Case report
A 23-year-old primigravida mother, of Iraqi origin, was referred to fetal cardiology at 29 weeks gestation for concerns of an abnormal heart on screening ultrasound. She was married to a first cousin, with no history of known genetic disease in the families. The pregnancy had been uncomplicated. Initial fetal echocardiogram showed normal segmental anatomy and an enlarged heart with a cardiothoracic ratio of 70%. This prompted follow-up assessment, revealing abnormal great arteries; the branch pulmonary arteries were elongated and tortuous with similar tortuosity of the aorta, vessels branching off the aorta, and the ductus arteriosus. There was no stenosis noted and the aortic root was not dilated (Fig 1a, b, c). The cardiac size remained slightly enlarged throughout pregnancy. Given the echocardiographic findings suggestive of a connective tissue disorder and the history of consanguinity, an underlying genetic diagnosis was suspected and an amniocentesis was performed. The fetus tested homozygous for p.S81R pathogenic variant in the SCL2A gene, which provides evidence for the diagnosis of arterial tortuosity syndrome (ATS). Cascade testing revealed the same variant in the mother.
The mother underwent a whole-body computed tomography scan which showed severe tortuosity of her arterial tree. She delivered uneventfully by spontaneous vaginal delivery at term in a high-risk maternity unit. The baby appeared well after birth. Cardiovascular exam was significant for an ejection systolic murmur in the upper left sternal border radiating to both axillae. Echocardiogram showed that the branch pulmonary arteries were long and tortuous with mild flow acceleration. The aorta was also tortuous in its course with no coarctation. Ventricular function was normal without any signs of raised right ventricular (RV) systolic pressure. Screening computed tomography scan at 4 months of age showed diffuse aortopathy characterised by multiple arterial tortuous branches involving the whole arterial system (Fig 2c and d). Areas of stenosis were predominantly seen in the left sub-clavian artery, superior mesenteric artery, and the coeliac axis. Pulmonary arteries were enlarged with areas of stenosis and tortuosity seen within them. The baby was followed closely by the cardiac team. An echocardiogram at 11 months of age revealed progressive and severe bilateral pulmonary artery stenosis. RV dilatation and supra-systemic RV pressures were also noted. A repeat cardiac computed tomography scan showed significant progression of disease in the pulmonary arteries. There was now marked tortuosity of the right and left mediastinal portions of the branch pulmonary arteries with severe stenosis. During this time, she started to have episodes of severe cyanosis similar to hypoxic spells following which she was taken up for an operative procedure.

Figure 1. Fetal echocardiograms: (a) dilated and tortuous course of the ductus arteriosus, (b) modified three-vessel view showing tortuous and redundant right, left, and branch pulmonary arteries, and (c) elongated unobstructed aorta making a sharp hair pin bend around the right pulmonary artery. Ao = aorta; LPA = left pulmonary artery; LVOT = left ventricular outflow tract; Pas = pulmonary arteries; PDA = patent ductus arteriosus; RPA = right pulmonary artery; SVC = superior caval vein.
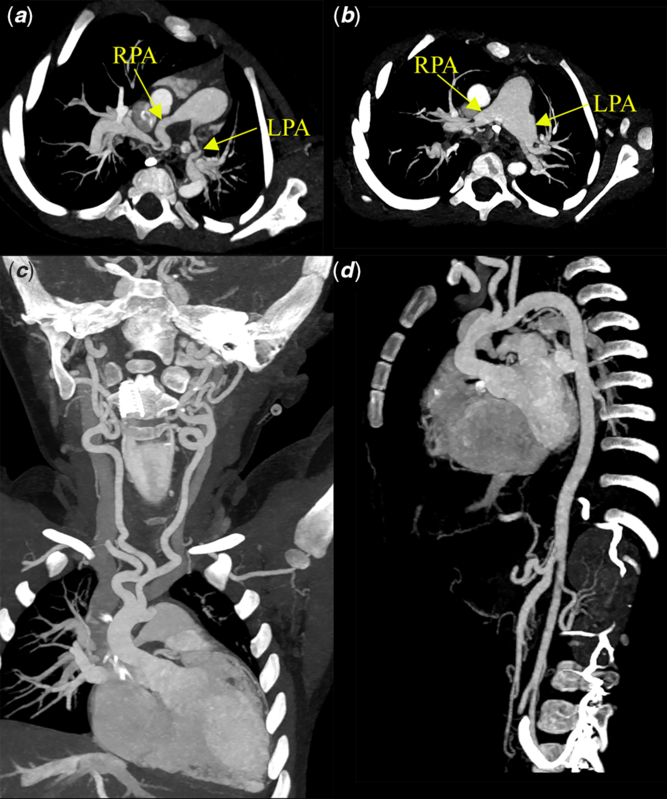
Figure 2. Computed tomography angiograms: (a) pre-operative image showing elongated and tortuous right, left, and branch pulmonary arteries. The left pulmonary artery appears kinked due to its redundant course, (b) post-operative image showing the plicated pulmonary arteries with the left pulmonary artery kinking no longer seen, (c) abnormal head and neck vessels exhibiting characteristic tortuosity, and (d) tortuous involvement of the vessels arising from the abdominal aorta.
Extensive pulmonary artery dissection into the lobar and segmental arteries was done. The redundant pulmonary artery was excised and comprehensive segmental pulmonary arterioplasty was undertaken. Immediate post-operative angiography showed a widely patent main pulmonary artery and branches with mild narrowing noted at the anastomosis sites. The RV pressures measured invasively were less than half systemic. She was discharged on the 6th post-operative day following an uneventful recovery. On follow-up, at 18 months of age, she was developing and growing appropriately. Her echocardiogram showed normal RV systolic pressures. Of note, the baby initially did not have obvious dysmorphic features in infancy, but they are becoming clearer with age. A flat nasal bridge, convex nose, and a high arched palate were noted at 11 months, and at 18 months, her skin has started to become lax.
Discussion
Ertugrel from Turkey first described a case of severe tortuosity of the arterial tree in a 10-year-old girl in 1967. Reference Ekici, Uçar, Fitöz, Atalay and Tutar1 The disease has since been predominantly reported from people originating in the Middle East and its surroundings. Alterations in the SCL2A gene causing a loss of function in the GLUT10 transporter are thought to be responsible for ATS. Reference Németh, Marcolongo and Gamberucci2 It is characterised by widespread tortuosity of the large- and medium-sized arteries. The pulmonary artery and the aorta are commonly involved; however, the mesenteric, coronary, and limb arteries may also be involved. Reference Beyens, Albuisson and Boel3,Reference Callewaert, De Paepe, Coucke, Adam, Ardinger and Pagon4 These arteries are at a higher risk of dissecting or undergoing aneurysmal changes. Often the vessels are stenotic, causing the respective ventricular chamber to be hypertensive. Reference Callewaert, De Paepe, Coucke, Adam, Ardinger and Pagon4,Reference Marwah, Shah, Suresh and Maheshwari5 The intra-cardiac anatomy is usually normal. Despite few reports of antenatal suspicion of ATS, to our knowledge none have been confirmed. Reference Al-Obaidly, Kamal and Al-Musafri6 This syndrome can also be present with central nervous system findings like aneurysms in the circle of Willis. The syndrome is usually accompanied with facial dysmorphism and skin changes similar to other connective tissue disorder. Ocular anomalies like keratoconus and strabismus may be present. Reference Naunheim, Walcott, Nahed, MacRae, Levinson and Ogilvy7 The treatment for ATS is multi-disciplinary. There is a need for constant cardiac surveillance as the above case demonstrates. Abdulmohsen et al published favourable post-operative results for seven patients at a mean follow-up at 17.6 months with all patients remaining asymptomatic. Reference Al-Khaldi, Mohammed, Tamimi and Alharbi8 A successful hybrid approach in a 4 year old with combination of angioplasty, stent deployment, and surgical arterioplasty has been reported by Vicchio and team from Italy. Reference Vicchio, Santoro, Carrozza and Caianiello9
In this case report, the diagnosis of the fetus led to a maternal diagnosis which was vital for both their safety. Favourable outcomes in our case and above described cases at follow-up give some reassurance that the disease process might not be aggressive in medium term once anatomical corrections have been undertaken. However, longer follow-up will be required to help understand the natural history of ATS post intervention.
Conclusion
ATS should be suspected antenatally when long and tortuous great arteries are seen. Close follow-up is essential to pick up cardiac dysfunction promptly. Interventions with good short and intermediate term outcomes are possible. We report the first known case of antenatally diagnosed ATS along with its natural history from 29 weeks of gestation to 18 months of life.
Acknowledgements
We would like to acknowledge Dr. Jim Potts with his help photo editing the radiology images.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of Interest
None.
Ethical Standards
Informed verbal consent was taken from the child’s guardian. All efforts have been taken to maintain the patient confidentiality during the writing of this document.





