will specifically focus on cariprazine, based on its prominent affinity for DA D3Rs over other DA receptor subtypes.
Cariprazine
Cariprazine is a piperazine derivative developed by Gedeon-Richter in Hungary. In 2015, the drug was approved in the USA for the treatment of schizophrenia and for the treatment of acute manic or mixed episodes associated with bipolar I disorder. Cariprazine has a unique pharmacodynamic profile rendering it different from other typical and atypical APDs.Reference Miyamoto, Duncan, Marx and Lieberman42 Indeed, it is a partial agonist at DA D2Rs and D3Rs as well as 5-HT1A receptors while acting as antagonist at 5-HT2A and 5-HT2B receptors. Moreover, it shows low to moderate affinity for other neurotransmitter receptors that may be responsible for the occurrence of important side effects.Reference Kiss, Horvath and Nemethy43
Cariprazine shares unique pharmacological signatures with two other DA partial agonists: aripiprazole and brexpiprazole in terms of their partial agonist activity at DA D2Rs, D3Rs, and 5-HT1ARs, as well as antagonistic activity at 5-HT2ARs. However, cariprazine has the strongest affinity for DA D3Rs, as partial agonist, followed by aripiprazole and brexpiprazole, whereas brexpiprazole has the strongest affinity for DA D2Rs, as a partial agonist, followed by aripiprazole and cariprazine.Reference Frankel and Schwartz41, Reference Veselinovic, Paulzen and Grunder44–Reference Stahl46 Cariprazine’s selective actions as a potent DA D3R partial agonist [intrinsic activity of 0.70]Reference Kiss, Horvath and Nemethy43 may stabilize abnormalities in DA neurotransmission in different brain regions including the cerebral cortex and therefore may improve negative symptoms and cognitive deficits in schizophrenia patients. The activity of cariprazine on 5-HT1ARs and 5-HT2ARs may further improve psychotic or manic symptoms while maintaining a benign EPS profile.Reference Frankel and Schwartz41, Reference Veselinovic, Paulzen and Grunder44, Reference Goff45
Cariprazine is safe and effective at the dose range of 1.5–6 mg daily. It is mainly metabolized by CYP3A4 (and, to a lesser extent, CYP2D6), generating two active metabolites (desmethyl cariprazine and di-desmethyl cariprazine). The steady state is reached around weeks 1–2 for cariprazine and desmethyl cariprazine and around weeks 4–8 for di-desmethyl cariprazine.Reference Nakamura, Kubota, Iwakaji, Imada, Kapas and Morio47 The presence of these two active metabolites may prolong the efficacy of the parent compound, although more information is needed with respect to the efficacy of such metabolites. The pharmacokinetic of cariprazine and its metabolites are not affected in a clinically relevant degree by CYP2D6 poor metabolizer status, age, weight, sex, or race.Reference Garnock-Jones48 Cariprazine and its metabolites are weak inhibitors of CYP1A2, CYP2C9, CYP2D6, CYP3A4, CYP2C19, and CYP2E1. Moreover, pharmacokinetic interactions of cariprazine with substrates of these enzymes are not likely to occur, while the dose of cariprazine has to be reduced if co-administered with a strong CYP3A4 inhibitor such as ketoconazole. The association with CYP3A4 inducers, such as carbamazepine, is not recommended.
Since 2008, several studies and reviews have been published on the efficacy, safety, and tolerability of cariprazine in humans.Reference Garnock-Jones48, Reference De Berardis, Orsolini and Iasevoli49 As an example, cariprazine was effective in adult patients diagnosed with schizophrenia and generally well tolerated in three 6-week randomized double-blind, placebo-, and/or active-controlled phase-II and phase-III studies. The treatment was not associated with alterations in metabolic parameters, prolactin production, prolongation of QT interval, or substantial increases in body weight.Reference Calabrese, Keck and Starace50 Nevertheless, the incidence of akathisia and EPS was higher with cariprazine than with placebo. Accordingly, open-label extension studies (NCT00839852–NCT01104792) reported that both low and high doses of cariprazine were generally well tolerated and did not result in any new safety concerns.Reference Durgam, Cutler and Lu51–Reference Cutler, Durgam and Wang55 Moreover, cariprazine treatment was generally associated with a low rate of sedation and weight gain.Reference Ketter, Sachs and Durgam56–Reference Nasrallah, Earley and Cutler58
Notably, one of the main unmet needs in schizophrenia is the limited ability of APDs to improve negative symptoms. It is important to underline that negative symptoms may be distinguished between primary (an integral part of the disease) and secondary that develop as a consequence of the positive symptoms or as side effects of some APDs.Reference Buchanan, Breier, Kirkpatrick, Ball and Carpenter59 Therefore, it is important to perform clinical trials that enroll only patients with primary negative symptoms. In this context, a recent randomized, placebo-controlled clinical trial that compared the effects of cariprazine vs. risperidone on negative symptoms in schizophrenia patients found a significant superiority of one APD versus another given in monotherapy.Reference Nemeth, Laszlovszky and Czobor10 Cariprazine showed a significant effect on primary negative symptoms after 14 weeks of treatment and continued to improve until the endpoint, at week 26. The reduction of the Positive and Negative Syndrome Scale factor score for negative symptoms (PANSS-FSNS) was paralleled by a greater improvement in functioning (self-care, interpersonal relationship, and social activities), with a consequent increase in the quality of lifeReference Nemeth, Molnar and Akehurst60 as previously shown for aripiprazole but not with paliperidoneReference Potkin, Loze and Forray9 emphasizing the added value of the partial agonism activity.
Molecular Effects of Cariprazine
A series of studies examined the long-term effects of cariprazine administration on regulation of different DA (D1, D2, and D3), 5-HT (5-HT1A and 5-HT2A), and glutamate (NMDA and AMPA) receptor subtypes in rat forebrain regions that represent limbic, cortical, and extrapyramidal brain systems, and then compared the effects to other typical and atypical antipsychotics on the same receptors from previous studies to determine if cariprazine would induce atypical-like effects on forebrain neurotransmitter receptors.
DA receptors
Long-term administration of cariprazine resulted in significant increases in DA D3R levels in olfactory tubercle, Islands of Calleja, and shell of NAc.Reference Choi, Adham, Kiss, Gyertyan and Tarazi61 Cariprazine-induced increases in forebrain DA D3Rs appear to be unique to this drug since the repeated administration of other typical and atypical APDs including haloperidol, fluphenazine, clozapine, olanzapine, risperidone, and asenapine failed to alter levels of DA D3Rs.Reference Tarazi, Florijn and Creese62–Reference Tarazi, Moran-Gates, Wong, Henry and Shahid65 It appears that cariprazine with its potent DA D3R affinity and occupancyReference Kiss, Horvath and Nemethy43, Reference Gyertyan, Kiss and Saghy66 is able to replace endogenous DA and occupy DA D3Rs to the level required to trigger receptor upregulation.Reference Zhang, Weiss, Tarazi, Kula and Baldessarini67 DA receptor upregulation is typically observed with repeated administration of DA receptor antagonists. However, despite its DA D3R partial agonist activity, cariprazine increased DA D3Rs, which may suggest it is acting as an antagonist at D3Rs in vivo. Cariprazine may normalize disturbances in DA D3R-mediated neurotransmission in patients with schizophrenia and bipolar mania, and improve their mood, cognitive, and executive functions.Reference Harvey, Wingo, Burdick and Baldessarini68, Reference Baldessarini, Tarazi, Brunton, Lazo and Parker69
Repeated administration of cariprazine also increased DA D2Rs in frontal cortex and hippocampus, an effect shared by other atypical APDs.Reference Tarazi, Zhang and Baldessarini64, Reference Tarazi, Moran-Gates, Wong, Henry and Shahid65 Such changes may contribute to the beneficial therapeutic effects of cariprazine in schizophrenia and bipolar mania. Higher doses of cariprazine increased DA D2Rs in basal ganglia, which may account for the higher incidence of akathisia (9% vs. 5%) and extrapyramidal disorder (12% vs. 5%), compared with placebo-treated patients, in clinical trials.Reference Citrome70, Reference Citrome71
Serotonin and glutamate receptors
The long-term effects of cariprazine were not limited to DA receptors. Repeated administration of cariprazine increased 5-HT1ARs in the cerebral cortex, an effect consistent with the effects of other atypical APDs such as olanzapine, risperidone, quetiapine, and asenapine on the same receptor in the same brain.Reference Tarazi, Moran-Gates, Wong, Henry and Shahid65, Reference Choi, Adham, Kiss, Gyertyan and Tarazi72, Reference Tarazi, Zhang and Baldessarini73 The effects of these APDs may result from direct blockade of 5-HT1A receptors or from secondary effects as a result of combined actions of these drugs on D2 and 5-HT2A receptors. These effects further validate cortical 5-HT1AR as common targets that mediate the beneficial actions of cariprazine and other atypical antipsychotics. Interestingly, long-term administration of cariprazine did not alter 5-HT2AR levels in the cerebral cortex. In contrast, several other atypical APDs significantly decreased these receptors in the same brain region, suggesting that 5-HT2ARs are less likely to contribute to the mechanism of action of cariprazine in vivo.Reference Tarazi, Moran-Gates, Wong, Henry and Shahid65, Reference Choi, Adham, Kiss, Gyertyan and Tarazi72, Reference Tarazi, Zhang and Baldessarini73 It is possible that long-term cariprazine treatment with the selected doses did not achieve the in vivo occupancy of 5-HT2A receptors needed to trigger changes in the concentrations of these receptors in different brain regions.
Long-term administration of cariprazine decreased NMDA receptors in caudate putamen and NAc, an effect shared by atypical and not typical APDs.Reference Choi, Adham, Kiss, Gyertyan and Tarazi72, Reference Tarazi, Baldessarini, Kula and Zhang74–Reference Tarazi, Florijn and Creese76 Downregulation of striatal NMDA receptors by cariprazine and several atypical APDs may contribute, at least in part, to the benign extrapyramidal profile of atypical antipsychotic agents.Reference Tarsy, Baldessarini and Tarazi77 Cariprazine also decreased NMDA and increased AMPA receptors in the hippocampus, which may improve psychotic symptoms by normalizing abnormalities in hippocampal Glu neurotransmission postulated to occur in schizophrenia patients.Reference Choi, Adham, Kiss, Gyertyan and Tarazi72, Reference Tsai and Coyle78
Behavioral Effects of Cariprazine
Acute administration of cariprazine was effective in behavioral tests with face validity for the positive symptoms of schizophrenia, including the blockade of amphetamine-induced hyperactivity, inhibition of apomorphine-induced climbing, as well as antagonism of the locomotor stimulating effect of non-competitive NMDA antagonists.Reference Gyertyan, Kiss and Saghy66
Cariprazine’s effects on cognitive functions were investigated using animal models based on the administration of the muscarinic antagonist scopolamine or the non-competitive NMDA receptors antagonist phencyclidine (PCP). Acute injection of cariprazine was able to normalize scopolamine-induced deficits in a water labyrinth task with a bell-shaped dose-response pattern, while risperidone, olanzapine, and aripiprazole were less effective.Reference Gyertyan, Kiss and Saghy66 Moreover, acute cariprazine pretreatment (0.08–0.15 mg/kg) significantly attenuated deficits on social recognition memory (hippocampal/perirhinal function), spatial working memory (SWM), and extradimensional attention set-shifting (prefrontal cortex-dependent), disrupted by acute PCP treatment.Reference Zimnisky, Chang, Gyertyan, Kiss, Adham and Schmauss79 Importantly, the positive effects of cariprazine were not observed when the drug was given to PCP-treated DA D3R KO mice, suggesting that, despite the complex mechanisms through which PCP elicits cognitive deficits, DA D3R modulation is critical in mediating the effects of cariprazine.Reference Zimnisky, Chang, Gyertyan, Kiss, Adham and Schmauss79
A recent study has shown that 5 days of PCP injection increased incorrect, premature, and timeout responses in the 5-choice serial reaction task.Reference Barnes, Young, Markou, Adham, Gyertyan and Kiss80 Interestingly, and different from aripiprazole, a 3-day treatment with cariprazine at a dose of 0.03 mg/kg attenuated PCP-induced deficits without producing non-specific response suppression.Reference Barnes, Young, Markou, Adham, Gyertyan and Kiss80 Neill and colleagues have also produced evidence on the ability of cariprazine to normalize the behavioral abnormalities observed after a sub-chronic treatment with PCP in female rats. PCP-induced alterations in cognition and social behavior, which were still present one week at the end of PCP administration, were normalized by a single dose of cariprazine (0.05 mg/kg) administered 1 h before the behavioral tests. Interestingly, risperidone (0.16 mg/kg) was only able to attenuate the PCP-induced avoidance, suggesting a larger effect of cariprazine.Reference Neill, Grayson, Kiss, Gyertyan, Ferguson and Adham81 The efficacy of cariprazine was also investigated in an experimental model that combines PCP treatment and social isolation. This model is of particular interest since the manipulations are conducted early in life and caused long-term neurodevelopmental, behavioral, structural, and neurochemical alterations with a translational relevance for a spectrum of symptoms seen in schizophrenia.Reference Reinwald, Becker and Mallien82 Interestingly, a single dose of cariprazine (0.1–0.3 mg/kg) or aripiprazole (3 mg/kg) reduced the hyperactivity and reversed the cognitive deficits in the novel object recognition (NOR) test that were observed in rats exposed to a combination of PCP and social isolation.Reference Watson, King, Gyertyan, Kiss, Adham and Fone83 Moreover, only cariprazine was able to correct pro-social behavioral and body-sniffing, which may reflect a potential effectiveness of cariprazine, but not aripiprazole, in treating negative symptoms.Reference Watson, King, Gyertyan, Kiss, Adham and Fone83
Recent studies have also shown that cariprazine is able to exert antidepressant- and anxiolytic-like behaviors in stress-based models, which mimic an important etiological mechanism for clinical depression and anxiety.Reference Duric, Banasr and Franklin84, Reference Papp, Gruca, Lason-Tyburkiewicz, Adham, Kiss and Gyertyan85 In particular, prolonged treatment with cariprazine was able to normalize the anhedonic-like behavior, measured as reduction of sucrose intake, induced by chronic stress exposure, an effect that appears to rely on the ability to modulate D3Rs.Reference Duric, Banasr and Franklin84, Reference Papp, Gruca, Lason-Tyburkiewicz, Adham, Kiss and Gyertyan85 Indeed, even if DA D3R knock-out (KO) mice do not exhibit anxiety and depressive-like behavior,Reference Chourbaji, Brandwein and Vogt86, Reference Leggio, Micale and Drago87 and the effect of prolonged stress exposure is similar between wild-type and DA D3R KO mice, cariprazine treatment was not able to normalize anhedonia in transgenic mice exposed to chronic stress.Reference Duric, Banasr and Franklin84 Cariprazine, similar to aripiprazole, was also able to attenuate the anxiolytic-like behavior in chronically stressed rats, as indicated by its ability to reduce drinking latency in the novelty-induced hypophagia test.Reference Duric, Banasr and Franklin84 Furthermore, it has been recently demonstrated that cariprazine, possibly through the modulation of D3Rs, increases DA, serotonin, and norepinephrine efflux in rat NAc and hippocampus, an effect that may also contribute to the procognitive, prosocial, and antipsychotic-like actions of cariprazine in animal models.Reference Meltzer, Huang, He, Kiss, Farkas and Adham88
Conclusions
In summary, cariprazine represents a novel APD with a peculiar receptor signature that is primarily characterized by a partial agonism at DA D3Rs and D2Rs, with higher affinity for the former receptor subtype when compared to partial agonists such as aripiprazole and brexpiprazole. Interestingly, preclinical studies, as summarized in Table 1, have clearly demonstrated the efficacy of cariprazine not only in regulating positive symptoms but also on negative symptoms and cognitive deterioration of schizophrenia. While the precise mechanism of action of cariprazine remains to be determined, its high affinity for the DA D3Rs is likely to play a key role, as supported by studies conducted in DA D3 KO mice.
Similar to other APDs, cariprazine improves positive symptoms primarily through its activity on D2R. Conversely, the partial agonism at the D3R may represent the main mechanism through which the drug ameliorates negative symptoms and cognitive deficits. Indeed, several evidences support the concept that D3Rs can participate in the complex and heterogeneous alterations of the dopaminergic system in schizophrenia. In particular, an overexpression of the D3Rs on the dopaminergic neuron projecting from the VTA to the PFC, therefore acting as autoreceptor, may reduce the dopaminergic activity leading to a hypofunction at cortical level. Such defects can be ameliorated by cariprazine that, by modulating these receptors, will ultimately improve the treatment of negative symptoms as well as cognitive deficits, which represent important elements for the functional disability found in schizophrenia patients (Figure 1).
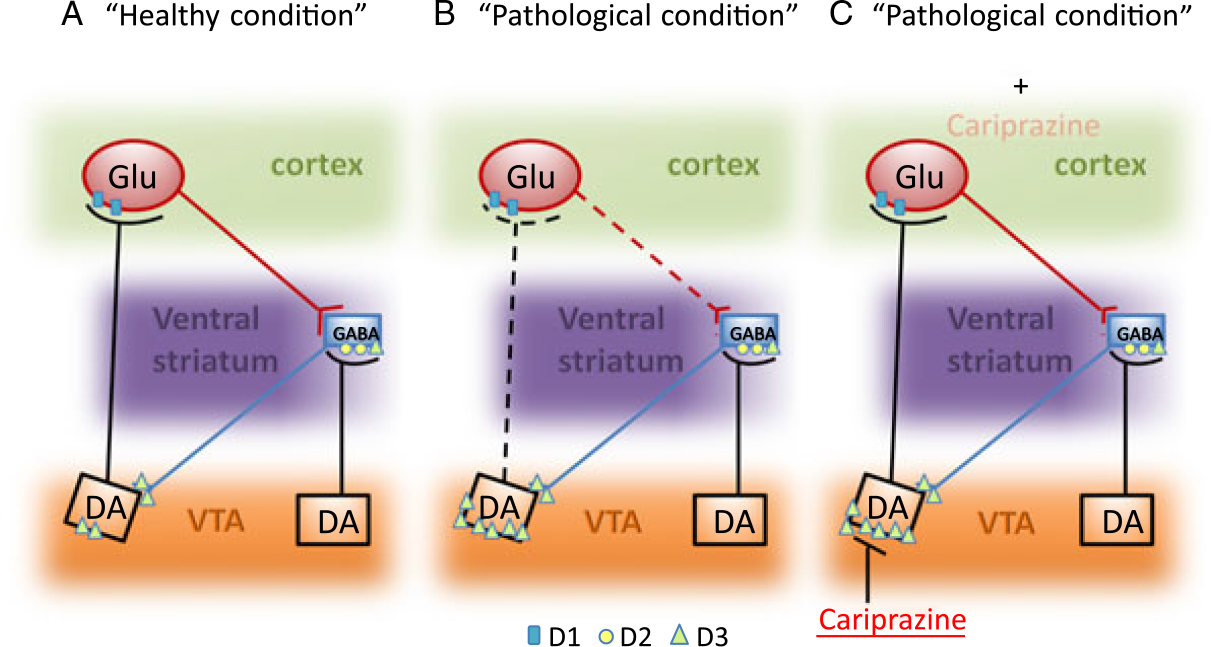
FIGURE 1. Schematic representation of DA D3R dysfunction in schizophrenia and the potential impact of cariprazine treatment. In pathological condition (panel B), as compared to the healthy condition (panel A), the overexpression of the D3Rs located in the DA neuron projecting from the VTA to the cortex leads to an inhibition of DA release in the prefrontal cortex, which may eventually reduce the Glu output. Cariprazine (panel C), by acting as partial agonist on the DA D3Rs of the mesocortical pathways, may reduce the “pathologic” inhibition thus leading to a normalization of DA release within the prefrontal cortex.
TABLE 1. Summary of the D3 receptor preclinical studies

Disclosures
Marco A. Riva received compensation as speaker/consultant from Lundbeck, Otzuka, Sumitomo Dainippon Pharma, and Sunovion, and received research grants from Lundbeck, Sumitomo Dainippon Pharma, and Sunovion. Frank I. Tarazi received research grants from Lundbeck and Sunovion. Giorgio Racagni received consulting fees and/or honoraria from Indena, Servier, and Recordati. Francesca Calabrese has nothing to declare.




