INTRODUCTION
Polychaetes in the family Serpulidae are often important components of marine communities in terms of numerical abundance or biomass, as ecosystem engineers that build persistent calcareous tubes adding structural complexity to the habitat, or as filter-feeders that link pelagic and benthic food webs. Serpulids are also used in biomonitoring and ecotoxicology assays due to their suitability and ecological relevance; larvae in particular are increasingly found to be suitable for this purpose due to the high sensitivity of this life history stage and readily available gametes and larvae (Dixon & Wilson, Reference Dixon and Wilson2000; Lewis & Watson, Reference Lewis and Watson2012). To fully understand the role of serpulid polychaetes in marine ecosystems, their potential for practical applications and the factors controlling their population dynamics, a detailed understanding of their reproductive biology is required. However, current knowledge about reproduction, development and settlement in serpulid polychaetes is largely based on the study of only a few common species; as reported by Kupriyanova et al. (Reference Kupriyanova, Nishi, Ten Hove and Rzhavsky2001), we observe that information on the life history of most serpulid species is still lacking. Some reproductive traits have been found to be common among the serpulid species studied to date. These include extended reproductive seasons with continuous reproduction by individuals, and also hermaphroditism, particularly protandric hermaphroditism in medium- and large-bodied species (Olive & Clark, Reference Olive, Clark and Mill1978; Kupriyanova et al., Reference Kupriyanova, Nishi, Ten Hove and Rzhavsky2001; Cotter et al., Reference Cotter, O'Riordan and Myers2003). As a limited number of serpulid species have been studied to date, however, it is not clear whether these traits are common in other serpulid species.
Spirobranchus cariniferus (Gray, 1843), a serpulid tubeworm previously identified as Pomatoceros caeruleus (Schmarda, 1861) (Glasby & Read, Reference Glasby and Read1998; Ten Hove & Kupriyanova, Reference Ten Hove and Kupriyanova2009), is distributed throughout New Zealand, colonizing the mid-intertidal zone of rocky, boulder and cobble shores (Morton & Miller, Reference Morton and Miller1973; Morton, Reference Morton2004; Schiel, Reference Schiel2006). This worm, which grows to lengths of about 4 cm, is gregarious and often occurs within a narrow vertical band in the intertidal zone at densities exceeding a thousand per m2 (personal observations). Despite its high abundance and broad distribution almost nothing is known of the biology of the species. The distribution of the species is described in textbooks and technical publications (e.g. Morton & Miller, Reference Morton and Miller1973; Hayward & Morley, Reference Hayward and Morley2004, Reference Hayward and Morley2008; Morton, Reference Morton2004; Schiel, Reference Schiel2006) and a few papers have reviewed the taxonomy of S. cariniferus (see above); other than these, the entire body of peer-reviewed literature on the biology of this species consists, to our knowledge, of a single paper reporting the proportion of S. cariniferus in Otago Harbour that are host to an apicomplexan gregarine parasite (Peoples et al., Reference Peoples, Randhawa and Poulin2011). The present study therefore examined S. cariniferus to explore current hypotheses regarding patterns in life history and larval ecology traits among serpulid polychaetes, and to expand our understanding of the reproductive ecology of this regionally abundant and potentially important species.
The specific goals of this study were to determine: (1) whether S. cariniferus has an extended spawning season by monitoring the proportion of individuals capable of spawning ripe gametes over a 5 month period ranging from mid-spring to mid-autumn; (2) whether S. cariniferus sex-ratios as well as the average and range of body sizes for each sex are consistent with protandric hermaphroditism; (3) the influence of algal diet on larval growth and time to reach competence; (4) the effectiveness of 3-isobutyl-1-methylxanthine (IBMX) as an inducer of metamorphosis; and (5) the effectiveness of conspecific tube and tissue homogenates as cues inducing settlement and metamorphosis in competent larvae. In addition, sampling for goals 1 and 2 was carried out in populations on the east and west coasts of New Zealand's North Island to determine if these life history traits are consistent over large dispersal distances. The phosphodiesterase inhibitor IBMX was tested as a metamorphic inducer based on evidence from previous studies that this compound induces metamorphosis in competent larvae of other polychaete species (e.g. Hydroides elegans (Haswell, 1883), Bryan et al., Reference Bryan, Qian, Kreider and Chia1997; Phragmatopoma californica (Fewkes, 1889), Pawlik, Reference Pawlik1990).
MATERIALS AND METHODS
The field sites selected for this study were located on the east and west coasts of New Zealand's North Island, in the vicinity of the city of Auckland (Figure 1). Most collections were from the Glendowie (east) and Mill Bay (west) sites. The shortest dispersal distance between east and west coast field sites, around Cape Reinga at the northern tip of North Island, is roughly 700 km. All laboratory experiments were carried out at the University of Auckland using natural seawater.
Fig. 1. Map of New Zealand's North Island, with an enlarged view of the north-central area showing each of the field collection sites. East coast: AB, Arkles Bay; GL, Glendowie; KB, Kawakawa Bay; TK, Te Kouma; WI, Whiritoa. West coast: MU, Muriwai; WA, Whatipu; MB, Mill Bay; GB, Green Bay; HB, Hillsborough Bay. Most collections of Spirobranchus cariniferus for the present study were made at the Glendowie site on the east coast and at Mill Bay on the west coast.
Spawning period, sex-ratio and adult body mass
The period during which Spirobranchus cariniferus is reproductive was examined by collecting samples of tubeworms from east and west coast sites, returning these to the laboratory and recording gamete production by individual worms. To this effect, worms were collected on 18 occasions over a 5 month period ranging from mid-spring (29 November 2011) to mid-autumn (26 April 2012). At low tide, clusters of intact tubes containing live worms were detached from rock surfaces, placed in a cooled container and returned to the laboratory. Worms were kept cool and moist, but out of water, until used in the spawning trials. Within 2–20 hours of being collected in the field, individual worms from a broad range of sizes were gently detached from other individuals, their tube was cracked or entirely removed and each worm was then placed in a separate vial containing 20 ml of seawater (19°C) that had been passed through a Waterco® filter bag rated at 5 µm pore size. This simple procedure, commonly used with serpulid polychaetes (Kupriyanova et al., Reference Kupriyanova, Nishi, Ten Hove and Rzhavsky2001), was sufficient to stimulate gamete release within the following 15 minutes. For each collection date, the percentage of worms capable of spawning was determined from samples of 14–30 individual worms, with the exception of the first sample, collected on 29 November 2011, in which only four worms were examined. These spawning trials also served to determine sex-ratios based on the type of gamete that was spawned by each individual, as there were no obvious morphological differences between males and females.
To determine if mature males and females differ in average body size, as would be expected if individuals are protandric hermaphrodites, the body mass of each worm without its tube was measured to the nearest 0.1 mg after briefly blot-drying on adsorbent paper; body mass measurements were carried out immediately after 10 of the 18 spawning trials.
Effect of algal diet on larval growth
To determine the effect of diet on larval growth, we reared S. cariniferus larvae in five different microalgal treatments, including three single-algal treatments and two multi-algal treatments: (1) Isochrysis galbana Parke, 1949; (2) Dunaliella tertiolecta Butcher, 1959; (3) Chaetoceros muelleri Lemmermann, 1898; (4) I. galbana and D. muelleri; and (5) I. galbana, D. tertiolecta and C. muelleri. The three algal species used in this study have been shown to differ significantly in terms of total lipid and polysaccharide content and also in terms of their content in highly unsaturated fatty acids (HUFAs) and polyunsaturated fatty acids (PUFAs) (D'Souza & Loneragan, Reference D'Souza and Loneragan1999) and thus might be of different nutritional value to S. cariniferus larvae.
Spirobranchus cariniferus were collected from Muriwai (Figure 1), returned to the laboratory and induced to spawn. This was accomplished by dividing large clusters of worms into smaller clusters of 2–6 worms, breaking the tubes of these worms and placing a few of these small clusters together in fingerbowls with filtered seawater for 15 minutes, during which time most worms released gametes. For this experiment, we obtained gametes from ≥15 females and ≥10 males. The worms were then removed from the fingerbowls, the water was swirled for a few seconds to mix the gametes and these were left to fertilize for 10 minutes. Since S. cariniferus egg diameter is 50–60 µm, we passed the water containing the gametes through a 100 µm screen to filter out large particles, then filtered onto a 25 µm screen to retain the eggs and separate these from water containing excess sperm and, finally, we gently washed the eggs into a 1 l beaker filled with seawater. This beaker was placed in a controlled temperature room at 18.5°C to allow embryonic development for 36 hours, based on preliminary larval cultures showing that trochophores hatch sometime after 24 hours but before 36 hours after fertilization. A large number (>10,000) of newly hatched trochophores was obtained from this culture, of which 12 haphazardly sampled larvae were immediately fixed in 0.5% paraformaldehyde and their trochus diameter measured to the nearest 2.5 µm under a compound microscope at 400×; at that age, the larvae are virtually spherical such that trochus diameter is roughly equivalent to body length. An additional 400 larvae were haphazardly collected and transferred into the algal treatments.
The algal diet experiment was carried out in Cellstar® 6-well cell culture plates; the trays were held at 18.5°C, without aeration or water motion. The experimental design consisted of the five algal treatments described above, with four replicate wells per treatment and 20 larvae per replicate well in 10 ml of algal solution. Since the goal of this experiment was to assess the effect of algal type, each algal treatment consisted of high algal densities (~0.5–1 × 106 cells/ml), providing larvae with ad libitum access to algae. For treatments 1 to 3, consisting of a single algal species, a stock suspension of the algae was prepared by diluting concentrated, rapidly growing algal cultures in filtered seawater. The two multi-algal treatments were prepared by mixing equal amounts of the relevant unialgal stock suspensions.
Every 3 days, 60% of the water in each well was replaced with fresh algal suspension, and the water and larvae transferred to the wells of clean 6-well plates. Three days after the start of the feeding trials (i.e. day 5 after fertilization), two larvae were haphazardly selected from each replicate well, fixed in 0.5% paraformaldehyde and their body length measured at 400×. The same was repeated on days 8 and 11, except that we measured three larvae per well on those dates.
Effectiveness of IBMX and conspecific homogenates in inducing metamorphosis
This experiment was designed to address two goals: (1) establish a method of determining when S. cariniferus larvae have reached competence; and (2) determine the response of competent larvae to tissue and tube material of conspecific adults. Spirobranchus cariniferus adults were collected from the Glendowie site (Figure 1); procedures for spawning, fertilization and embryonic development were carried out as described above for the algal diet experiment. After 36 hours, ~50 ml of water from the beaker containing newly hatched trochophore larvae was transferred to a clean beaker, in which we added 550 ml filtered seawater containing equal amounts of I. galbana, D. tertiolecta and C. muelleri as food. We changed the water and provided new algae every 3 days.
To test the effectiveness of IBMX as a pharmacological inducer of metamorphosis, we exposed S. cariniferus larvae to IBMX concentrations of 10−4 M and 10−5 M, as described by Bryan et al. (Reference Bryan, Qian, Kreider and Chia1997), and also to filtered seawater without IBMX (control). Larvae were first exposed to treatment solutions 8 days after fertilization, when the larvae in the culture had reached a body length of ~180 µm and therefore starting well before they had reached the largest size observed in the algal diet experiment (~300 µm). Larvae in this culture grew more slowly than in the algal diet experiment, likely because larval densities in this culture (~10 larvae per ml) were higher than in the algal diet experiment (two larvae per ml). This experiment consisted of three metamorphosis treatments (control and two IBMX concentrations), with five replicates per treatment and 10 larvae per replicate, and was carried out in wells of 6-well cell culture plates. A clean glass coverslip was added to the bottom of each well as an alternate settlement surface to the plastic wells. Larvae were left in the treatment solutions for 24 hours, after which they were examined to determine if they had begun metamorphosis.
Once larvae were found to metamorphose in response to IBMX, we then examined the response to surfaces coated with tissues or with calcareous tube material of conspecific S. cariniferus adults to determine if these constitute cues for larval settlement. Since larvae may become responsive to natural cues some time after becoming responsive to IBMX (e.g. 24 hours later in H. elegans: Pechenik & Qian, Reference Pechenik and Qian1998), we began this experiment 2 days after the larvae had been found to respond to IBMX. This second metamorphosis experiment consisted of four treatments: control (no added cue), adult tissues, adult crushed tube material, and a mix of adult tissues and tube material. To prepare the last three treatments, we collected live adult worms from the Glendowie field site on day 15 of the larval culture, carefully removed adult worms from their calcareous tubes and separately homogenized adult tissues and tubes with mortar and pestle. The tissue homogenate was prepared by grinding 12 entire adult worms together (~0.5 g) in 3 ml of filtered seawater and then passing through a 100 µm screen to remove coarse fragments. The tube homogenate was prepared by grinding 4.5 g of tube material (4–8 tubes), adding 4 ml of filtered seawater and then passing the suspension through a 100 µm screen to remove coarse fragments. The last treatment was prepared by combining an equal quantity of each of the above tissue and tube filtrates. These three treatment suspensions were then painted onto glass coverslips, five replicates per treatment, and allowed to dry for 3 hours so the material would adhere to the surface. The coated coverslips were then gently rinsed in filtered seawater for a few seconds to remove unattached material and were then placed in the wells of 6-well cell culture plates with 10 ml of filtered seawater and ten 15 day old larvae. Control replicates received clean coverslips, filtered seawater and 10 larvae. Each well was then examined again after 48 hours for metamorphosed individuals.
RESULTS
Spawning period, sex-ratio and adult body mass
The reproductive season of Spirobranchus cariniferus had already begun by late November 2011, when our spawning survey began. The proportion of individuals capable of spawning ripe gametes then remained consistently high from late November to early April; throughout that period, 75–100% of individuals released gametes (Figure 2A). The proportions then dropped in mid-April, and by the end of April only 40% of individuals released gametes. In addition, although the number of gametes produced by each individual was not counted, it was apparent that most females that did spawn in mid or late April released very few oocytes (~10–50), whereas most females spawning during the November–early April period released abundant oocytes, from several hundred up to a few thousand oocytes per female. Males also seemed to release substantially less sperm material in mid and late April than during the November to early April period.
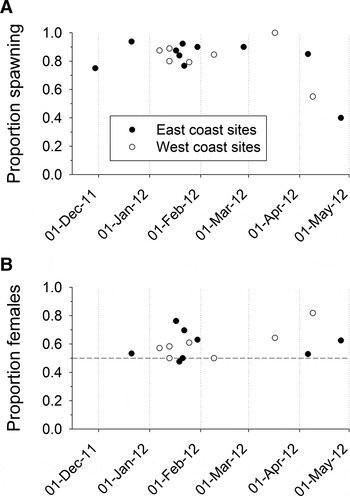
Fig. 2. (A) Proportion of Spirobranchus cariniferus adults, collected from east and west coast sites over the period 29 November 2011 to 26 April 2012, that released gametes in the laboratory; (B) proportion of spawning S. cariniferus that released oocytes.
The proportion of individuals releasing gametes was very similar for S. cariniferus collected on the east and west coasts of North Island (Figure 2A), consistent with the hypothesis of similar spawning periods on both coasts. The first collections from the west coast, however, were carried out in early January 2012, so it is not known whether the proportion of individuals capable of releasing ripe gametes was comparable for the two coasts prior to January.
On both coasts, sex-ratios tended to be slightly female-biased, with the proportion of individuals releasing oocytes ranging from 50 to 82% (Figure 2B). Overall sex-ratios, obtained by pooling data from all sampling dates for each coast, were 59.6% female to 40.4% male for the east coast, and 58.3% female to 41.7% male on the west coast; these values were significantly different from a 50:50 sex-ratio for east coast populations (χ 2 goodness of fit test: χ 2 = 5.769, df = 1, N = 156, P < 0.016) and almost significantly different from a 50:50 ratio for west coast populations (χ 2 = 3.806, df = 1, N = 139, P = 0.051). The female-biased sex-ratio was apparent even among the smallest worms; of the 26 worms <20 mg that we weighed, three (12%) did not release any gametes, 10 (38%) released sperm and 13 (50%) released oocytes.
Individuals reached maturity at small sizes; even the smallest individuals examined in the survey (11 mg, corresponding to a body length of about 1.5 cm) released gametes. A random complete block analysis of variance (ANOVA), using collection site as blocking variable, revealed no significant difference (F 2,249 = 1.305, N = 262, P = 0.273) in average body mass among individuals releasing sperm, oocytes, or not releasing any gametes (Table 1). In addition, the sizes of males, females and non-spawning individuals each spanned the full range of worm sizes that were tested in our survey (Table 1). None of the 279 individuals that were individually tested in our spawning surveys released both types of gametes, which would have been indicative of simultaneous hermaphroditism.
Table 1. Sizes of Spirobranchus cariniferus that were induced to release gametes in the laboratory.
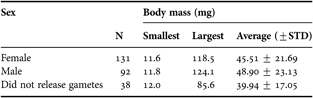
N, sample size; STD, standard deviation.
Effect of algal diet on larval growth
Larval development was significantly affected by algal diet. Larvae in all treatments grew rapidly up to day 8 after fertilization, after which average larval size stabilized or increased moderately (Figure 3). By day 8, average larval body length differed significantly among treatments (ANOVA; F 4,15 = 3.08, N = 20, P = 0.049); those provided with a mixed diet of Isochrysis galbana, Dunaliella tertiolecta and Chaetoceros muelleri were significantly larger than those fed only D. tertiolecta as determined by a Tukey honestly significant difference (HSD) multiple comparisons test. Larvae fed a mixed diet of I. galbana and D. tertiolecta, as well as those fed monoalgal diets of I. galbana or C. muelleri, had intermediate sizes. Larvae fed D. tertiolecta did continue to grow after day 8, but were still smaller on day 11 than in all other treatments except for the C. muelleri treatment (ANOVA; F 4,12 = 7.47, n = 16, p = 0.003, followed by Tukey HSD test).
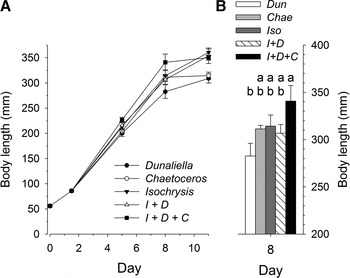
Fig. 3. Algal diet experiment: (A) body length of Spirobranchus cariniferus larvae as a function of age since fertilization for each of the 5 algal diet treatments; (B) average body length of larvae in the algal diet treatments 8 days after fertilization; values with the same letter are not significantly different as determined by a Tukey honestly significant difference multiple comparisons test. In both graphs, error bars represent standard error.
Effectiveness of IBMX and conspecific homogenates in inducing metamorphosis
IBMX at a concentration of 10−4 M was a potent inducer of metamorphic transformations in S. cariniferus larvae. No larvae metamorphosed in the trials on days 8 and 10 after fertilization, but almost all of the larvae in the 10−4 M IBMX treatment metamorphosed on days 13 and 15 (Figure 4A). The 10−4 M IBMX treatment was the only one to induce metamorphosis in a substantial proportion of larvae; few or no larvae in the control and 10−5 M IBMX treatments metamorphosed. Larvae that settled in the IBMX treatments completed most of the changes typically associated with this transition in serpulid polychaetes (Kupriyanova et al., Reference Kupriyanova, Nishi, Ten Hove and Rzhavsky2001): S. cariniferus larvae stopped swimming, lost their prototroch, secreted mucus that loosely attached the body of the juvenile worm to the bottom or side surfaces of the well and differentiated the head region into a branchial crown. IBMX did not, however, induce complete metamorphosis; none of the larvae in the IBMX treatments on days 13 or 15 secreted a calcareous tube, even several days after metamorphosis. In the 4–7 days following metamorphosis, these larvae did not grow and most had died by day 7 after metamorphosis.
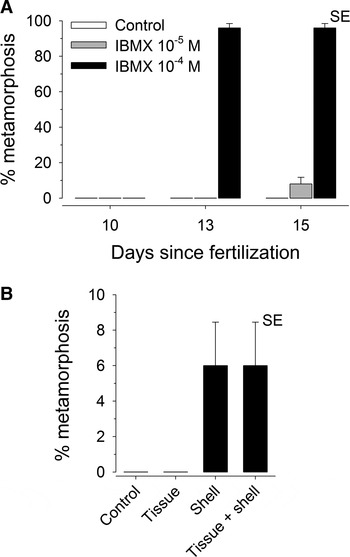
Fig. 4. Metamorphosis experiment: (A) percentage of Spirobranchus cariniferus larvae that metamorphosed when exposed to 3-isobutyl-1-methylxanthine as a function of age since fertilization; (B) percentage of 15 day old larvae that metamorphosed when exposed to surfaces coated with tissue or tube homogenate. In both graphs, error bars represent standard error (SE).
We then tested the effectiveness of conspecific tube and tissue homogenates as inducers of settlement, using 15 day old larvae from the above culture which had just been shown to have reached competence. No larvae metamorphosed in the control and tissue homogenate treatments during the 48 hours trial, but a small proportion of larvae did metamorphose in the tube homogenate treatment and in the tissue + tube treatment (Figure 4B), suggesting a modest effect of tube homogenate as inducer of metamorphosis. In addition, those larvae that did metamorphose completed full metamorphosis, including the secretion of a calcareous tube; these juveniles were reared for a further 3 weeks, during which all individuals survived and grew, reaching a size of ~5–6 mm tube length.
DISCUSSION
Spawning period, sex-ratio and adult body mass
Spirobranchus cariniferus has an extended spawning season, which in 2011 and 2012 spanned at least part of the austral spring as well as the entire summer. Most S. cariniferus already carried ripe gametes in late November when this study began, suggesting that by then the reproductive season had already been under way for some time. At any given time between late November and early April, most individuals (75–100%) were capable of releasing gametes. In addition, we obtained gametes from S. cariniferus on several occasions from December 2011 to early April 2012 to rear larvae for this and for a separate study (unpublished); abundant viable larvae were consistently obtained on all occasions, indicating that ripe gametes were being produced throughout the summer. The drop in proportion of S. cariniferus individuals capable of spawning in mid-April 2012, combined with the very small amount of gametes released by spawning individuals at that time, suggests the reproductive season was coming to an end in late April. That would be consistent with other serpulid species in which spawning ends in late summer or autumn (e.g. Ficopomatus enigmaticus (Fauvel, 1923) in England, Dixon, Reference Dixon1981; Hydroides ezoensis Okuda, 1934 in Japan, Miura & Kajihara, Reference Miura and Kajihara1981; Spirobranchus tetraceros (Schmarda, 1861) in Egypt, Selim et al., Reference Selim, Naby, Gab-Alla and Ghobashy2005).
From January to April 2012, the proportions of individual S. cariniferus releasing gametes was similar for populations from the east and west coasts of New Zealand's North Island, suggesting that reproductive seasons may be similar on both coasts. Our sampling of west coast populations only began in early January, however, so it is not clear whether the reproductive season began at the same time on both coasts. Our finding of an extended spawning season in S. cariniferus, spanning several months and during which a high proportion of adults are mature and may spawn repeatedly, is consistent with reports for several other serpulid polychaetes (Kupriyanova et al., Reference Kupriyanova, Nishi, Ten Hove and Rzhavsky2001) such as Ficopomatus enigmaticus in England (Dixon, Reference Dixon1981), Pomatoceros lamarkii (Quatrefages, 1866) in Ireland (Cotter et al., Reference Cotter, O'Riordan and Myers2003), P. triqueter (Linnaeus, 1758) in Ireland (Cotter et al., Reference Cotter, O'Riordan and Myers2003) and Portugal (Fragoso & Icely, Reference Fragoso and Icely2009), Serpula vermicularis Linnaeus, 1767 in Scotland (Chapman et al., Reference Chapman, Moore, Harries and Lyndon2007) and more specifically in various species of the genus Spirobranchus such as S. giganteus (Pallas, 1766) in Puerto Rico (Allen, Reference Allen1957), S. tetraceros in Egypt (Selim et al., Reference Selim, Naby, Gab-Alla and Ghobashy2005) and S. corniculatus (Grube, 1862) in Australia (Smith, Reference Smith and Hutchings1984).
Sex-ratios were most often slightly female-biased for populations on both coasts. Male and female worms also had the same average body size, and each sex had size-ranges spanning the broad range of sizes that were examined. Although a number of large-bodied serpulid species have been found to be protandric hermaphrodites, suggesting this might be a common life history trait in large-bodied Serpulidae (Kupriyanova et al., Reference Kupriyanova, Nishi, Ten Hove and Rzhavsky2001), our results do not support the hypothesis that S. cariniferus are protandric hermaphrodites. Protandric hermaphroditism is often associated with differences in average body size between sexes (Dixon, Reference Dixon1981; Castric-Fey, Reference Castric-Fey1984; but see Cotter et al., Reference Cotter, O'Riordan and Myers2003) and male-biased sex-ratios among small adult size-classes (Castric-Fey, Reference Castric-Fey1984). In our samples of S. cariniferus, however, males and females did not differ in body size, and there were slightly more females than males among the smallest worms (<20 mg body mass). Our results for this species are consistent with gonochorism, but might also be consistent with simultaneous hermaphroditism in which individuals release sperm and oocytes on different dates.
Effect of algal diet on larval growth
Spirobranchus cariniferus was found to be particularly amenable to laboratory and experimental studies, being highly abundant on mid-intertidal rocky shores, having a broad spawning season and being very easy to rear in the laboratory on a variety of algal diets. However, algae are not all of equal value to S. cariniferus larvae. Larvae grew faster on a mixed diet of Isochrysis galbana, Chaetoceros mulleri and Dunaliella tertiolecta than on a unialgal diet of D. tertiolecta. The mixed algal diet did not support significantly faster larval growth than diets of I. galbana or C. mulleri alone, however, and combining I. galbana and D. tertiolecta did not enhance larval growth above that observed when only I. galbana was provided, suggesting the observed differences in larval growth were primarily due to D. tertiolecta being of lower nutritional value to S. cariniferus larvae than the other algae. Isochrysis galbana and C. mulleri appear to be of similar nutritional value to S. cariniferus larvae, and might provide some complementary nutrients to S. cariniferus larvae, whereas D. tertiolecta are a lesser quality food source supporting slower growth and resulting in either an extended time to reach competence or small size at competence. This finding is consistent with reports that prawn larvae (Penaeus spp.) fed D. tertiolecta also have reduced growth and survival relative to larvae fed other algal species (Kurmaly et al., Reference Kurmaly, Jones, Yule and East1989; D'Souza & Loneragan, Reference D'Souza and Loneragan1999).
Effectiveness of IBMX and conspecific homogenates in inducing metamorphosis
Our study revealed IBMX to be an effective indicator of competence in S. cariniferus larvae. IBMX solutions at a concentration of 10−4 M induced metamorphosis in virtually all larvae aged 13 days and 15 days but induced no detectable response in younger larvae. The same concentration of IBMX also induces metamorphosis in high proportions of Hydroides elegans larvae (Bryan et al., Reference Bryan, Qian, Kreider and Chia1997) and Phragmatopoma lapidosa californica larvae (Pawlik, Reference Pawlik1990). Given that larvae may become responsive to IBMX some time before becoming responsive to natural settlement cues (e.g. 1 day earlier in H. elegans: Pechenik & Qian, Reference Pechenik and Qian1998) and that 15 day old S. cariniferus larvae did settle and metamorphose on adult tube material in the second metamorphosis experiment, our results indicate that competence in S. cariniferus can be reached within 13–15 days after fertilization. The time required to complete larval development, however, appears to vary depending on the conditions experienced during larval development, such as diet and larval density. In our earlier algal diet experiment, larvae in two of the algal treatments reached full size (~300–330 µm body length) in as little as 8 days, after which size stabilized, whereas in the other three treatments growth continued up to day 11. In addition, most larvae reared for the metamorphosis experiments, which were held at the higher density of ~10 larvae per ml compared to 2 per ml in the algal diet experiment, reached full sizes (~300 µm body length) and competence after 13 days. Time to reach competence may therefore vary from 8–15 days depending on the conditions experienced during larval development.
IBMX has been reported to induce metamorphosis in a variety of species, including polychaetes (Pawlik, Reference Pawlik1990; Bryan et al., Reference Bryan, Qian, Kreider and Chia1997; the present study), barnacles (Clare et al., Reference Clare, Thomas and Rittschof1995; Holm et al., Reference Holm, McClary and Rittschof2000), abalone (Baxter & Morse, Reference Baxter and Morse1987) and bivalves (Dobretsov & Qian, Reference Dobretsov and Qian2003; Mesías-Gansbiller et al., Reference Mesías-Gansbiller, Bendimerad, Román, Pazos, Sánchez and Pérez- Parallé2008; Sanchez-Lazo & Martinez-Pita, Reference Sánchez-Lazo and Martínez-Pita2012). Spirobranchus cariniferus larvae that responded to IBMX completed most behavioural and anatomical changes associated with metamorphosis except that, unlike H. elegans larvae (Bryan et al., Reference Bryan, Qian, Kreider and Chia1997; Pechenik & Qian, Reference Pechenik and Qian1998), S. cariniferus larvae responding to IBMX never secreted a calcareous tube and, when left in the wells with algal food they did not grow and died within 7 days. Although metamorphosis without tube formation has not been reported for other serpulid species, IBMX has been found to have toxic effects at similar concentrations on larvae of mussels (Sanchez-Lazo & Martinez-Pita, Reference Sánchez-Lazo and Martínez-Pita2012) and oysters (Sriyutha Murthy et al., Reference Sriyutha Murthy, Venugopalan, Nair and Subramoniam1999).
Larvae did not metamorphose in the controls of either the first or second metamorphosis experiments, suggesting S. cariniferus larvae require particular chemical cues to trigger metamorphosis. In the second experiment, larvae attached and metamorphosed only in response to crushed tube material from conspecific adults. In addition, those individuals responding to crushed tube material produced a calcareous tube, grew and remained healthy during the following 3 weeks. These findings are consistent with a report by Marsden (Reference Marsden1991) that 7–9 day old late metatrochophore larvae of S. polycerus augeneri Ten Hove, 1970, a congeneric species that is also gregarious and intertidal, are attracted to odours of empty tubes from conspecific adults. Our findings contrast, however, with those of Bryan et al. (Reference Bryan, Qian, Kreider and Chia1997) who found that larvae of the serpulid tubeworm H. elegans settle and metamorphose in response to conspecific adult tissue homogenate but not to tube homogenate. The reason for this difference in response by S. cariniferus and H. elegans is not clear, but may be related to differences in habitat (H. elegans are subtidal) and in composition of the biofilm community present on the adult tube and body, since serpulid polychaetes use specific bacterial strains as cues for settlement and metamorphosis (Hadfield et al., Reference Hadfield, Unabia, Smith, Michael, Thompson, Nagabhushanam, Sarolini and Fingerman1994; Toonen & Pawlik, Reference Toonen and Pawlik1996; Hamer et al., Reference Hamer, Walker and Latchford2001; Lau et al., Reference Lau, Harder and Qian2003; Hadfield, Reference Hadfield2011).
In our study, the proportion of S. cariniferus larvae settling in response to crushed tube material from conspecific adults was low (only 6%), but this contrasted with the tissue homogenate and control treatments in which no larvae settled. This suggests the settlement cue, perhaps one or more compounds produced by bacteria developing on S. cariniferus tubes, was in low concentration in our treatments. Low concentration of the cue could have resulted from dilution or from chemical breakdown during preparation of the treatment coverslips. In some serpulid species, the settlement-inducing effect of biofilms on larvae can be significantly diminished by exposure to certain environmental conditions, for example by exposure of the biofilm to ultraviolet radiation (e.g. H. elegans: Hung et al., Reference Hung, Thiyagarajan, Wu and Qian2005) or to air drying for 1–2 h (P. lamarkii: Hamer et al., Reference Hamer, Walker and Latchford2001). Homogenates in our experiment were not exposed to ultraviolet radiation, but the treatment surfaces were dried for 3 hours before exposing to larvae. However, H. elegans and P. lamarkii are primarily subtidal in distribution and their larvae settle on biofilms that are not normally exposed to aerial conditions, whereas S. cariniferus primarily colonizes the mid-intertidal zone where adult tubes are repeatedly exposed to air and sunlight at low tide and often dry out. It would seem maladaptive for S. cariniferus larvae to seek cues that lose potency when dried or exposed to light. Alternatively, S. cariniferus larvae may have responded to the coarse texture of the crushed tube material rather than to a chemical cue. In any case, the settlement response of competent S. cariniferus larvae to conspecific tube material is consistent with the highly gregarious distribution of juvenile and adult S. cariniferus in the field and merits further investigation as a mechanism responsible for the distribution of individuals.
ACKNOWLEDGEMENTS
We thank E. Duder, B. Patel, J. Peters and M. Hudson for providing technical support for this project and V. Ward for help with map design. This research was funded by an NSERC grant and PA fund support from TRU to L.A.G.







