Introduction
Several species in the family Phytoseiidae are important natural enemies used to control mite pest outbreaks in many crops (McMurtry & Croft, Reference McMurtry and Croft1997). Specific diagnostic is, thus, of primary importance for the success of biological control programs. This family is widespread all over the world and includes three sub-families and more than 2000 valid species (Chant & McMurtry, Reference Chant and McMurtry2003a,Reference Chant and McMurtryb, Reference Chant and McMurtry2004a,Reference Chant and McMurtryb, Reference Chant and McMurtry2005a,Reference Chant and McMurtryb, Reference Chant and McMurtry2006a,Reference Chant and McMurtryb,Reference Chant and McMurtry2007; Moraes et al Reference Moraes, McMurtry, Denmark and Campos2004; Kreiter & Tixier, Reference Kreiter and Tixier2006). Species of the genus Phytoseiulus Evans (sub-family Amblyseiinae) are the most frequently used for the biological control of mite pests, especially Phytoseiulus persimilis Athias-Henriot, a species that has been widely released in greenhouses all over the world. This paper focuses on specimens morphologically assigned to the species Phytoseiulus longipes Evans. In recent surveys carried out in Brazil to look for efficient enemies for controlling Tetranychus evansi Baker & Pritchard, an invasive pest in Africa and Europe, a strain of P. longipes was collected on Solanaceous plants infested by the mite pest. Further laboratory experiments showed the efficiency of this strain to eat and develop on both T. evansi and T. urticae (Ferrero et al., Reference Ferrero, Moraes, Kreiter, Tixier and Knapp2007; Furtado et al., Reference Furtado, Moraes, Kreiter, Tixier and Knapp2007). This result was quite surprising, as a previous study, carried out on specimens of P. longipes initially collected from South Africa and mass-reared in the laboratory, showed that this species was not able to develop and reproduce when fed on T. evansi (Moraes & McMurtry, Reference Moraes and McMurtry1985). Until 2008, P. longipes, thus, was not considered an efficient predator of T. evansi. Since then, other surveys have been performed; and two other populations of P. longipes have been found, in Chile and Argentina. In laboratory experiments, Ferrero et al. (Reference Ferrero, Kreiter and Tixier2008) have shown the ability of the Argentinean population to feed, develop and reproduce on T. evansi and T. urticae. However, the same tests conducted on the Chilean population of P. longipes showed the opposite results (Ferrero, unpublished data). Despite the different feeding habits, all the specimens have been morphologically assigned to the same species, P. longipes. However, several studies have already shown that morphologically similar specimens can belong to different species (Mahr & McMurtry, Reference Mahr and McMurtry1979; McMurtry et al., Reference McMurtry, Mahr and Johnson1976, Reference McMurtry, Badii and Congdon1985; McMurtry & Badii, Reference McMurtry and Badii1989; Tixier et al., Reference Tixier, Kreiter, Cheval and Auger2003, Reference Tixier, Kreiter, Croft and Cheval2004, Reference Tixier, Kreiter, Barbar, Ragusa and Cheval2006, Reference Tixier, Guichou and Kreiter2008). Furthermore, no study, so far, has reported such intra-specific variation in the feeding habits of Phytoseiidae mites. As molecular markers can be of great help to differentiate cryptic species (Hebert et al., Reference Hebert, Cywinska, Ball and deWaard2003), the aim of this study was to determine, using combined morphological and molecular analyses, whether the specimens identified as P. longipes and collected in South Africa, Brazil, Argentina and Chile actually belong to the same species.
Material and methods
Origin of specimens examined
The origin of the specimens of P. longipes considered, the number of females measured and the number of the DNA sequences analysed are outlined in table 1. Once collected, the specimens were maintained in laboratory colonies and reared on T. urticae until morphological and molecular analyses (for 15 days for all populations except those from South Africa). The South African population has been mass-reared for several decades in the USA (Biotactics® 25139 Briggs Road, Romoland, CA, 92585, USA) and is the same population that was used in the study by Moraes & McMurtry (Reference Moraes and McMurtry1985) (Moraes, personal communication). Although it would have been interesting to also consider a freshly collected field population from South Africa, several recent attempts to retrieve this population have been unsuccessful.
Table 1. Characteristics of collection localities of the different populations of Phytoseiulus longipes studied.

Morphological analysis
At least 14 females per strain were mounted on slides in Hoyer's medium and measured with a phase and differential interference contrast microscope (Leica DMLB, Leica Microsystèmes SAS, Rueil-Malmaison, France) (40× magnification) (table 1). Terminology for setal notation used in this paper follows that of Lindquist & Evans (Reference Lindquist and Evans1965) as adapted by Rowell et al. (Reference Rowell, Chant and Hansell1978) for the Phytoseiidae. A total of 32 characters were taken into account. As dorsal seta lengths are usually considered in phytoseiid mites' taxonomy, the 14 dorsal idiosomal setae of the collected females were measured: j1, j3, j4, j6, J5, z2, z4, z5, Z1, Z4, Z5, s4, r3 and R1. Other morphological characters, such as macroseta length of the basitarsus IV, dimensions (length and width) of: the dorsal shield, the sternal shield (distances between seta insertions), the ventrianal shield and the spermatheca, were also taken into account. All measurement values are given in micrometers.
Molecular analysis
DNA was individually extracted from several females per strain, according to the DNA extraction protocol described by Tixier et al. (Reference Tixier, Kreiter, Barbar, Ragusa and Cheval2006) . The DNA fragment used is the 12S rRNA gene, which seems to be useful for species diagnostic (Murrel et al., Reference Murrell, Campbell and Barker2001; Jeyaprakash & Hoy, Reference Jeyaprakash and Hoy2002; Okassa et al., Reference Okassa, Tixier, Cheval and Kreiter2009). Ten specimens of P. persimilis, collected in Montpellier on Phaseolus vulgaris L., were also analysed as a control in order to assess interspecific genetic distances (accession number in Genbank FJ952540, FJ952541, FJ952542, FJ985106, FJ985107, FJ985108, FJ985109, FJ985110, FJ985111, FJ985112). An out-group species was selected from the sub-family Amblyseiinae and the genus Neoseiulus Hughes: Neoseiulus californicus (McGregor). The number of specimens analysed in each population of P. longipes is shown on table 1 along with their Genebank accession numbers.
The primers used to amplify the 12S rDNA were those proposed by Jeyaprakash & Hoy (Reference Jeyaprakash and Hoy2002) for the Phytoseiidae: 5′-3′ TACTATGTTACGACTTAT and 3′-5′ AAACTAGGATTAGATACCC. The PCR was performed in a total volume of 25 μl containing 2 μl of mite DNA, 1 μl of DNTP (2.5 Mm for each nucleotide), 2.5 μl of Taq buffer, 1 μl of each primer (100 μM), 0.5 μl of Taq (Qiagen, 5 U per μl) and 18.9 μl of water. Thermal cycling conditions were as follows: 95°C for 1 min., followed by 35 cycles of 94°C for 30 s, 40°C for 30 s and 72°C for 1 min., and an additionnal 5 min. at 72°C. Electrophoresis was carried out on a 1.5% agarose gel in 0.5×TBE buffer during 30 min. at 100 volts. PCR products were sequenced using the dynamic ET terminator cycle sequencing kit. The sequencer used was the Megabase 1000 apparatus. All DNA fragments were sequenced along both strands. Sequences were aligned and analysed with Mega 4.1. (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007).
Statistical analysis
Morphological data
ANOVA and Tukey HSD mean comparison tests were performed (R Development Core Team, 2009) to determine differences in measurements among the different populations studied. A multifactorial analysis and a discriminant analysis (StatSoft France, 2005) were performed in order to determine if the combination of morphological characters would enable us to differentiate among the four populations.
Molecular data
Sequences were analysed using Mega 4.1 (Tamura et al., Reference Tamura, Dudley, Nei and Kumar2007). The distance matrix was constructed using the Jukes & Cantor (Reference Jukes, Cantor and Munro1969) model, as the transition/transversion rate is 1. A neighbour joining (NJ) tree was constructed. Support was determined using 1000 bootstrap replicates. Even if the NJ algorithm is relatively fast and performs well when the divergence between sequences is low, a potentially serious weakness is that the observed distances are not accurate reflections of their evolutionary distances; multiple substitutions at the same site (i.e. homoplasy) can obscure the true distance and make sequences seem artificially close to each other (Holder & Lewis, Reference Holder and Lewis2003). For this reason, a Bayesian analysis was also performed (Jordal & Hewitt, Reference Jordal and Hewitt2004; Nylander et al., Reference Nylander, Ronquist, Huelsenbeck and Nieves-Aldrey2004). The best-fit substitution model was determined by Modeltest 3.06 (Posada & Crandall, Reference Posada and Crandall1998) through hierarchical likelihood-ratio tests (LRTs). The GTR model of evolution was selected by the LRTs with a proportion of invariable sites and a gamma distribution. The GTR model was implemented in MrBayes 3.1 (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003). The number of categories used to approximate the gamma distribution was set at four, and four Markov chains were run for 1,000,000 generations. Stabilization of model parameters (burn-in) occurred at around 250,000 generations. The results are presented in the form of a 50% majority-rule consensus tree (in which trees corresponding to the burn-in period are discarded) and the support for the nodes of this tree is given by posterior probability estimates for each clade.
Results and discussion
Morphological analysis
Significant differences among specimens from the four localities were observed for 17 of the 32 characters considered (table 2). These differences were very small and standard errors within strains are low. This was confirmed by the results of the discriminant analysis (table 3); all individuals were well classified in their original population (except one), suggesting a greater morphological homogeneity within populations than between populations.
Table 2. Means (Standard Error) of morphological measurements for the four strains of Phytoseiulus longipes considered, and results of the ANOVA. The letters show the differences from the Tukey HSD test (DSL: Dorsal Shield Length; DSW: Dorsal Shield Width; VAS: Ventrianal Shield; StIV, length of the macroseta on the basitarsus IV). Mean, min, max, standard error (SE) and variation coefficient (VC%=SD*100/mean) for the 70 specimens of P. longipes considered.

Table 3. Classification given by the discriminant analysis with 32 characters on four populations of Phytoseiulus longipes. The percentage of well-classified individuals in their original population is represented in %.

The Chilean population differs from the others because of its lower j6 length, longer st1-st1, st2-st2, st3-st3 distances and higher metapodal plate 2 length. Furthermore, the mean lengths of the setae Z4 and Z5 are longer for the populations from Chile and South Africa than for the populations from Brazil and Argentina. On the two axes of the multifactorial analysis (fig. 1) showing 33.13% of the total variation, the Chilean and South African populations are the most distant. The two populations collected in Brazil and Argentina are grouped together and have an intermediate position between the populations from South Africa and Chile. The same observation can be seen in the canonical analysis (fig. 2).

Fig. 1. Scatter plots of the first two multifactorial axes for 32 morphological characters of the four strains of Phytoseiulus longipes considered. Percentages in axis refer to the amount of variation accounted for by the first and second axis in the multifactorial analysis (a, Phytoseiulus longipes from Argentina; b, Phytoseiulus longipes from Brazil; c, Phytoseiulus longipes from Chile; sa, Phytoseiulus longipes from South Africa).
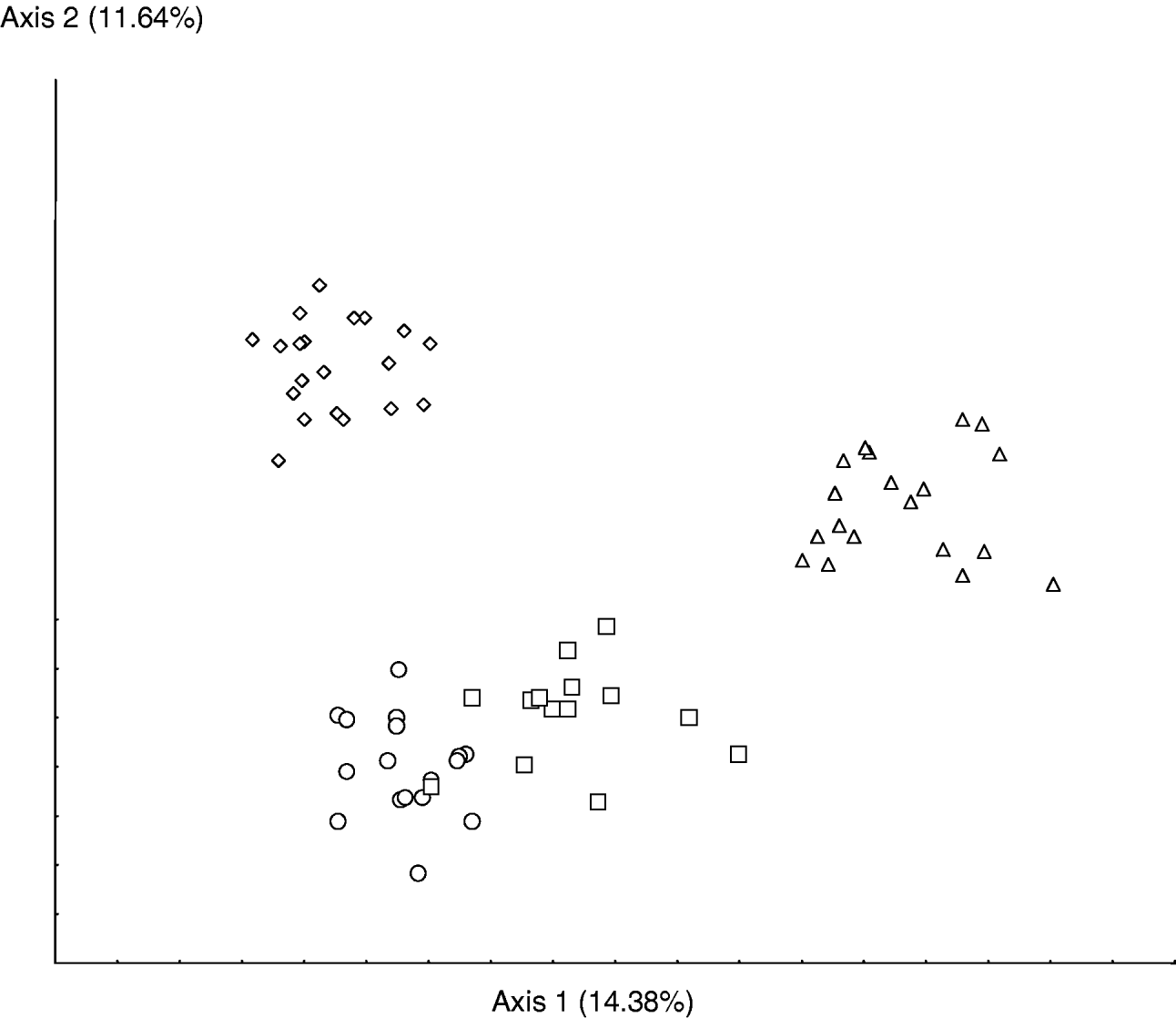
Fig. 2. Scatter plots of the first two canonical analysis axes for 32 morphological characters of the four strains of Phytoseiulus longipes considered. Percentages in axis refer to the amount of variation accounted for by the first and second axis in the multifactorial analysis (○, Phytoseiulus longipes from Argentina; □, Phytoseiulus longipes from Brazil; ◊, Phytoseiulus longipes from Chile; ▵, Phytoseiulus longipes from South Africa).
The four populations of P. longipes studied show different mean measurements and could be morphologically differentiated thanks to a combination of characters. Even if those differences are very small, several studies have already shown that some morphologically similar specimens can belong to different species (McMurtry et al., Reference McMurtry, Mahr and Johnson1976, Reference McMurtry, Badii and Congdon1985; Mahr & McMurtry, Reference Mahr and McMurtry1979; McMurtry & Badii, Reference McMurtry and Badii1989; Tixier et al., Reference Tixier, Kreiter, Cheval and Auger2003, Reference Tixier, Kreiter, Croft and Cheval2004, Reference Tixier, Kreiter, Barbar, Ragusa and Cheval2006, Reference Tixier, Guichou and Kreiter2008). Furthermore, the two populations that are able to feed on T. evansi (from Argentina and Brazil) are morphologically closer to one another than to the two populations that do not feed on T. evansi. However, these latter populations (from Chile and South Africa) are not morphologically similar.
Molecular analysis
A fragment of 388 bp was amplified for the 12S rDNA gene. DNA analysis showed quite similar and constant rates of nucleotide substitutions for all the populations and species studied. Among the amplified 388 bp, 380 were aligned. A BLAST search of the Genbank database showed that the sequences blasted with other 12S rDNA sequences of Phytoseiidae. The best query coverage (100%) was obtained with P. persimilis, Iphiseius degenerans (Berlese), Neoseiulus fallacis (Garman) and Neoseiulus californicus.
The NJ tree and the bayesian analysis show a clear separation between the specimens of P. longipes and those of P. persimilis (figs 3 and 4). The mean genetic distance among the specimens of P. persimilis was 0, whereas this mean distance was 11.8% between P. persimilis and P. longipes (table 4). Nucleotide divergence among P. longipes specimens was low (mean: 0.4%; min=0; max=1%) (table 4). In another study also using the 12S rDNA fragment, Okassa et al. (Reference Okassa, Tixier, Cheval and Kreiter2009) observed genetic distances ranging from 14 to 22% between species of the same genus (Euseius Wainstein) and ranging from 0 to 3% between populations of a same species. Jeyaprakash & Hoy (Reference Jeyaprakash and Hoy2002) obtained an interspecific distance of 9% between two morphological similar species of the genus Neoseiulus (N. californicus and Neoseiulus fallacis) using this same DNA fragment. The weak genetic distances observed between the four populations of P. longipes considered here, thus, suggest that all specimens belong to the same species, despite their different feeding habits on T. evansi. This result is in accordance with the morphological data. However, differentiation between the specimens collected in Brazil/Argentina and Chile/South Africa is observable in the NJ analysis. This difference is also found, to a lesser extent, in the Bayesian analysis; but, here, only the specimens from Chile and South Africa are included in a same sub-clade.

Fig. 3. Neighbour joining tree based on genetic distances (Jukes & Cantor, Reference Jukes, Cantor and Munro1969) between the specimens of Phytoseiulus longipes collected in Argentina, Brazil, Chile and South Africa and specimens of P. persimilis collected on bean at Montpellier (France) with the 12S rDNA fragment. Numbers at nodes correspond to bootstrap values.

Fig. 4. Bayesian analysis tree (GTR) calculated for ‘no gap’ data set with 12S rDNA data on the specimens of Phytoseiulus longipes collected in Argentina, Brazil, Chile and South Africa and specimens of P. persimilis collected on bean at Montpellier (France). Values below branches indicate posterior probabilities.
Table 4. Mean distances of Jukes & Cantor (Reference Jukes, Cantor and Munro1969) for the rDNA 12S gene for the four populations of Phytoseiulus longipes and one population of Phytoseiulus persimilis.

Conclusion
The main conclusion of this study is that the four populations of P. longipes discovered so far belong to the same species. Even if morphological differences exist, they are small; and the low genetic distances between these different populations clearly correspond to intraspecific variation. Intraspecific variation of numerous morphological characters from a great number of specimens for the four known populations of P. longipes has also been assessed for the first time. The present paper, therefore, provides an exhaustive redescription of the species that should be helpful for avoiding misidentifications. Indeed, as already mentionned for other species of Phytoseiidae mites, this study emphasizes high intraspecific variation of setae lengths, a character regularly used to distinguish between species (i.e. Tixier et al., Reference Tixier, Kreiter, Cheval and Auger2003, Reference Tixier, Kreiter, Croft and Cheval2004, Reference Tixier, Kreiter, Barbar, Ragusa and Cheval2006, Reference Tixier, Guichou and Kreiter2008).
The existence of different feeding habits among populations of the same species of Phytoseiidae is quite new for this family. In the present study, weak morphological and molecular differentiation was found between specimens able to develop, feed and reproduce on T. evansi and those which are not. Further experiments, such as cross breeding tests, would be interesting to carry out in order to determine if partial mating isolation exists between populations feeding on different prey species. Furthermore, because the differences we found are small, the use of more discriminant molecular markers (such as microsatellites or the sequencing of more variable DNA fragments such as cytb mtDNA) is required to confirm these preliminary results. The weak differences between these populations could be linked to different factors, such as prey and host plant and/or geographic isolation. Indeed, in the present study, the two populations (from Brazil and Argentina) feeding on T. evansi are geographically very close (<50 km between the two collection sites). However, the two populations that are not able to feed and develop on T. evansi are geographically distant (South Africa and Chile). Local geographic differentiation could explain differences found in the two localities in Brazil and Argentina. Another possibility is that the host plants where the phytoseiids occur play a role in their genetic differentiation. Indeed, the populations from Brazil and from Argentina occur on the same host plants, and they are genetically closer to each other than to the other two populations. Host plants are known to play an important role in Phytoseiid behavioural and life history traits, both in terms of their chemical composition and because of their physical structures (trichomes, domatia) (Walter, Reference Walter1992; Walter & O'Dowd, Reference Walter and O'Dowd1992; Karban et al., Reference Karban, Loeb, Walker and Thaler1995; Walter, Reference Walter1996; Sabelis, Reference Sabelis, Needham, Mitchell, Horn and Welbourn1999; Seelmann et al., Reference Seelmann, Auer, Hoffmann and Schausberger2007; Ferreira et al., Reference Ferreira, Eshuis and Janssen2008). In addition, solanaceous plants are known to be unfavourable plant supports for many arthropod species (Jarosik, Reference Jarosik1990; Skirvin & Fenlon Reference Skirvin and Fenlon2001; Kennedy, Reference Kennedy2003; Koller et al., Reference Koller, Knapp and Schausberger2007), and only a low number of Phytoseiid mite species are naturally encountered on these plants (Moraes et al., Reference Moraes, McMurtry and Denmark1986). The two populations found on Solanaceous plants were able to develop on those plants (Ferrero et al., Reference Ferrero, Moraes, Kreiter, Tixier and Knapp2007, unpublished data). However, laboratory experiments showed that the Chilean population could also develop on tomato when fed with T. urticae (Ferrero et al., Reference Ferrero, Kreiter and Tixier2008), whereas these specimens died on tomato when fed on T. evansi. The South African population also developed well when fed T. urticae on Solanum douglasii Dunal, but incurred high mortality when fed T. evansi on the same plant support (Moraes & McMurtry, Reference Moraes and McMurtry1985). Thus, it seems that the plant support is not a limiting factor and can not account for the differentiation we found among the four populations considered. It is possible that the prey regime accounts for genetic differences among populations, much the same way as differences in host plants can account for genetic differences among herbivore species, including phytophagous mites (Agrawal et al., Reference Agrawal, Vala and Sabelis2002; Tajima et al., Reference Tajima, Ohashi and Takafuji2007; Kant et al., Reference Kant, Sabelis, Haring and Schuuring2008). Possibly, within the species P. longipes, populations are further specialized in a subset of the species' diet. Eubanks et al. (Reference Eubanks, Blair and Abrahamson2003) provided ecological evidence that two subpopulations of a predatory beetle associated with different host races of an insect herbivore are themselves host races. This study is one of the first study that demonstrate that other animals than herbivorous ones, whose life histories are closely associated with a single resource, may also diversify in response to a shift in resource use. The present results do not allow us to accurately characterize the factors affecting inter-populational differentiation. To do so, more populations combining different characteristics would be required. However, up to now, only the four populations we studied are known. Similarly, to determine the relative influence of the different factors, especially the effect of the plant support and prey species on biological parameters of development, laboratory experimental studies are currently being planned. These studies will be of primary importance to ensure the success of biological control programs and to develop strains adapted both to crops and prey species.
Acknowledgements
We are very grateful to Salvatore Ragusa (University of Palermo, Italy), Eddie Ueckermann (Plant Protection Research Institute, South Africa), Markus Knapp (ICIPE, Kenya) and Gilberto de Moraes (ESALQ, Brazil) for sending mites and to Isabelle Olivieri (University of Montpellier, France) for useful discussions. We also thank Karen McCoy (CNRS, Montpellier, France) for her very useful comments and English improvements.










