Introduction
In modern livestock production intensive animal rearing systems, such as those practiced in pig production, are being questioned by animal welfare groups since these animals are bred, housed and fattened for slaughter in total confinement, an environment perceived to be stressful. Consequently, animals may be more susceptible to stress, which may affect health, growth performance and meat quality. Various stressors during weaning and growth may also affect the health status of productive animals. Weaning is a stressful event that may cause oxidative stress and subsequent manifestation of pathological conditions (Zhu et al., Reference Zhu, Zhao, Chen and Xu2012).
In recent years, many studies have shown the importance of applying alternative nutritional strategies in order to prevent and mitigate the detrimental effects of stress on animals as well as to optimize meat quality (Kotsampasi et al., Reference Kotsampasi, Christodoulou, Zotos, Liakopoulou-Kyriakides, Goulas, Petrotos, Natas and Bampidis2014). The current authors' research group has also performed several in vivo studies showing that by-products from olive oil and the wine industry improved antioxidant capacity, meat quality and welfare of chickens, pigs and lambs (Gerasopoulos et al., Reference Gerasopoulos, Stagos, Kokkas, Petrotos, Kantas, Goulas and Kouretas2015a, Reference Gerasopoulos, Stagos, Petrotos, Kokkas, Kantas, Goulas and Kouretas2015b, Reference Gerasopoulos, Stagos, Krouezas, Karaveli, Barda, Gkika, Mitsiou, Petrotos, Goulas and Kouretas2016; Kafantaris et al., Reference Kafantaris, Kotsampasi, Christodoulou, Kokka, Kouka, Terzopoulou, Gerasopoulos, Stagos, Mitsagga, Giavasis, Makri, Petrotos and Kouretas2017, Reference Kafantaris, Stagos, Kotsampasi, Hatzis, Kypriotakis, Gerasopoulos, Makri, Goutzourelas, Mitsagga, Giavasis, Petrotos, Kokkas, Goulas, Christodoulou and Kouretas2018; Kerasioti et al., Reference Kerasioti, Terzopoulou, Komini, Kafantaris, Makri, Stagos, Gerasopoulos, Anisimov, Tsatsakis and Kouretas2017; Makri et al., Reference Makri, Kafantaris, Stagos, Chamokeridou, Petrotos, Gerasopoulos, Mpesios, Goutzourelas, Kokkas, Goulas, Komiotis and Kouretas2017).
The cheese-making process produces huge amounts of by-products. One of these, whey protein, is found in the watery portion when milk is separated from the curds. Whey is processed into various food products or discarded into the ecosystem, causing serious pollution problems. Whey protein concentrate (WPC) exhibits antioxidant properties and is rich in the sulphur-containing amino acids (cysteine, methionine), beta-lactoglobulin and immunoglobulins (Walzem et al., Reference Walzem, Dillard and German2002). Several studies, performed by the current authors' research group, have shown that whey protein administration improved antioxidant capacity in humans and cell lines through the activation of important antioxidant molecular mechanisms such as the nuclear factor-like 2 (Nrf2) pathway (Kerasioti et al., Reference Kerasioti, Kiskini, Veskoukis, Jamurtas, Tsitsimpikou, Tsatsakis, Koutedakis, Stagos, Kouretas and Karathanos2012, Reference Kerasioti, Stagos, Jamurtas, Kiskini, Koutedakis, Goutzourelas, Pournaras, Tsatsakis and Kouretas2013, Reference Kerasioti, Stagos, Priftis, Aivazidis, Tsatsakis, Hayes and Kouretas2014, Reference Kerasioti, Stagos, Georgatzi, Bregou, Priftis, Kafantaris and Kouretas2016a, Reference Kerasioti, Stagos, Tzimi and Kouretas2016b).
Thus, the aim of the current study was to evaluate the effect of feed supplemented with whey protein concentrate on piglets' redox status, faecal microbiota and fatty acid profile of meat. The hypothesis was that WPC supplementation might have potential beneficial effects on (i) animal health, by enhancing various antioxidant mechanisms in blood and tissues as well as by improving the balance between probiotic and potentially pathogenic microorganisms, and (ii) meat quality, by improving fatty acid (FA) composition.
Materials and methods
Animals and diets
Forty-eight piglets (Topigs 20 × TN Talent, weaned at 20 days of age) were selected from the pigsty of the Technical Education Institute of Thessaly (Larissa, Greece) and used in a 30-day feeding trail. Piglets were allotted to two dietary treatments based on their initial body weight (BW) using a randomized complete block design. Each dietary treatment consisted of six replications, with four piglets per pen. The experimental dietary treatments included: (i) control (basal diet) and (ii) WPC (diet supplemented with sheep/goat whey protein concentrate). Sheep/goat whey protein concentrate was obtained from the Hellenic Protein S.A. (Tavros, Greece). The nutritional content (g/kg dry matter) and amino acid composition of sheep/goat whey protein concentrate are presented in Table 1, while ingredient and chemical composition (g/kg dry matter) of the experimental diets are summarized in Table 2. Throughout the experimental trial, piglets were housed under controlled environmental conditions (12-h light/dark cycle, temperature 27–33 °C, humidity 50–70%). Each pen was equipped with a feeder and a nipple drinker and all piglets were provided with ad libitum access to feed and water. Diets were formulated to have equal levels of metabolizable energy (ME) and amino acids, according to local practice and experience. The EvaPig software (version 1.4.0.0, INRA, AFZ and Ajinomoto Animal Nutrition Europe, available from http://www.evapig.com/x-home-en), which uses information from the INRA–AFZ (Institut National de la Recherche Agronomique – Association Française de Zootechnie, Paris, France), was used to obtain the complete amino-acid profile of the experimental feeds and to standardize values on a standardized ileal digestible (SID) basis. Tables of feedstuff composition (Sauvant et al., Reference Sauvant, Perez and Tran2004) were used to calculate the nutritional values from the feed ingredients. Metabolizable energy was also calculated using the EvaPig software.
Table 1. Nutritional content and amino acid composition of sheep/goat whey protein concentrate (WPC)
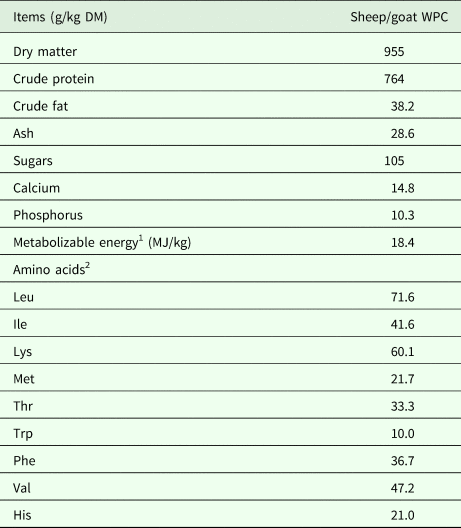
1 Calculated using EvaPig (1.4.0.0).
2 Standardized ileal digestible (SID) amino acids.
DM, dry matter.
Table 2. Experimental diets for the study

1 Calculated using EvaPig (1.4.0.0).
2 Standardized ileal digestible (SID) amino acids.
3 Concentrations (mg/ml) that caused 50% (IC50) scavenging of 2,2-diphenyl-1-picrylhydrazyl radical.
4 Significantly different from values of the control feed (P < 0.05).
DM, dry matter; HCL, hydrochloride; WPC, sheep/goat whey protein concentrate.
Blood, tissues and faecal collection
Blood samples were collected at three different time-points: 20, 35 and 50 days of age. The first blood sampling, at day 20, was performed in order to determine piglets' redox status before feeding with the diets. In particular, at day 20, piglets were restrained manually and blood samples from 24 piglets were collected from the anterior vena cava. At subsequent time points (35 and 50 days of age), blood and tissue samples were collected from 24 piglets (i.e. 12 piglets from each group). The collection and processing of blood samples, as well as the collection and homogenization of tissues (i.e. brain, heart, kidney, liver, lung, quadriceps muscle, pancreas, spleen and stomach), were performed as described previously (Gerasopoulos et al., Reference Gerasopoulos, Stagos, Petrotos, Kokkas, Kantas, Goulas and Kouretas2015b). For tissue collection (35 and 50 days of age), piglets were transported and slaughtered in a fully automated slaughter complex (Slaughter Houses of Larissa S.A., Girtoni, Greece). For blood collection, 4 ml of blood was collected from jugular vein and placed in vacutainer tubes with ethylenediamine tetraacetic acid (EDTA). Tissues were removed quickly by specialized personnel and snap-frozen in liquid nitrogen. In preparation for tissue biochemical analysis, a mortar and pestle was used for crushing and grinding the samples. One part of tissue powder was then homogenized with two parts (weight/volume) of 0.01 M phosphate buffered saline pH 7.4 (138 mM sodium chloride [NaCl], 2.7 mM potassium chloride [KCl] and 1 mM EDTA) and a cocktail of protease inhibitor tablet (Complete Mini, Roche, Germany) was added. The homogenate was vortexed vigorously and a brief sonication treatment on ice was applied. The homogenate was then centrifuged at 12 000 g for 30 min at 4 °C and the supernatant was collected and stored at −80 °C. Faecal samples were collected by specialized personnel at two different time-points (35 and 50 days of age) from 12 piglets of each group (samples were collected from the same animals at both 35 and 50 days of age). The procedure of faecal collection was performed as described previously (Kafantaris et al., Reference Kafantaris, Kotsampasi, Christodoulou, Kokka, Kouka, Terzopoulou, Gerasopoulos, Stagos, Mitsagga, Giavasis, Makri, Petrotos and Kouretas2017). Piglets were restrained manually and faeces were collected with sanitized disposable gloves from the rectum of each animal separately to avoid contamination from the ground. A quantity of approximately 40 g of faeces was collected in sterile 50-ml plastic containers which were fully filled up to the top of the cup in order to eliminate air and maintain anaerobic conditions during the maintenance of the samples. The samples were kept refrigerated for 1–2 days before being transferred to the laboratory where the microbiological analyses were carried out (within 2 days of collection).
Determination of antioxidant capacity of the diets
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the diets was evaluated as described previously (Kafantaris et al., Reference Kafantaris, Kotsampasi, Christodoulou, Kokka, Kouka, Terzopoulou, Gerasopoulos, Stagos, Mitsagga, Giavasis, Makri, Petrotos and Kouretas2017). Briefly, 1.0 ml of a freshly prepared methanolic solution of DPPH radical (100 µM) was mixed with the tested samples at different concentrations (0–30 mg/ml). The contents were mixed vigorously, incubated at room temperature in the dark for 20 min and the absorbance was recorded at 517 nm. In order to compare the radical scavenging efficiency of the diets, the IC50 value showing the concentration that caused 0.50 scavenging of DPPH radicals was calculated from a graph plotting inhibition proportion against extract concentration.
Oxidative stress biomarkers methods
Reduced glutathione (GSH), catalase activity (CAT), total antioxidant capacity (TAC), thiobarbituric acid reactive substances (TBARS), protein carbonyls (CARB) and H2O2 decomposition activity were assessed as described previously (Makri et al., Reference Makri, Kafantaris, Stagos, Chamokeridou, Petrotos, Gerasopoulos, Mpesios, Goutzourelas, Kokkas, Goulas, Komiotis and Kouretas2017). All reagents were purchased from Sigma-Aldrich (Munich, Germany). For the determination of GSH (Reddy et al., Reference Reddy, Murthy, Krishna and Prabhakar2004), 20 µl of erythrocyte lysate or tissue homogenate (diluted 1 : 2) treated with 5% TCA was mixed with 660 µl of 67 mmol/l sodium potassium phosphate (pH 8.0) and 330 µl of 1 mmol/l 5,5′-dithiobis-2 nitrobenzoate. Samples were incubated in the dark at room temperature for 10 min and the absorbance was read at 412 nm. The GSH concentration was calculated on the basis of calibration curve made using commercial standards (Sigma-Aldrich, Munich, Germany).
For the determination of CAT and H2O2 decomposition activity (Aebi, Reference Aebi1984), 4 µl of erythrocyte lysate (diluted 1 : 10) or 40 µl of tissue homogenate (diluted 1 : 2) were added to 2991 or 2955 µl, respectively, of 67 mmol/l sodium potassium phosphate (pH 7.4) and samples were incubated at 37 °C for 10 min. A total of 5 µl of 30% hydrogen peroxide (H2O2) were added to the samples, and the change in absorbance was immediately read at 240 nm for 2 min. Calculation of catalase activity was based on the molar extinction coefficient of H2O2 (43.6 M/cm).
Determination of TAC was based on the method of Janaszewska and Bartosz (Reference Janaszewska and Bartosz2002). Briefly, 20 µl of plasma or 40 µl tissue homogenate (diluted 1 : 10) were added to 480 µl or 460 µl, respectively, of 10 mmol/l sodium potassium phosphate (pH 7.4) and 500 µl of 0.1 mmol/DPPH free radical and samples were incubated in the dark for 60 min at room temperature. Samples were centrifuged for 3 min at 20 000 g, and the absorbance was read at 520 nm.
For the determination of TBARS (Keles et al., Reference Keles, Taysi, Sen, Aksoy and Akçay2001), 100 µl of plasma or 50 µl of tissue homogenate (diluted 1 : 2) was mixed with 500 µl of 35% TCA and 500 µl of Tris–hydrochloride (HCl) (200 mmol/l; pH 7.4) and incubated for 10 min at room temperature. One milliliter of 2 mol/l sodium sulphate (Na2SO4) and 55 mmol/l thiobarbituric acid solution was added and the samples were incubated at 95 °C for 45 min. The samples were cooled on ice for 5 min and vortexed after addition of 1 ml of 70% TCA. The samples were centrifuged at 15 000 g for 3 min and the absorbance of the supernatant was read at 530 nm.
Protein carbonyl determination was performed as described previously (Patsoukis et al., Reference Patsoukis, Zervoudakis, Panagopoulos, Georgiou, Angelatou and Matsokis2004), Briefly, 50 µl of 20% TCA was added to 50 µl of plasma or tissues homogenate (diluted 1 : 2), and this mixture was incubated in an ice-bath for 15 min and then centrifuged (15 000 g, 5 min, 4 °C). The supernatant was discarded, and 500 µl of 10 mmol/l 2,4-dinitrophenylhydrazine (in 2.5 N hydrochloric acid [HCl]) per sample (500 µl of 2.5 N HCl for the blank) was added to the pellet. The samples were incubated in the dark at room temperature for 1 h with intermittent vortexing every 15 min and were centrifuged (at 15 000 g for 5 min at 4 °C). Proteins were then precipitated with 10% TCA and washed three times with ethanol-ethyl acetate (1 : 1 v/v). The supernatant was discarded, and 1 ml of 5 mol/l urea (pH 2.3) was added, vortexed and incubated at 37 °C for 15 min. The samples were centrifuged (at 15 000 g for 5 min at 4 °C) and the absorbance was read at 375 nm.
Each assay was performed in triplicate and within 3 months of blood and tissue collection. Samples were stored in multiple aliquots at −80 °C and thawed only once before analysis. All measurements were conducted on a Hitachi U-1900 ratio beam spectrophotometer (serial no. 2023-029; Hitachi, Tokyo, Japan).
Microbiological analysis of faecal microbiota
For each sampling day (i.e. 35 and 50 days of age), the average values of 12 individual animals from each group and the standard error of each microbial population density (Enterobacteriacae, sulphite-reducing Clostridium, Campylobacter jejuni, Bifidobacterium spp., lactic acid bacteria and Escherichia coli) were estimated. In addition, the faecal samples were tested for the presence of Salmonella. Microbiological analysis of faecal microbiota was performed as described previously (Kafantaris et al., Reference Kafantaris, Kotsampasi, Christodoulou, Kokka, Kouka, Terzopoulou, Gerasopoulos, Stagos, Mitsagga, Giavasis, Makri, Petrotos and Kouretas2017). Briefly, Enterobacteriaceae were plated in violet red bile agar with a double layer at 37 °C for 24 h; Escherichia coli were cultivated on SMAC-BCIG agar at 37 °C for 24 h; sulphite-reducing Clostridium were cultivated on TSC agar under anaerobic conditions in anaerobic jars with Anaerocult A at 37 °C for 48 h; Campylobacter jejuni was spread on Campylobacter selective agar with Campylobacter selective supplement under microaerophilic conditions in anaerobic jars with Anaerocult C at 37 °C for 72 h; Bifidobacterium spp. were cultivated in Bifidobacterium selective agar with Bifidobacterium selective supplement under anaerobic conditions in anaerobic jars using Anaerocult A at 37 °C for 72 h; lactic acid bacteria were cultivated in MRS agar at 37 °C for 72 h. The results were expressed as log colony forming units (CFU)/g of fresh matter.
Fatty acid methyl ester synthesis
Meat samples for determination of the FA profile were collected at 50 days of age from 24 piglets (12 piglets from each group). The method of fatty acid methyl ester (FAME) synthesis (Gerasopoulos et al., Reference Gerasopoulos, Stagos, Krouezas, Karaveli, Barda, Gkika, Mitsiou, Petrotos, Goulas and Kouretas2016) was applied as follows: in 0.5 ml of homogenized tissue (quadriceps muscle), 1 ml methanolic solution of tridecanoid acid (C13 : 0) was added at a concentration of 600 µg/ml, as an internal standard. Subsequently, 0.4 ml of 10 N potassium hydroxide (KOH) and 2.7 ml of pure methanol were added. For proper hydrolysis of samples, the tubes were placed in a water bath at 55 °C for a period of 1.5 h and stirred vigorously every 20 min. For the correct composition of FA methyl esters, 0.3 ml 24 N sulphuric acid (H2SO4) was added and the tubes were placed in a water bath at 55 °C for a period of 1.5 h, with vigorous stirring every 20 min. Finally, 3 ml hexane were added as solvent and the samples were vortexed for 3 min before being centrifuged at 6000 g, 15 min at room temperature. The supernatant (2 ml) was placed in gas chromatography (GC) vials and stored at −20 °C until GC/MS analysis. Fatty acid methyl esters were analysed using a GC-MS Varian CP-3800 chromatograph (Varian Inc, Palo Alto, CA, USA) and a capillary column (Agilent J&W 112-88A7: 804.11246 HP-88 250°C: length 100 m × internal diameter 0.25 mm and film thickness 0.25 µm; Agilent, Frankfurt, Germany). The various FAs were identified by comparison with standard FA methyl esters from Supelco (Bellefonte, PA, USA): 37 Component FAME Mix (product number 47885-U) and PUFA 2 (product number 47015-U). The National Institute of Standards and Technology database was used to identify FAME.
Statistical analysis
Data were analysed by one-way analysis of variance (ANOVA). The experimental data were expressed as mean ± SE and statistical significance was considered at P < 0.05. Data were analysed using SPSS, version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Assessment of antioxidant capacity of the diets
The antioxidant capacity of the diets was evaluated according to the DPPH radical scavenging assay. The IC50 values for scavenging of DPPH radical were 18.6 and 9.6 mg/ml for control and WPC experimental diet, respectively (Table 2). The results showed higher (at least 2-fold) antioxidant capacity of the diet supplemented with WPC than the control diet.
Assessment of oxidative stress markers in blood and tissues
The results from the assessment of oxidative stress biomarkers in blood showed that at 35 days, CAT activity was decreased significantly (P < 0.05), by 30%, in erythrocytes of the WPC group compared with the control group (Table 3). At 35 days of age, total antioxidant capacity was decreased significantly (P < 0.05), by 11%, in plasma of the WPC group compared with the control group. Moreover, at 35 and 50 days, CARB concentration was decreased significantly (P < 0.05), by 21 and 16%, respectively, in plasma of the WPC group compared with the control group. However, there were no significant differences between the two groups for GSH in erythrocytes or TBARS in plasma (Table 3). Moreover, statistical analysis between the three time-points within the same group showed that GSH concentration was increased significantly (P < 0.05), by 256 and 200% in erythrocytes of the control group at 35 and 50 days, respectively, compared with 20 days. Moreover, a significant (P < 0.05) reduction of 27% was found for GSH concentration in erythrocytes of the WPC group at 50 days compared with 35 days. Meanwhile, a significant (P < 0.05) increase of 28% was seen in catalase activity at 50 days compared with 20 days in the control group. Furthermore, CAT activity was increased significantly (P < 0.05) by 33% at 50 days compared with 35 days in the WPC group. Total antioxidant capacity was increased significantly (P < 0.05) by 50 and 33% in plasma of the control group at 35 and 50 days, respectively, compared with 20 days, whereas TBARS concentration was decreased significantly (P < 0.05) in plasma of the control group by 26 and 42% at 35 and 50 days, respectively, compared with 20 days. Finally, CARB concentration was increased significantly (P < 0.05) by 11% in plasma of the WPC group at 50 days compared with 35 days.
Table 3. Effects on oxidative stress markers in blood samples of growing piglets
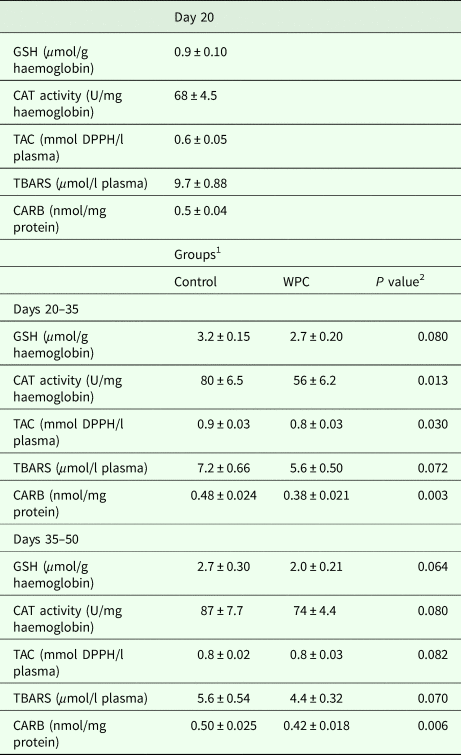
1 Control, standard diet; WPC, diet supplemented with sheep/goat whey protein concentrate. Piglets/group n = 12 (at each time-point).
2 Significantly different from values of control group (P < 0.05).
GSH, reduced glutathione; CAT, catalase activity; TAC, total antioxidant capacity; DPPH, 2,2-diphenyl-1-picrylhydrazyl; TBARS, thiobarbituric acid reactive substances; CARB, protein carbonyls; WPC, sheep/goat whey protein concentrate.
Data expressed as mean ± SE.
The feed supplemented with WPC enhanced the antioxidant mechanisms and improved the redox status of piglets in almost all the tested tissues. In particular, at 35 days of age, GSH concentration was increased significantly (P < 0.05) in the WPC group compared with the control group, with increases of 20, 33, 25, 27, 50, 33 and 200% seen in the liver, heart, quadriceps muscle, brain, lungs, stomach and pancreas, respectively (Table 4). At 50 days of age, GSH was also increased significantly (P < 0.05) in the WPC group compared with the control group: at this stage, the increases were 128, 25, 74 and 153% in the liver, heart, kidneys and pancreas, respectively. However, at 50 days, GSH was decreased significantly (P < 0.05) in spleen and stomach by 28 and 47%, respectively, in the WPC group compared with the control group (Table 4).
Table 4. Effects of whey protein concentrate on oxidative stress markers, reduced glutathione (GSH) and hydrogen peroxide (H2O2) decomposition activity, in tissues of growing piglets

1 Control, standard diet; WPC, diet supplemented with sheep/goat whey protein concentrate. Piglets/group n = 12 (at each time-point).
2 Significantly different from values of control group (P < 0.05).
Data expressed as mean ± SE.
At 35 days of age, H2O2 decomposition activity was decreased significantly (P < 0.05) in the liver, quadriceps muscle, brain and stomach (by 21, 46, 25 and 26%, respectively), whereas it was increased significantly (P < 0.05) in spleen (by 17%), in the WPC group compared with the control group (Table 4). At 50 days, H2O2 decomposition activity was increased significantly (P < 0.05) in the quadriceps muscle, lungs and pancreas (by 66, 40 and 145%, respectively), whereas it was decreased significantly (P < 0.05) in liver and brain, by 18 and 33%, respectively, in the WPC group compared with the control group (Table 4).
Total antioxidant capacity was increased significantly (P < 0.05) in the pancreas at 35 days of age, by 21%, in the WPC group compared with the control group. However, at the same time point, TAC was decreased significantly (P < 0.05) in the brain (by 15%), in the WPC group compared with the control group (Table 5). At 50 days of age, TAC was increased significantly (P < 0.05) in the pancreas by 41% and in the kidneys by 24%, whereas, in heart, brain and spleen, TAC was decreased significantly (P < 0.05), by 22, 13 and 14%, respectively, in the WPC group compared with the control (Table 5).
Table 5. Effects of whey protein concentrate on total antioxidant capacity (TAC) and thiobarbituric acid reactive substances (TBARS)

1 Control, standard diet; WPC, diet supplemented with sheep/goat whey protein concentrate. Piglets/group n = 12 (at each time-point).
2 Significantly different from values of control group (P < 0.05).
Data expressed as mean ± SE.
Piglets fed with the diet supplemented with WPC exhibited decreased oxidative stress-induced damage to lipids. In particular, TBARS concentration was decreased significantly in most of the tested tissues in WPC group compared with the control group. Thus, at 35 days, TBARS concentration was decreased significantly (P < 0.05) in the liver, heart, quadriceps muscle and brain by 37, 30 40 and 35%, respectively, in the WPC group compared with the control group (Table 5). At 50 days of age, TBARS concentration was also decreased significantly (P < 0.05) in the WPC group compared with the control group: in the liver by 37%, in the heart by 24%, in the stomach by 49% and in the pancreas by 47% (Table 5).
Finally, like TBARS, at 35 days, CARB concentration was decreased significantly (P < 0.05) in the liver, spleen and stomach, by 50 21 and 25%, respectively, in the WPC group compared with the control group (Table 6). At 50 days, protein carbonyls concentration was also decreased significantly (P < 0.05) in the WPC group compared with the control group: in the heart by 25%, in the quadriceps muscle by 20%, in the kidneys by 38%, in the stomach by 62% and in the pancreas by 54% (Table 6).
Table 6. Effects of whey protein concentrate on oxidative stress marker protein carbonyls (CARB), in tissues of growing piglets

1 Control, standard diet, WPC, diet supplemented with sheep/goat whey protein concentrate. Piglets/group n = 12 (at each time-point).
2 Significantly different from values of control group (P < 0.05).
Data expressed as mean ± SE.
Microbiological analysis in faecal samples
The results from the microbiological analysis in faecal samples showed that the diet supplemented with WPC had no effect on the population density of Enterobacteriaceae and Escherichia coli in the faeces (Table 7). Also, no differences between the control and the WPC group were observed regarding the population density of sulphite-reducing Clostridium during the sampling period. Salmonella was absent in 10 g of faeces of the experimental groups. In contrast, at 35 and 50 days of age, the population density of Campylobacter jejuni was decreased significantly (P < 0.05) (by up to 1.0 log CFU/g) in the WPC group compared with the control group (Table 7). Interestingly, the population density of lactic acid bacteria in the faeces exhibited a significant increase (P < 0.05) (by up to 1.0 log CFU/g) at 35 days after consumption of WPC. In a similar manner, at 50 days, the population density of Bifidobacterium spp. was increased significantly (P < 0.05) (by up to 1.2 log CFU/g) in the WPC group compared with the control group (Table 7).
Table 7. Effects of whey protein concentrate (WPC) on faecal microbiota

1 Control, standard diet; WPC, diet supplemented with sheep/goat whey protein concentrate. Piglets/group n = 12 (at each time-point).
2 Significantly different from values of control group (P < 0.05).
At all time-points faecal samples were collected from the same animals. The results were expressed as log colony forming units (CFU)/g of fresh matter. Data expressed as mean ± SE.
Assessment of fatty acids in meat
The effect of feed supplemented with WPC on the FA composition in piglets' meat is presented in Table 8. The inclusion of WPC in the diet increased significantly (P < 0.05) the content of pentadecanoic acid (C15 : 0), palmitelaidic acid (C16 : 1trans-9), vaccenic acid (C18 : 1), linolelaidic acid (C18 : 2n-6 trans-9,12), γ-linolenic acid (C18 : 3n-6), α-linolenic acid (ALA; C18 : 3n-3), eicosapentaenoic acid (EPA; C20 : 5n-3) and docosahexaenoic acid (DHA; C22 : 6n-3). In contrast, the content of lauric acid (C12 : 0) was decreased significantly (P < 0.05) in the WPC group compared with the control group. Interestingly, the content of n-3 FAs and total polyunsaturated fatty acids (PUFA) was increased significantly (P < 0.05), whereas n-6/n-3 ratio was decreased significantly (P < 0.05) in the WPC group compared with the control group.
Table 8. Fatty acid profile in meat samples

1 Control, standard diet; WPC, diet supplemented with sheep/goat WPC. Piglets/group n = 12. Meat samples were collected at 50 days of age.
2 Significantly different compared with control group (P < 0.05).
3 Represents other known and unidentified fatty acids.
Data presented as mean ± SE. P values included.
ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; SFA, total saturated fatty acids; MUFA, total monounsaturated acids; PUFA, total polyunsaturated fatty acids.
Discussion
Effect of feed supplemented with whey protein concentrate on piglets' redox status
The results from the current study showed that the administration of feed supplemented with WPC improved the antioxidant mechanisms of piglets. In particular, the experimental feed supplemented with WPC increased significantly GSH in almost all the tested tissues both at 35 and 50 days of age. However, at 50 days of age, WPC inclusion in piglets' diet decreased significantly GSH in spleen and stomach in WPC group compared with control group. This result may be explained by the fact that endogenous GSH concentration in spleen and stomach of piglets at 50 days was high and so administration of WPC, instead of increasing the biochemical mechanisms responsible for GSH synthesis, decreased them. A recent study has also reported that the administration of methionine increased GSH in broilers with low concentration of GSH, but decreased its concentration in broilers with high GSH (Chen et al., Reference Chen, Chen, Zhang and Zhou2013). Moreover, DeLeve and Kaplowitz (Reference DeLeve and Kaplowitz1990) reported that increased concentration of GSH may inhibit its synthesis, through inhibition of glutamate-cysteine ligase (GCL), one of the major enzymes responsible for GSH synthesis. The main enzymes responsible for GSH synthesis are glutamate cysteine ligase (GCL) and glutathione synthetase (GSS) (Aquilano et al., Reference Aquilano, Baldelli and Ciriolo2014). The high content of WPC in sulphur-containing amino acids such as cysteine and methionine may also account for the increase in GSH since cysteine is an indispensable amino acid precursor in cellular glutathione biosynthesis (Liu et al., Reference Liu, Hyde, Simpson and Barycki2014). Recently, it was found that sheep/goat whey protein increased GSH in muscle and endothelial cells (Kerasioti et al., Reference Kerasioti, Stagos, Priftis, Aivazidis, Tsatsakis, Hayes and Kouretas2014, Reference Kerasioti, Stagos, Georgatzi, Bregou, Priftis, Kafantaris and Kouretas2016a).
Apart from increases in GSH, inclusion of WPC in piglets' diet increased H2O2 decomposition activity, especially at 50 days of age. In particular, H2O2 decomposition activity was increased in quadriceps muscle, lungs and pancreas in the WPC group compared with the control group. This result may be explained by the fact that, at 50 days, the administration time of the feed was longer than at 35 days and so it was expected the antioxidant effects to be enhanced. Indeed, the results confirmed this hypothesis since most of the redox biomarkers including H2O2 decomposition were improved at 50 days compared with 35 days of age. This effect was observed in some tissues since the bioactive compounds of WPC exhibited tissue specificity. This is probably due to physiological and biochemical differences as well as to differences in the content of antioxidant enzymes and molecules between different tissues. Interestingly, a recent study showed that sheep/goat whey protein supplementation had similar effects in tissues of rats (Kerasioti et al., Reference Kerasioti, Stagos, Tsatsakis, Spandidos, Taitzoglou and Kouretas2018). Another study has also shown that the administration of WPC increased CAT activity and prevented myoblast toxicity (Xu et al., Reference Xu, Liu, Xu and Kong2011).
The experimental feed supplemented with WPC increased TAC in some of the tested tissues, indicating an enhancement of the piglets' total antioxidant mechanisms. The slight decrease in TAC in brain, heart and spleen of the WPC group did not seem to indicate pro-oxidant effect since CARB and TBARS were also decreased at the same time. The decrease in TAC may be explained by the fact that the increase of some antioxidant molecules (e.g. GSH) caused a decrease of other antioxidant molecules as a compensation effect. Moreover, the TAC assay has the disadvantage that it is based on an assessment of the amount of only the reductants and not the oxidants, while the redox status is based on both. Thus, even if TAC is reduced, this does not mean necessarily that oxidative stress occurs. Moreover, the current results in TAC may indicate that the bioactive compounds of WPC act in a tissue-specific manner, as it has also been mentioned above and reported by a previous study (Kerasioti et al., Reference Kerasioti, Stagos, Tsatsakis, Spandidos, Taitzoglou and Kouretas2018). In addition, several in vivo studies have shown that the bioactive compounds with antioxidant proprieties, such as polyphenols, have also exhibited tissue specificity (Kafantaris et al., Reference Kafantaris, Kotsampasi, Christodoulou, Kokka, Kouka, Terzopoulou, Gerasopoulos, Stagos, Mitsagga, Giavasis, Makri, Petrotos and Kouretas2017, Reference Kafantaris, Stagos, Kotsampasi, Hatzis, Kypriotakis, Gerasopoulos, Makri, Goutzourelas, Mitsagga, Giavasis, Petrotos, Kokkas, Goulas, Christodoulou and Kouretas2018; Kerasioti et al., Reference Kerasioti, Terzopoulou, Komini, Kafantaris, Makri, Stagos, Gerasopoulos, Anisimov, Tsatsakis and Kouretas2017; Makri et al., Reference Makri, Kafantaris, Stagos, Chamokeridou, Petrotos, Gerasopoulos, Mpesios, Goutzourelas, Kokkas, Goulas, Komiotis and Kouretas2017).
Apart from the enhancement of the antioxidant mechanisms, the feed supplemented with WPC seemed to protect the piglets from oxidative stress-induced damage. In particular, lipid peroxidation shown by TBARS was decreased significantly in several tissues (four out of nine tested tissues) in the WPC group compared with the control group. In a previous study, it was found that TBARS concentration was decreased in muscle and endothelial cells after treatment with sheep/goat WPC (Kerasioti et al., Reference Kerasioti, Stagos, Priftis, Aivazidis, Tsatsakis, Hayes and Kouretas2014, Reference Kerasioti, Stagos, Georgatzi, Bregou, Priftis, Kafantaris and Kouretas2016a). Furthermore, another study has reported that WPC reduced lipid peroxidation under various oxidative stressors in rats (Haraguchi et al., Reference Haraguchi, Silva, Neves, dos Santos and Pedrosa2011).
Similarly, WPC inclusion in piglets' diet had a protective role against protein oxidation, as shown by the decrease in CARB in many of the tested tissues. The results showed that this effect was more intense after 30 days of feeding with the experimental diet. The concentration of CARB was also decreased in plasma of the WPC group compared with the control. Moreover, other studies have also reported beneficial effects of dairy by-products on protein oxidation in animals. For example, the administration of hydrolysed WPC reduced CARB in muscle of exercised rats (Wang et al., Reference Wang, Niu, Lu, Li and Li2014). Specifically, the reduction of protein oxidation and lipid peroxidation in muscle tissue is an important finding, since meat consists of the edible part of the animal. Thus, the results of the current study suggested that the supplementation of pigs' feed with WPC increased oxidative stability of meat, which in turn may improve its quality.
Effect of whey protein concentrate on faecal microbiota
Based on the results of the microbiological analysis, it seems that at 35 and 50 days of age, the population density of Campylobacter jejuni was decreased significantly in the WPC group at both 35 and 50 days. Also, a 1.1 log reduction was observed in the population of Enterobacteriacae at 35 days, in parallel to an equivalent increase of the population of lactic acid bacteria. It has been shown previously that supplementation of lactic acid bacteria (which are naturally present in high numbers in whey) can alter animal gut microbiota by inhibiting Enterobacteriacae and Campylobacter species or other potential human pathogens found in animal faeces, due to the production of lactate, acetate, hydrogen peroxide, ethanol and acetaldehyde (Klewicki and Klewicka, Reference Klewicki and Klewicka2004; Giang et al., Reference Giang, Viet, Ogle and Lindberg2010; Santini et al., Reference Santini, Baffoni, Gaggia, Granata, Gasbarri, Di Gioia and Biavati2010). The population density of beneficial lactic acid bacteria was increased significantly after the addition of WPC to the piglets' diet, especially at 35 days of age. Similarly, the beneficial and probiotic Bifidobacterium reached a significantly higher population density at 50 days of age in the WPC group compared with the control group. The somewhat lower population of lactic acid bacteria in the gut of the WPC group at 50 days (which is still higher than in the control group) compared with 35 days of the WPC group, with a concomitant increase of Bifidobacteria from 35 to 50 days in the WPC group, may be due to the fact that not all lactic acid bacteria can colonize the gut permanently or long-term by attaching to enterocytes, while all Bifidobacteria are well adapted as permanent gut microbiota and usually more resistant to exposure to intestinal bile salts compared with lactic acid bacteria (although this resistance can be strain-dependent) (Ibrahim and Bezkorovainy, Reference Ibrahim and Bezkorovainy1993; Kailasapathy and Chin, Reference Kailasapathy and Chin2000; Pereira and Gibson, Reference Pereira and Gibson2002). It is also possible that lactic acid bacteria gradually decrease from 35 to 50 days in the WPC group, giving way (and space for attachment on enterocytes) to Bifidobacteria, which simultaneously increase due to the presence of lactoferrin (an iron-binding protein) in whey, which can selectively and gradually benefit the growth of Bifidobacteria in the gut, in contrast to other more iron-dependent bacteria that are partly restricted (Chierici et al., Reference Chierici, Fanaro, Saccomandi and Vigi2003). Overall, the results suggested that the supplementation of pigs' feed with WPC can boost the population density of beneficial, potentially probiotic bacteria in the gut and at the same time control the population of some potential enteric pathogens.
Effect of whey protein concentrate on fatty acid composition of meat
The chemical profile analysis showed that the diet supplemented with WPC resulted in significantly higher content of vaccenic acid (VA; C18 : 1) compared with the control group. Vaccenic acid is a major trans-fatty acid in milk fat and is also the main intermediate product of biohydrogenation of polyunsaturated 18-carbon FAs to stearic acid (Ritvanen et al., Reference Ritvanen, Putkonen and Peltonen2012). The action of Δ-9-desaturase in the mammary gland converts a high proportion of stearic and trans-vaccenic acid to oleic acid and conjugated linoleic acid (CLA), respectively (Griinari et al., Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000). Vaccenic acid is the only known dietary precursor of CLA, but recent data have shown that consumption of this trans-fatty acid may impart health benefits beyond those associated with CLA (Field et al., Reference Field, Blewett, Proctor and Vine2009). Since vaccenic acid is the main trans-fat in dairy products, the statistically higher content of VA in the WPC group than in the control group may be explained by the fact that pigs consumed feed supplemented with WPC. Moreover, in the current study, the supplementation of WPC in piglets' diet affected the long-chain n-3 FA and consequently n-6/n-3 ratio. In particular, the feed supplemented with WPC significantly increased EPA, DHA and ALA compared with the control group. The significantly increased content of ALA FA could be explained by the fact that WPC acted as a probiotic. According to a previous study (Ross et al., Reference Ross, Van Nieuwenhove and González2012), the inclusion of a probiotic compound in animals' diet had health benefits on intestinal microbiota and affected the FA profile in meat. Moreover, the supplementation of feed with WPC indicated a beneficial effect on meat quality since the n-6/n-3 ratio was decreased significantly in the WPC group compared with the control group. This may be due mainly to the increased content of EPA, DHA and ALA. The fact that WPC inclusion in the diet decreased the n-6/n-3 ratio significantly is an interesting finding since several studies have shown that the consumption of meat characterized by a lower n-6/n-3 ratio provided beneficial effects on human health (Simopoulos, Reference Simopoulos2002).
In conclusion, the current study indicated that the inclusion of WPC in piglets' diet enhanced antioxidant mechanisms, prevented oxidative stress damage to lipids and proteins and improved the intestinal microbiota in favour of beneficial and potentially probiotic bacteria (Lactobacillus, Bifidobacterium species) and to the detriment of potential enteric pathogens, thus suggesting beneficial effects on animal well-being. These findings are important since weaning is a significant event and it is generally stressful for piglets, leading to oxidative stress and manifestation of physiological changes and diseases. In addition, WPC inclusion in the diet of piglets showed a significantly beneficial effect on meat quality by enriching animals' meat with n-3 polyunsaturated FAs. The exploitation of dairy by-products for developing farm animal feed is important since it would also provide ecological benefits by reducing the waste disposal and the resulting environmental problems. Consequently, supplementation of animal feeds with WPC could be an alternative nutritional strategy that would provide considerable multiple beneficial impacts for animals, consumers, environmental and agricultural economy.
Financial support
This research project was funded by the Hellenic Agricultural Organisation ‘DEMETER’ (NSRF 2007-2013). Also, it was funded in part by the MSc programmes ‘Biotechnology-Nutrition & Environment’ and ‘Toxicology’ in the Department of Biochemistry and Biotechnology at the University of Thessaly.
Conflicts of interest
None.
Ethical standards
The experiment was reviewed and approved by the institutional review board and the appropriate state authority. All procedures were performed according to EU Directive 2010/63/EU for animal experiments.










