INTRODUCTION
Archaeological sites often present useful materials for radiocarbon (14C) dating, such as wood, charcoal, shells and remains of animal or human bones. Independently of the kind of sample to be dated, it is necessary to isolate the original carbon of the sample, i.e. to remove the so-called contaminants, which are compounds that may have exchanged carbon with the environment and, therefore, may not record the actual age of the organism. Sample preparation protocols are specific for each kind of material, depending on their chemical composition. Although some of these materials require easier and more straightforward protocols, the choice of material to be used in each context should take into account the availability of samples, the reliability and state of degradation of remains and the information they provide for the archaeological context. Radiocarbon dating of bone requires caution but as long as the original chemical fraction can be successfully isolated, it represents the most reliable record to investigate human activities at archaeological sites (Stafford Jr. et al. Reference Stafford, Hare, Currie, Jull and Donahue1991; Saliege et al. Reference Saliege, Perason and Paris1995; Zazzo et al. Reference Zazzo, Saliège, Person and Boucher2009) or animal presence (Zazzo and Saliège Reference Zazzo and Saliège2011) up to 50 ka BP. The state of preservation of this material depends on the environmental conditions in the location where it is found (Zazzo et al. Reference Zazzo, Saliège, Person and Boucher2009; Zazzo and Saliège Reference Zazzo and Saliège2011). Very old bones are often poorly preserved, requiring extra care during chemical treatment and limiting the success of dating (Snoeck et al. Reference Snoeck, Staff and Brock2016). On the other hand, even recent bones can be degraded when burial soil has acidic pH (Van Klinken Reference Van Klinken1999; Higham et al. Reference Higham, Ramsey, Karavanic, Smith and Trinkaus2006), which is the case of the soils in some regions of Brazil.
Bone tissue is composed of organic and inorganic fractions with a ratio of approximately 20/80. The organic matter is mostly composed of collagen while the inorganic fraction consists of hydroxyapatite, the bone carbonate mineral phase (Stafford Jr. et al. Reference Stafford, Hare, Currie, Jull and Donahue1991). The latter represents most of the bone mass and is more susceptible to diagenesis (Zazzo et al. Reference Zazzo, Saliège, Person and Boucher2009) and contamination with secondary carbonates (Stafford Jr. et al. Reference Stafford, Hare, Currie, Jull and Donahue1991). Although some authors have reported efficient dating of hydroxyapatite (Saliege et al. Reference Saliege, Perason and Paris1995; Zazzo et al. Reference Zazzo, Saliège, Person and Boucher2009; Zazzo and Saliège Reference Zazzo and Saliège2011; Snoeck et al. Reference Snoeck, Staff and Brock2016), collagen is more commonly used for radiocarbon dating (Van Klinken Reference Van Klinken1999; Higham et al. Reference Higham, Ramsey, Karavanic, Smith and Trinkaus2006; Zazzo et al. Reference Zazzo, Saliège, Person and Boucher2009; Harvey et al. Reference Harvey, Egerton, Chamberlain, Manning and Buckley2016) because it does not exchange carbon with the surrounding environment (Hassan et al. Reference Hassan, Termine and Haynes1977). Collagen is a large molecule composed of a variety of amino acids (Schoeninger et al. Reference Schoeninger, Moore, Murray and Kingston1989; Stafford Jr. et al. Reference Stafford, Hare, Currie, Jull and Donahue1991). The presence of characteristic amino acids in bone collagen, such as proline and hydroxyproline, can be used to attest collagen integrity (Ho et al. Reference Ho, Marcus and Berger1969). In the case of poorly preserved bone (less than 5% of the original collagen), it is possible to isolate specific amino acids to be measured (Hassan and Hare Reference Hassan and Hare1978; Stafford Jr. et al. Reference Stafford, Hare, Currie, Jull and Donahue1991; Tripp et al. Reference Tripp, McCullagh and Hedges2006; McCullagh et al. Reference McCullagh, Marom and Hedges2010). This approach requires a more laborious and careful extraction (Hassan and Hare Reference Hassan and Hare1978). The more specific the compound to be isolated the lower is the yield in sample preparation. Fortunately, the accelerator mass spectrometry (AMS) technique enables the measurement of such small samples (Santos et al. Reference Santos, Southon, Griffin, Beaupre and Druffel2007). At the Radiocarbon Laboratory of Universidade Federal Fluminense (LAC-UFF) we have been preparing different sample materials for a variety of applications. Since the LAC-UFF is the only 14C-AMS facility in South America, there is a large demand for dating of all sort of materials, including bones. For this reason, we are working to expand our methods and protocols. In this paper, we report our preliminary results of bone tissue dating performed at LAC-UFF.
MATERIALS AND METHODS
Two samples from the Sixth International Radiocarbon Intercomparison (SIRI), labeled SIRI-B (mammal) and SIRI-C (Mammoth), plus a third sample of modern bone (cow bone) collected in Brazil in 2010, two other samples from the Centre for Nuclear Energy in Agriculture of the University of São Paulo (CENA-USP) previously dated by AMS at CAIS, University of Georgia, USA, and a fragment of human bone from a Brazilian shellmound were used in this preliminary test. The SIRI-B sample is a bone from the North Sea with approximately 40,000 years BP. SIRI-C is a background (~50 ka) sample from Latton Quarry, Wiltshire/Gloucestershire (Scott et al. Reference Scott, Cook and Naysmith2014, Reference Scott, Naysmith and Cook2017). The other two samples, named CENA 913 (SC-URU-27), from Urubici, Santa Catarina state of Brazil, and CENA 920 (C7D7, 50–60 cm), from an archaeological site in Rio Grande do Sul state—395 “Deobaldino,” Sto. Antonio da Patrulha—were previously dated, returning 1180 ± 20 BP and 2790 ± 40 BP, respectively and last a fragment of human bone from the Amourins site, a Brazilian shellmound located near the Guanabara Bay region, Rio de Janeiro state previously dated from 4100–3900 cal BP, based on a chronological model built.
In order to compare the ages of collagen and hydroxyapatite and also to evaluate the contamination during the sample chemical preparation, the modern bone was submitted to both collagen and hydroxyapatite extractions. Since the other samples do not have a consensus value in their ages for hydroxyapatite, they were submitted only to collagen extraction.
Collagen Extraction
For the collagen extraction we followed a protocol based on Longin (Reference Longin1971) plus an ultrafiltration step recommended by Brown et al. (Reference Brown, Nelson, Vogel and Southon1988).
The pretreatment involved acid/base/acid (ABA) steps in ca. 600 mg of crushed bone prior to collagen extraction. In this step the liquids are removed by decantation without filter. The first acid wash is called the decalcification step and it was performed by the addition of approximately 3 mL of 0.5M hydrochloric acid (0.5M HCl) during 36 hr; this step was followed by a sodium hydroxide treatment (0.1M NaOH, 30 min) for the removal of humic acids from the burial environment (Brock et al. Reference Brock, Higham, Ditchfield and Ramsey2010) and then a second acid treatment (0.5M HCl, 15 min). All steps were performed at room temperature. After that, gelatinization was performed by adding 0.01M HCl (pH 3 solution) at 65°C for approximately 20 hr (we defined an upper limit of 24 hr), and finally the samples were filtrated as explained at the ultrafiltration step (Bronk Ramsey et al. Reference Bronk Ramsey, Pettitt, Hedges, Hodgins and Owen2000, Reference Bronk Ramsey, Higham, Bowles and Hedges2004; Higham et al. Reference Higham, Ramsey, Karavanic, Smith and Trinkaus2006; Brock et al. Reference Brock, Bronk Ramsey and Higham2007; Beaumont et al. Reference Beaumont, Beverly, Southon and Taylor2010).
Ultrafiltration
The extraction of collagen was performed using two filters: a Millex 0.45 µm, aiming to remove insoluble contaminants, and a VIVASPIN 30KD ultrafilter, to retain larger molecular weight materials. The latter filter contains a membrane composed by glycerol, which can contaminate the sample, but that is soluble in water. Although, on a general basis, it cannot be stated whether the contamination is modern or old due to origin from animals, plants or petroleum, this latter depleted in radiocarbon (Talamo and Richards Reference Talamo and Richards2011), some researches supposed to be modern contamination (Brock et al. Reference Brock, Bronk Ramsey and Higham2007; Wood et al. Reference Wood, Bronk Ramsey and Higham2010). Regardless of origin, such contamination need to be removed and monitored (Bronk Ramsey et al. Reference Bronk Ramsey, Higham, Bowles and Hedges2004; Brock et al. Reference Brock, Bronk Ramsey and Higham2007; Fülöp et al. Reference Fülöp, Heinze, John and Rethemeyer2013). Because both filters may contain exogenous carbon, they need to be carefully cleaned before use. The cleaning processes of the filters consist in several rinses in UP H2O, as well as centrifugation and ultra-sonication followed as described in Bronk Ramsey et al. (Reference Bronk Ramsey, Higham, Bowles and Hedges2004) and Brock et al. (Reference Brock, Bronk Ramsey and Higham2007). After the first filter, the sample is transferred to a VIVASPIN 30 KD ultrafilter and centrifuged at 3000 RPM in cycles of 10 minutes until 0.5–1.0 mL of solution remains. The solution is stored in a bottle with the same amount of ultrapure water in the freezer for 48 hr before freeze-drying, which occurs for ca. 48 hr (Brock et al. Reference Brock, Higham, Ditchfield and Ramsey2010).
Hydroxyapatite
The chemical pretreatment for hydroxyapatite was performed in an initial amount of approximately 600 mg. The organic matter was removed by the addition of 1.5% sodium hypochlorite (1.5% NaClO) during 48 hr followed by a treatment with 0.1M HCl during 12 hr, both at room temperature. The sample was converted to CO2 by acid hydrolysis in phosphoric acid (85% H3PO4) (Snoeck et al. Reference Snoeck, Staff and Brock2016).
Conversion to Carbon Dioxide
At LAC-UFF, the conversion of inorganic materials to carbon dioxide is typically performed by acid hydrolysis in evacuated septum sealed vials. When performed in organic materials, the conversion occurs by combustion in torch sealed tubes (Oliveira et al. Reference Oliveira, Macario, Carvalho, Moreira and Alves2020) this issue. For collagen fraction, approximately 5 mg of the extracted and dried collagen was placed in a 9-mm quartz tube containing prebaked (at 900°C for 3 hr) CuO (Fisher Scientific, carbon compounds 0.0004%) and silver wire (Aldrich ≥ 99.99% 0.5 mm diameter). Glass wool is inserted at the opening of the tube to prevent the sample from being sucked out of the tube when evacuated. In the case of hydroxyapatite samples, the pretreated material was placed in vials which were then closed with a rubber stopper. The tubes of both types were pumped out using a stainless steel vacuum line (Macario et al. Reference Macario, Oliveira, Carvalho, Santos and Xu2015) either by means of a needle or through ultra-Torr connections. Combustion tubes are sealed with an oxi-acethylene torch and heated in a muffle oven at 900°C for 3 hr. Carbonate vials are injected with 1 mL of 85% H3PO4 and left reacting overnight at room temperature. The gas is purified in a stainless steel vacuum line (Macario et al. Reference Macario, Oliveira, Carvalho, Santos and Xu2015, Reference Macario, Alves, Oliveira, Moreira and Chanca2016) using cryogenic traps (dry ice/ethanol and liquid nitrogen) and transferred into graphitization tubes.
Graphitization and Measurement
The graphite is produced by Zn/TiH2 reduction in independently sealed Pyrex™ tubes at 550°C during 7 hr (Macario et al. Reference Macario, Alves, Oliveira, Moreira and Chanca2016). The Pyrex tubes are prebaked (at 550°C for 7 hr) and prepared before CO2 purification. The so-called graphitization tubes consist of 9-mm Pyrex tubes containing zinc and titanium hydride and 6 mm Pyrex tubes inside the first one containing ca. 5 mg of iron following the procedure described in Macario et al. (Reference Macario, Oliveira, Moreira, Alves and Carvalho2017) and Xu et al. (Reference Xu, Trumbore, Zheng, Southon and McDuffee2007). The samples were measured in the 0.5 MeV Accelerator Mass Spectrometry Center for Applied Isotope Studies (CAIS), Athens, Georgia, USA (Cherkinsky et al. Reference Cherkinsky, Culp, Dvoracek and Noakes2010; Ravi Prasad et al. Reference Ravi Prasad, Cherkinsky, Culp and Dvoracek2015) and in a NEC 250kV Single Stage Accelerator System (SSAMS) (Linares et al. Reference Linares, MacArio, Santos, Carvalho and Dos Santos2015).
RESULTS AND DISCUSSION
The collagen yield of samples varied between 2 and 4 %wt. This characterizes a very satisfactory yield, since collagen contents superior to 1 %wt are accepted as parameters for good preservation of bone (Ambrose Reference Ambrose1990). The yield can be calculated by the ratio between the values of the sample mass after chemical treatment (collagen extracted) and before chemical treatment, whose values are shown in Table 1.
Table 1 Radiocarbon results expressed in 14C ages (BP) and pMC. Mass values calculated before and after chemical treatment. The masses after chemical treatment are the results of collagen extracted. *The previous results for SIRI-B and SIRI-C are reported by Scott et al. (Reference Scott, Naysmith and Cook2017).

It was not possible to calculate the yield to the hydroxyapatite for the modern bone because there are no quality parameters associated with it.
The samples CENA 913 (1180 ± 20 cal BP) and CENA 920 (2790 ± 40 cal BP) were previously measured by collagen extraction without ultrafiltration step in the University of Georgia (UGAMS), Georgia. The previously results and the bones measured at LAC-UFF are summarized in Table 1.
In Figure 1 it is possible to see the percentage of modern carbon (pMC) from SIRI samples and blanks measured in our laboratory. The result for sample C is consistent with a background sample. The apparent difference is easily understood when we take a look at the pMC values of blank samples at LAC-UFF, which are, for instance, 1.061 ± 0.026. There is no significant difference between the hydroxyapatite and collagen radiocarbon ages applied for modern bone and, in this case, both methods can be performed. In Figures 2–8 we can see the calibrated results for bones samples. The results were calibrated using the OxCal v4.2.3 calibration software (Bronk Ramsey Reference Bronk Ramsey2009, Reference Bronk Ramsey2013). The IntCal 13 (Reimer et al. Reference Reimer, Bard, Bayliss, Beck and Blackwell2013) was used in order to calibrate SIRI samples; the post bomb atmospheric SH1-2 curve (Hua et al. Reference Hua, Barbetti and Rakowski2013) for the modern sample and SHCal13 atmospheric curve (Hogg et al. Reference Hogg, Hua, Blackwell, Niu and Buck2013) was used to calibrate the remaining bones from South America. The values presented are not corrected for background.

Figure 1 Blank and bone samples results in pMC (%) versus sample number. The triangle represents SIRI sample B, while the square is SIRI sample C, and dots are blank samples measured at LAC-UFF.

Figure 2 Calibrated date from SIRI sample B.

Figure 3 Calibrated date from SIRI sample C.
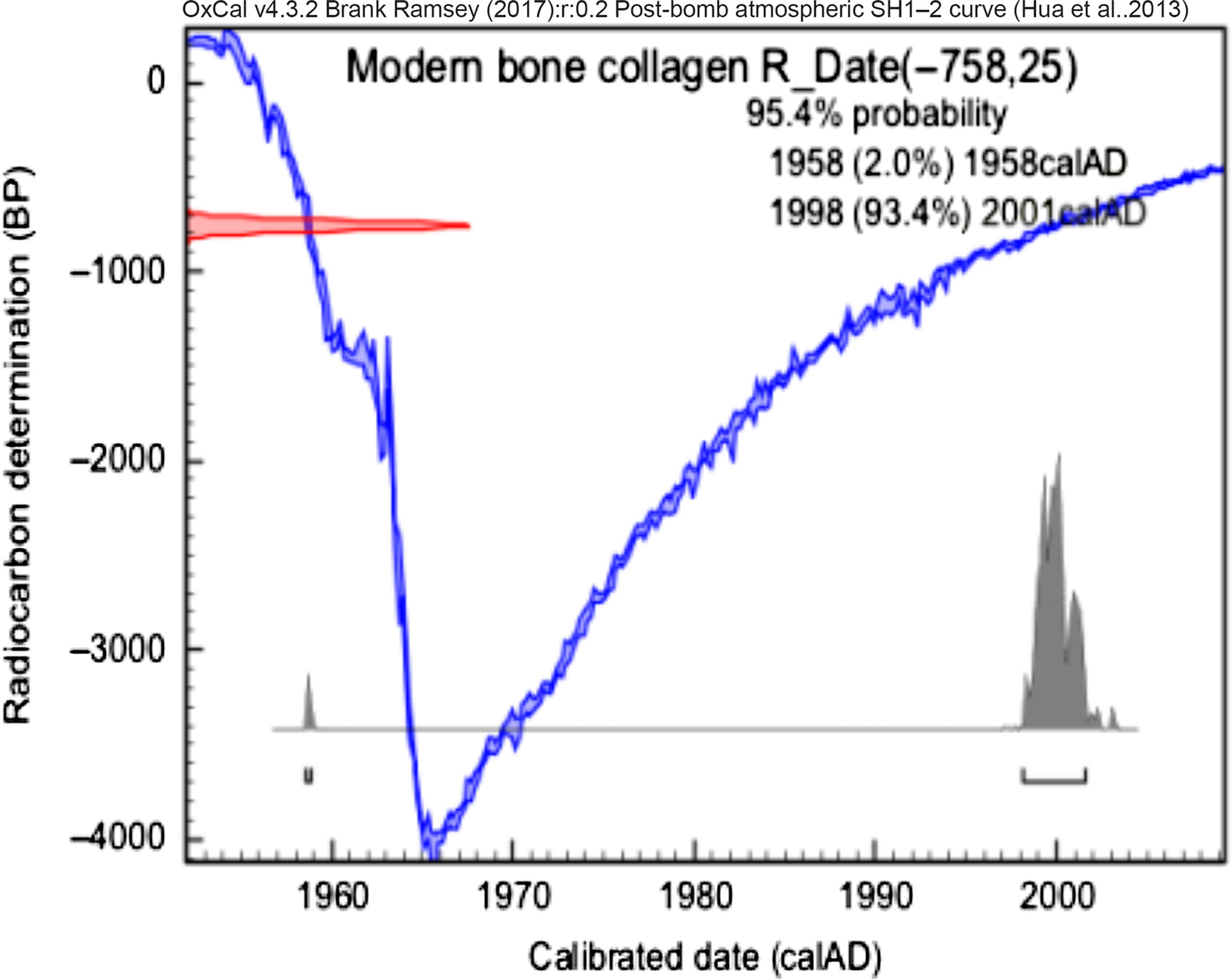
Figure 4 Calibrated date from collagen extraction of modern sample.

Figure 5 Calibrated date from hydroxyapatite extraction of modern sample.

Figure 6 Calibrated date from collagen extraction of CENA913.
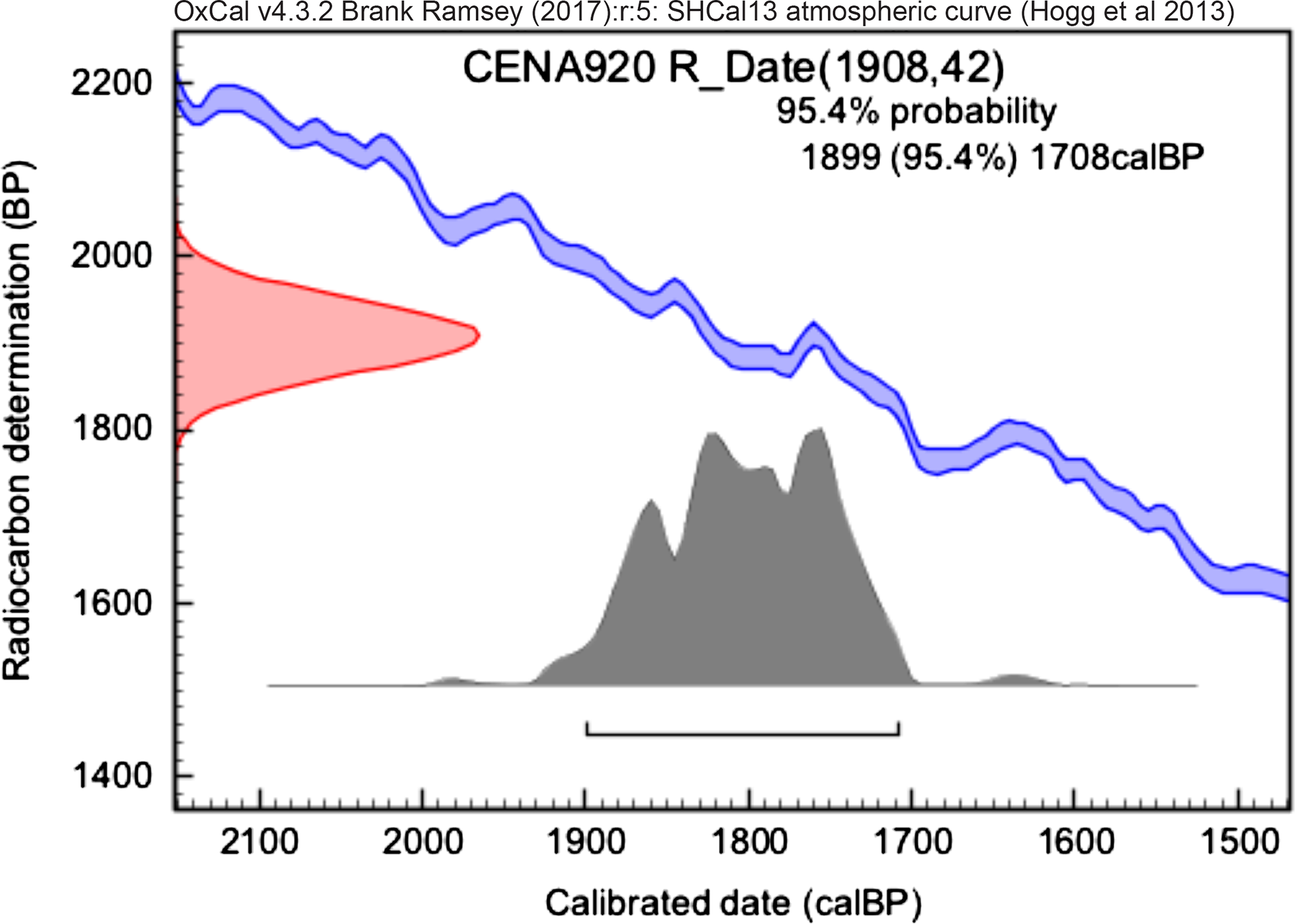
Figure 7 Calibrated date from collagen extraction of CENA920.
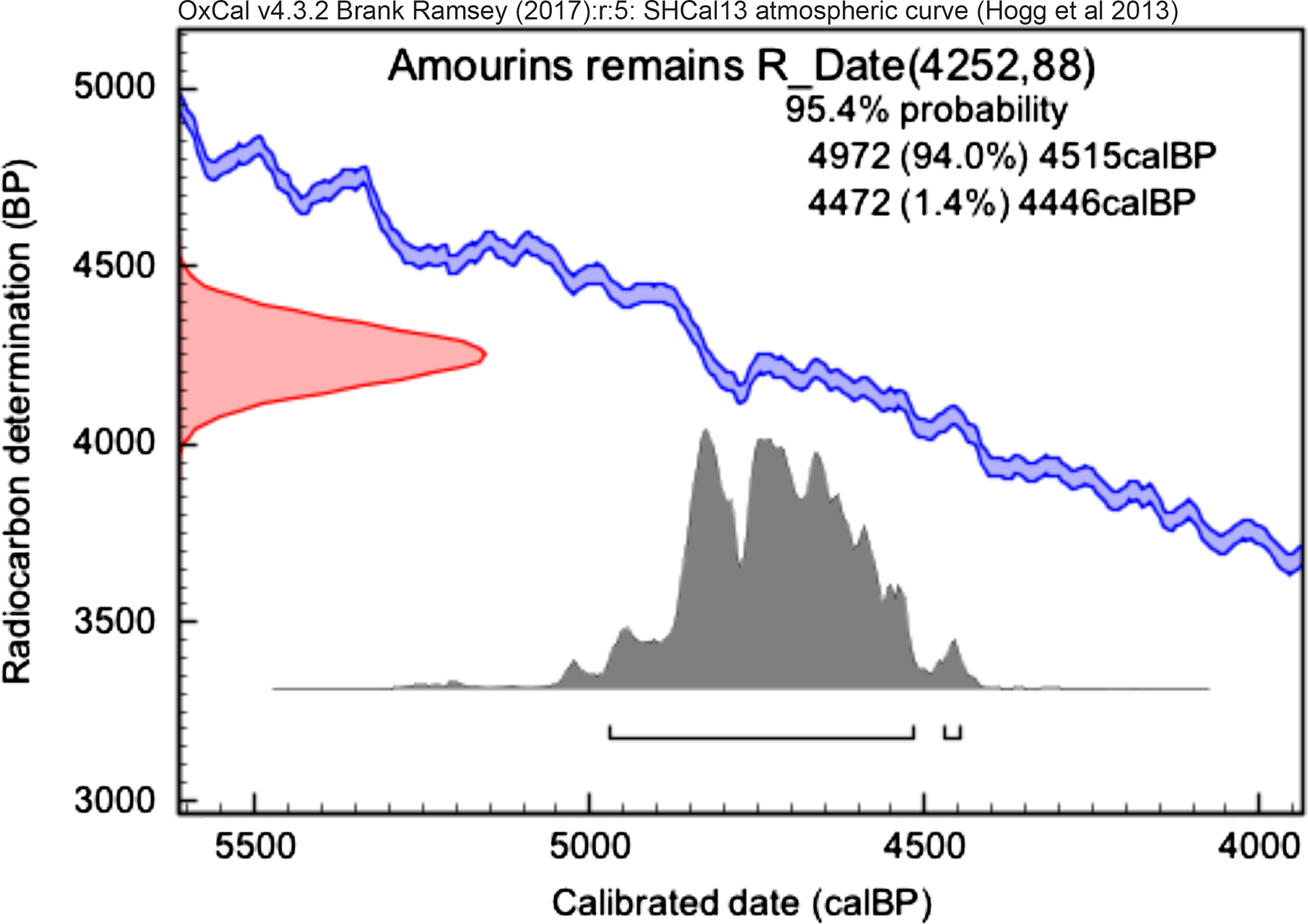
Figure 8 Calibrated date from collagen extraction of Amourins remains.
Compared with the report by Scott et al. (Reference Scott, Cook and Naysmith2014, Reference Scott, Naysmith and Cook2017), the SIRI samples’ results are in agreement with the previous report. Although there are no consensus values published yet, (Szidat et al. Reference Szidat, Vogel, Gubler and Lösch2017) showed that the sample SIRI-C with F14C of approximately 0.002 reveal evidence of background sample, while the age of sample SIRI-B was reported by Bronk Ramsey et al. (Reference Bronk Ramsey, Higham, Bowles and Hedges2004) as approximately 40 kBP. For the latter, Szidat et al. (Reference Szidat, Vogel, Gubler and Lösch2017) found values near 30 kBP and suggested investigating contamination issues. Crann et al. (Reference Crann, Murseli, Xiaolei, Ian and Kieser2017) reported for collagen extraction dates 38,300 ± 300 BP and 44,100 ± 300 BP for SIRI-B and SIRI-C respectively.
The context where the mammoth from the North Sea was collected is an issue discussed by van der Plicht and Palstra (Reference van der Plicht and Palstra2016). They showed results from SIRI samples and reported a 14C date of 39,860 (+350, –310) BP for mammoth femur (SIRI-C), and dates 39,820 (+350, –310) and 39,520 (+340, –300) BP for the sample B measured in the Center for Isotope Research, University of Groningen. Note that the asymmetric errors in BP occur because 14C activities were measured near the detection limit. They report that background samples are not representative for any contamination due to degradation or any carbon exchange process. Therefore, when dating background samples, especially bone samples, extra care should be taken, from chemical treatment to the measurement. Huels et al. (Reference Huels, van der Plicht, Brock, Matzerath and Chivall2017) reported difficulty in comparing between laboratories for bone samples, dated near background (~50 ka). In fact, measurements of background samples requires more effective calculations for background correction. The ultrafiltration method was established by Brown et al. (Reference Brown, Nelson, Vogel and Southon1988) in addition to collagen extraction proposed initially by Longin (Reference Longin1971). In general, different laboratories follow Longin (Reference Longin1971) with some differences in molarity, temperature and duration of chemical procedure (Higham et al. Reference Higham, Ramsey, Karavanic, Smith and Trinkaus2006; Snoeck et al. Reference Snoeck, Staff and Brock2016). Although there is no consensus about the use of ultrafilters (Hüls et al. Reference Hüls, Grootes and Nadeau2009; Fülöp et al. Reference Fülöp, Heinze, John and Rethemeyer2013; Fewlass et al. Reference Fewlass, Tuna, Fagault, Hublin and Kromer2019), this method has been widely used (Zazzo et al. Reference Zazzo, Saliège, Person and Boucher2009). Considering the samples from CENA-USP, the dates obtained are of the same order of magnitude as expected. However, the results indicate that different sample preparation protocols led to significant differences in the determined ages. The radiocarbon laboratory at CENA-USP is a reference in liquid scintillation in Brazil (Pessenda and Camargo Reference Pessenda and Camargo1991; Macario et al. Reference Macario, Gomes, Anjos, Carvalho and Linares2013). The samples described in this paper had insufficient collagen for dating by liquid scintillation and were sent as natural sample to CAIS. The collagen extraction at CAIS does not use the ultrafiltration step during chemical pretreatment. It has been discussed in some studies the differences of the results for bones samples with and without ultrafiltration step (Higham et al. Reference Higham, Ramsey, Karavanic, Smith and Trinkaus2006; Wood et al. Reference Wood, Bronk Ramsey and Higham2010). Higham et al. (Reference Higham, Ramsey, Karavanic, Smith and Trinkaus2006) showed that ages of bones samples dated using ultrafiltration are usually older and more accurate than non-ultrafiltered ones (Higham et al. Reference Higham, Ramsey, Karavanic, Smith and Trinkaus2006). This is supported by the fact that the CENA samples prepared at LAC-UFF (i.e., with ultrafiltration step) indicated results older than the samples analyzed in CAIS.
The result for the collagen extracted from human fragment found in Amourins site is in agreement with the expected for the studied sector of Amourins shellmound. This sector dates from 4100 to 3900 cal BP, based on a chronological model built from the analysis of charcoal samples found in different stratigraphic layers (Brandão et al. in prep.).
CONCLUSIONS AND FUTURE DIRECTIONS
In this preliminary work, we have successfully extracted both the collagen fraction of bone tissue with yield between 2 and 4 %wt and the hydroxyapatite fraction on modern cow bone. The results for the recent bone show that no detectable dead carbon contamination was added by using ultrafilters, as the hydroxyapatite and collagen ages do not differ significantly. For the old samples, the obtained results are consistent with our background levels. In order to better determine the age of samples near background levels it will be crucial to reduce our lower laboratory background. In the future, we are going to prepare a larger set of samples ranging from modern to background ages comparing the two protocols (for hydroxyapatite and collagen extraction) in order to verify the accuracy of both methods and evaluate any possible differences. We are also going to prepare bones from the Megafauna period (between 20 kBP and 10 kBP) in order to evaluate the effect of the acidity in Brazilian soils on radiocarbon dating and the dating of specific amino acids.
ACKNOWLEDGMENTS
The authors would like to thank Brazilian financial agencies CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, 305079/2014-0, INCT-FNA, 464898/2014-5), FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, E-26/110.138/2014) for their support and Brazilian financial agency CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for post-doctoral fellowship at Brazilian AMS Radiocarbon Laboratory (LAC-UFF). Dr. Alexandre Percequillo, ESALQ/USP, Piracicaba, São Paulo, Brazil, and Luciana Cristina de Almeida from Santa Catarina State, Brazil, are thanked for providing bone samples.












