Introduction
Although a great deal of research has been directed towards the management of herbivorous insects on crop plants, little of this research has focused on the behaviour of herbivores within whole plants. More explicit studies of intra-host plant movement, in particular, are required to help us understand how and why a larva ‘chooses’ its feeding site, and thus inflicts possible economic damage to crop plants. Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae)-Pisum sativum (L.) was used in this study as a model herbivore-host system to explore in some detail the behavioural mechanisms responsible for caterpillar macro-movement within plants. Helicoverpa armigera is an economically-important herbivore in many countries from the Cape Verde Islands in the East Atlantic, through Africa, Asia and Australasia, to the South Pacific Islands, and from Germany in the north to New Zealand in the south (Reed & Pawar, Reference Reed and Pawar1982). It attacks a wide variety of agricultural and horticultural plants as well as indigenous species (Zalucki et al., Reference Zalucki, Daglish, Firempong and Twine1986, Reference Zalucki, Murray, Gregg, Fitt, Twine and Jones1994), mostly in the semi-arid tropics (Reed & Pawar, Reference Reed and Pawar1982). The garden pea, P. sativum, was chosen as the model host plant since H. armigera survive well on this host; the plant has a relatively simple linear structure, and several strains and mutants of varying plant attributes were available.
The distribution of young H. armigera larvae on whole plants has been described for cotton, tomato, mung bean and pigeon pea. First instars were sampled from the fruiting structures of cotton over a season and were found mostly on small squares (immature flowers whose surrounding bracts form a ‘square’) and never on open flowers or bolls (Wilson & Waite, Reference Wilson and Waite1982). Similarly, Hassan (Reference Hassan1983) observed on cotton that small squares were the preferred feeding sites of 1st instar larvae, and he attributed this finding to the fact that young fruiting structures were softer than more developed ones. Yang et al. (Reference Yang, Johnson and Zalucki2008) sampled entire conventional and Bt-expressing cotton plants at the squaring stage (just before flowering) and found that 1st instars were most often on squares (30% and 56%, respectively) and mature leaves (55% and 41%) rather than on immature leaves (14% and 0%) and terminals (1% and 2%). Hochberg (Reference Hochberg1987) found that larvae fed on a combination of leaves, flowers and fruit of the tomato, and suggested that the age of the plant component upon which larvae eclose is more important than the type of component (flowers vs. leaves) to their subsequent distribution. On mature pigeon pea plants, larvae collectively spent most of their time on the reproductive parts (flowers and pods); but, on mature mung bean, larvae were found less often on flowers or flower buds than leaves (Johnson & Zalucki, Reference Johnson and Zalucki2005). Whilst there is some evidence from laboratory studies that young H. armigera larvae are more likely to stay on the flowers of certain host plants once there (Rajapakse & Walter, Reference Rajapakse and Walter2007), there is little evidence from studies on whole plants that 1st instars are attracted to flowers or generally prefer to feed on flowers.
The intra-plant movement of 1st instars on a small number of hosts has been described in general terms (Hassan, Reference Hassan1983; Hochberg, Reference Hochberg1987; Johnson & Zalucki, Reference Johnson and Zalucki2005, Reference Johnson and Zalucki2007), but such movement is rarely quantified due to the laborious nature of the task. First instar H. armigera on plants cannot be videoed for later analysis because they are small (2 mm) objects on complex 3-D surfaces and do not contrast well with their background substrate. Hence, data must come from direct personal observation or by following silk trails (Johnson et al., Reference Johnson, Merritt, Cribb, Trent and Zalucki2006). In general, newly hatched larvae of H. armigera have demonstrated either little displacement (Hassan, Reference Hassan1983) or moved upwards on the main stem and outwards along the branches of the host plant (Hassan, Reference Hassan1983; Johnson & Zalucki, Reference Johnson and Zalucki2005, Reference Johnson and Zalucki2007). This behaviour is thought to be driven by light and the angle of the plant substrate (Perkins et al., Reference Perkins, Cribb, Hanan, Glaze, Beveridge and Zalucki2008). Johnson & Zalucki (Reference Johnson and Zalucki2005) found that a greater proportion of 1st instars (97%) moved upwards on flowering pigeon pea plants than on immature pigeon pea (71%), which suggests that the pigeon pea flowers may have been ‘drawing’ larvae upwards. Once there, all larvae stayed at the top of flowering plants, but only 58% stayed at the top of immature plants. The same effect was not found for mung bean, where 92% of 1st instars moved upwards on both flowering and non-flowering plants. However, of those larvae, 50% stayed at the top of flowering plants and only 24% at the top of immature plants (Johnson & Zalucki, Reference Johnson and Zalucki2005). Again, these studies support the idea that young larvae are less likely to leave the top of a plant if flowers are present.
Previous studies have shown that while the macro-architecture of plant components affects the likelihood and direction of 1st instar movement (Perkins et al., Reference Perkins, Cribb, Hanan, Glaze, Beveridge and Zalucki2008), the micro-architecture of leaf surfaces affects the speed of movement (unpublished data). To further investigate the mechanisms underlying larval movement, here, we also measured leaf nitrogen content, leaf water content and the hardness of various plant components. Emergent patterns in these plant attributes were related to quantified larval movement.
The specific aims of this study were: (i) to measure how far H. armigera moved from their eclosion site during the first stadium (three days); (ii) to record the plant component that larvae were located on at the end of the stadium; (iii) to determine how flowering affects the distance moved and distribution of 1st instars; and (iv) to determine how egg placement on differently aged leaves affects the distance moved and distribution of 1st instars. Because so many plant attributes can influence larval movement (see Zalucki et al., Reference Zalucki, Clarke and Malcolm2002, for a review), we took a novel approach and used plants that differed in one gene only (the gene for flowering) in order to compare flowering and non-flowering plants at the same age and at the same time. Data were collected by direct observation in glasshouse trials of H. armigera eggs eclosed on host P. sativum plants.
Materials and methods
Plants
Pisum sativum (L.), the garden pea plant, was grown in a glasshouse at the University of Queensland, St. Lucia, Australia. Genotypes L107 and K218 were grown from seed in 150 mm pots using commercial potting mix (Amgrow Black Label Pot ‘n’ Peat, Envirogreen Pty Ltd, Christensen Road, Stapylton 4207, Queensland) sterilized by steam. Plants were watered four times per week and fertilized once per week with a general purpose liquid fertilizer (N:P:K=20.8:3.3:17.4) (Flowfeed EX7, Growforce, www.growforce.com.au ).
Genotype L107 (cv. Torsdag) was a tall wild type strain used as the standard. Genotype K218 (dne) was a ‘day neutral’ tall early flowering mutant in the Torsdag background. The two genotypes differed only in the gene for flowering. Plants were used when 5–6 weeks old, at which time they had grown an average of 12 nodes. Whereas K218 peas were flowering when used in the experiment, L107 peas were not. This allowed direct comparison between flowering and non-flowering plants at the same age and time. The plants had a linear structure of a single main stem and leaves comprising leaflets and tendrils (fig. 1). Stipules (modified leaflets) almost fully encircled the stem at nodes where leaves joined the stem (fig. 1).
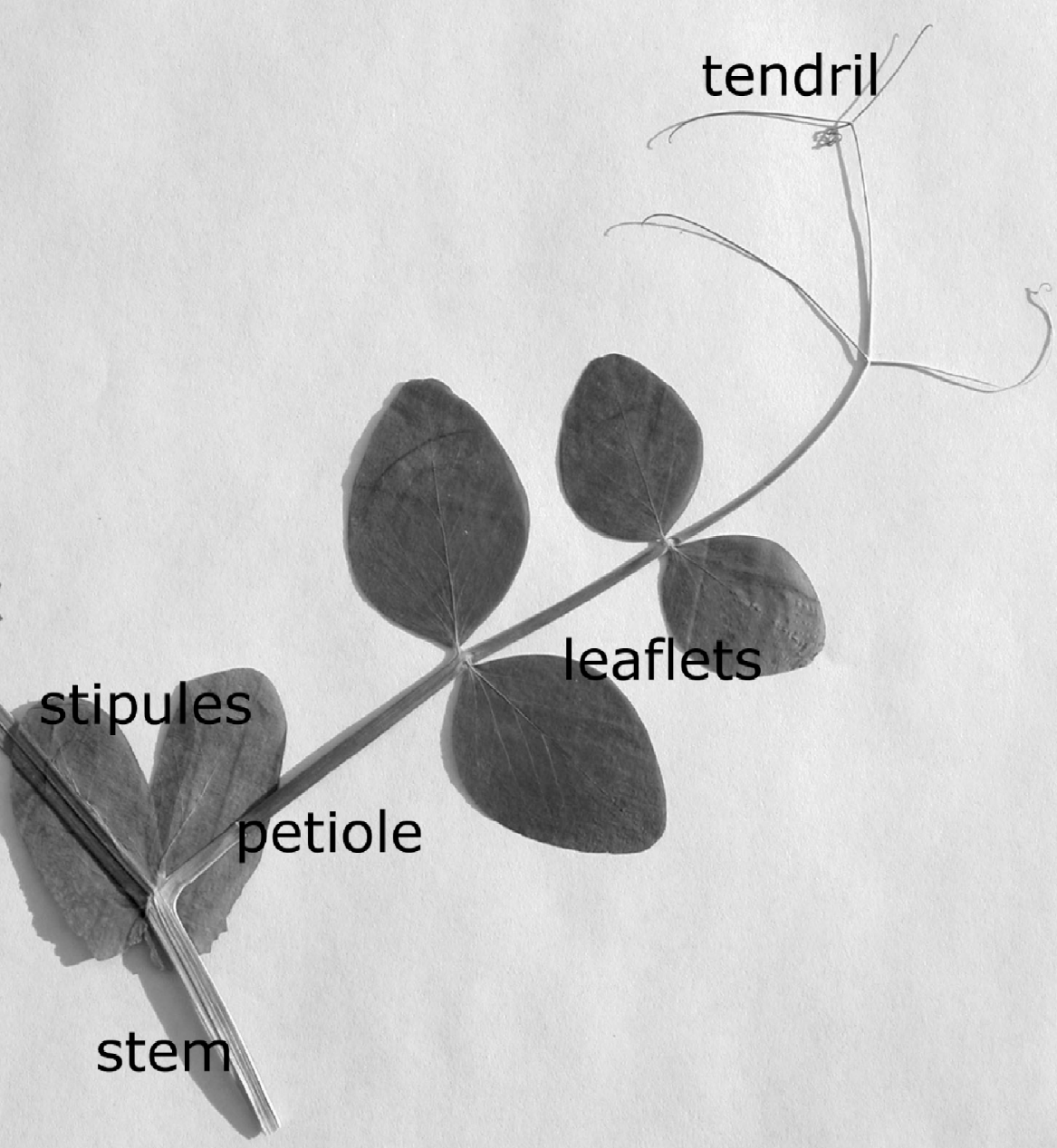
Fig. 1. A single node of the garden pea plant P. sativum showing stem, stipules, and a single leaf comprising petiole, leaflets and tendrils.
Insects
Helicoverpa armigera eggs were obtained from a culture maintained at the Queensland Department of Primary Industries and Fisheries, Toowoomba, Australia. Eggs were hatched and larvae reared to pupation at 25.0°C on a soyflour-based artificial diet (modified from Teakle & Jensen, Reference Teakle, Jensen, Singh and Moore1985). The diet was modified by substituting the formaldehyde with 2.6 ml of anti-fungal solution (42% propionic acid and 4% phosphoric acid in water). Pupae were placed in a cage and held at an average of 25.6°C, 46% RH, and a photoperiod of 12:12 h (L:D). After emergence, adults were provided with 10% sucrose solution and allowed to mate freely. Females oviposited on a fabric liner (Kimwipes, Kimberly-Clark Aust. Pty Ltd, www.kcprofessional.com) covering the top of the cage. Eggs on liners were placed in a sealed plastic bag and allowed to mature in the laboratory at 25.0°C. Over three days, eggs changed from a pearl-white colour when newly laid to a dark brown colour with a visible black head capsule when ready to hatch, at which stage they were used in experiments.
Experimental design of movement assays
Two groups of 20 or 30 pea plants were sown as above at either end of a large glasshouse: one group of L107 genotype (tall wild type) and one group of K218 genotype (tall early flowering). After 5–6 weeks' plant growth, a single H. armigera egg was placed on a leaflet in either the lower, middle or upper third of each plant using a minimal amount of hen albumen to attach the egg. The 1st instar larva on each plant was located and its position recorded daily for three days (until the end of the stadium). The design above was repeated five times over a year from August 2006 to August 2007 to obtain a data set of approximately 100 plants where the larva was located at the end of three days. Not all seeds germinated, and some assays had plants affected by powdery mildew. Eggs were placed on 138 plants in total. The placement of the groups of plants in the glasshouse was reversed for each assay and the results were pooled, as a Chi-squared test showed there was no association (at P=0.05) between assay number and data used for analyses below.
The net distance moved by each larva (the Euclidean distance from the egg to the end point of the larva after three days) was calculated. A generalized linear model incorporating a negative binomial distribution for the response variable was fitted to the raw data using R (R Development Core Team, 2007). The final plant component upon which the larva was found was also recorded to determine if larvae ‘preferred’ particular plant parts. Data were expressed as a multi-way frequency table of the final plant component (leaf, stipule, petiole, tendril, pod/bud/flower) against treatment factors (genotype and position of egg placement). A multinomial model was fitted directly to the data (excluding pod/bud/flower) using R as described by Venables & Ripley (Reference Venables and Ripley2002). The flower and pod components were not included in the analysis because genotype L107 plants were not flowering and, therefore, could not have these structures. A Chi-squared test was used to determine associations between genotype or the section of plant where the egg was placed and the number of larvae lost during the trials. Similarly, a Chi-squared test was used to determine associations between genotype or the section of plant where the egg was placed and the number of larvae leaving the natal leaf.
Measurement of plant attributes
Plant component hardness
The hardness of leaves, petioles and stems was measured using a Chatillon® AG dial tension gauge (AMETEK US Gauge Division, www.ametek.com) of 50 g or 150 g capacity. The tension gauges measured grams of force (applied through a probe) required to pierce the cuticle. By measuring the diameter of the probe, the force in pounds per square inch (psi) was calculated and then converted to kilopascals (kPa). Ten L107 pea plants were delineated into three regions: lower, middle and upper. A pair of adjacent leaflets from two nodes from each region of each plant was measured for hardness. Three measurements were taken each from the adaxial (upper) and abaxial (under) surface of the leaflets and were averaged to give a mean for each leaf surface for each node. The hardness of the petioles and stems was measured for the lower, middle and upper regions of five pea plants. An average of three measurements was taken to achieve each data point. All measurements were taken in the glasshouse where the plants had been raised from seed. A nested general linear model (GLM) was fitted to the data of leaf hardness. A paired t-test was used to test for differences in hardness between the adaxial and abaxial surfaces of adjacent leaflets within the same leaf. Two-way ANOVA was used to determine significant between- and within-plant variability of petiole and stem hardness.
Water content of leaves
Pea plants were moved from the glasshouse to the laboratory, watered well and left for two hours to hydrate. Individual pea leaflets were excised and weighed immediately using a Sartorius BP210 D balance (www.sartorius.com). Weighed leaflets were placed in open glass Petri dishes (60 mm×15 mm) in a Kangbao SDX 71C drying oven (Guangdong Kangbao Electrical Co., Ltd, www.kangbao.com.cn) for five days. Dried leaflets were removed from the oven and immediately weighed. The percent water content of leaflets was calculated as (fresh weight–dried weight)/fresh weight×100. Two leaflets from each of the three regions (lower, middle and upper) of 13 plants were sampled for water content. A GLM was fitted to the raw data.
Nitrogen content of leaves
Leaflets from each of the three regions (lower, middle and upper) of five plants were excised in the glasshouse and analysed for nitrogen content using a LECO CNS 2000 combustion analyser (LECO Australia Pty Ltd, www.leco.com.au) set at 1100°C and following standard protocols (Rayment & Higginson, Reference Rayment and Higginson1992). Results are automatically expressed as dry weight percentages. A two-way ANOVA was used to determine if the nitrogen content of leaves varied between and within plants.
Results
A total of 138 P. sativum plants had H. armigera eggs placed on them. Eggs did not hatch on 12 plants (9%), and larvae were lost from a further 27 plants (21%) over the three-day trial period. There was no association between the number of larvae lost and either genotype (P=0.22) or the region of the plant (P=0.34) where the eggs were placed.
Of 92 larvae for which complete movement data were obtained, 9 larvae (10%) had net displacement downwards on the plant, 45 larvae (49%) displaced upwards and 38 larvae (41%) did not move from the node where they eclosed. The net distance moved by 1st instar H. armigera over the stadium varied from zero to ten nodes. Egg placement was the only factor that significantly influenced the proportion of larvae leaving the natal leaf (χ22=7.848, P=0.02) and how far individual larvae moved (F2=7.909, P<0.01) (fig. 2). Larvae that hatched from eggs placed in the lower region of the plant moved away from the natal leaf (comprising a number of leaflets) more often than expected and moved (mean±SD) further, 3.11±2.74 nodes, than larvae from eggs in the middle region, 0.87±1.77 nodes, or upper region, 0.40±1.41 nodes, of the plant. There was no significant difference between the proportion of larvae leaving the natal leaf or the number of nodes moved by larvae on flowering compared to non-flowering plants (fig. 3).

Fig. 2. The net distance moved by 1st instar H. armigera hatched on P. sativum plants where eggs were placed on the lower, middle, or upper region of the plant. (□, lower third, n=26; ▪, middle third, n=31; ![]() , upper third, n=35).
, upper third, n=35).

Fig. 3. The net distance moved by 1st instar H. armigera on P. sativum plants. (□, genotype L107 non-flowering, n=49; ▪, genotype K218 flowering, n=43).
At the end of the stadium, 1st instars were found mostly on edible plant components: stipules, leaflets, tendrils and buds/flowers/pods; larvae were rarely found on inedible components (stems and petioles) (fig. 4). The multinomial model of best fit contained plant genotype as the only significant factor (ANOVA3, P Chi=0.03) affecting larval distribution. Larvae were found less often on the leaflets of flowering plants (35%) (and more often on stipules, tendrils and buds/flowers/pods) compared to non-flowering plants (65%). The region of egg placement within the plant did not affect the final distribution of 1st instars by plant component (P Chi=0.12).

Fig. 4. The components of P. sativum plants where larvae were found at the end of the 1st stadium (□, genotype L107, non-flowering, n=49; ▪, genotype K218, flowering, n=43).
The hardness of leaflets ranged from 1609 kPa to 3353 kPa. The average hardness of leaflets, petioles and stems from each vertical plant section is given in table 1. The adaxial (upper) surface was significantly softer than the abaxial (under) surface of paired leaflets (t=2.83, P<0.01), so each surface is tabled separately. The GLM fitted to the hardness of leaves data showed significant differences between plants, and vertical regions within plants, (adaxial surface: F9=10.82, P<0.01; F20=7.639, P<0.01, respectively, and abaxial surface: F9=10.86, P<0.01; F20=2.08, P=0.03, respectively). There was no consistent pattern of variation in leaf hardness between the vertical regions within plants; the lower, middle or upper region was hardest in different plants. The mean petiole hardness was 1938±288 SD kPa and mean stem hardness was 3476±254 SD kPa (table 1). Petiole hardness did not vary between plants but varied significantly between the lower, middle and upper regions within plants (F2=17.32, P<0.01). Petioles at nodes in the lower third of plants were the softest with either the middle or upper petioles being the hardest. Similarly, stem hardness varied significantly between plant regions (F2=23.26, P<0.01) but not between plants. Stems in the lower third of plants were softest with either the middle or upper stems the hardest.
Table 1. The mean surface hardness (kPa) of leaves, petioles and stems of the garden pea P. sativum across three regions: lower third of plant (lower); middle third of plant (middle); upper third of plant (upper).

SD, standard deviation; n, sample size.
Significant differences are presented in the text.
A GLM fitted to the water content of leaves data showed significant variation both between individual plants (F12=2.42, P=0.02) and between regions within plants (F2=161.12, P<0.01). There was a significant interaction between plant and region of plant (F24=4.11, P<0.01), but this could be attributed to a single plant. Water content decreased from the bottom to the top of 12 plants, but for one plant the upper region had the highest water content. Overall, the mean water content of leaves was: 87.40±1.34% SD for the lower regions, 85.13±1.11% SD for the middle regions and 83.17±1.33% SD for the upper regions. The nitrogen content of leaves also varied significantly both between (F4=14.90, P<0.01) and within (F2=23.75, P<0.01) pea plants. Within plants, the nitrogen content consistently increased from the bottom to the top of plants. The mean percentage of nitrogen (dry weight) was: 5.22±1.63% SD for lower region leaves, 6.10±1.39% SD for middle region leaves and 7.61±0.79% SD for upper region leaves.
Discussion
Data from 92 individual larvae moving on a pea plant were obtained from the glasshouse trials, and their net distance moved (in nodes) and final distribution were determined by direct observation. The egg non-viability rate of 9% was similar to that recently reported by Bisane & Katole (Reference Bisane and Katole2008) for eggs that were field collected (9.35%), laboratory cultured on pigeon pea (10.19%) and laboratory cultured on artificial diet (10.94%). The survival rate from egg hatch to the end of the 1st stadium was 79%. The survival rate of 1st instars in our glasshouse study cannot be compared to survival rates in the field because the conditions are very different. Under field conditions, wind, rain, heat, predators and competition from conspecifics all contribute to the high mortality of 1st instars (Zalucki et al., Reference Zalucki, Clarke and Malcolm2002). Losses could be due to predators such as spiders, although predators and parasitoids were removed from the glasshouse as far as practicable. It is worth noting that the rate of ‘drop off’ from pigeon pea plants in experimental field plots was 15% (Perovic et al., Reference Perovic, Johnson, Scholz and Zalucki2008), and this behaviour could account for the loss of larvae in our glasshouse trials in the absence of predators and adverse environmental conditions.
At the end of the first stadium, most larvae were found on an edible component at a feeding site, demonstrating that freely-roaming 1st instar H. armigera will naturally feed on the vegetative and reproductive parts of P. sativum. Only four larvae (two on flowering and two on non-flowering plants) from 92 were found on an uneaten component, the petiole, presumably in transit between feeding sites. After removing reproductive structures from the analysis, the genotype of the host plant still affected where the larva was found. On flowering plants, fewer larvae were found on the leaflets than on non-flowering plants, but more larvae were found on stipules and tendrils. The stipules and tendrils are the extremities of a pea leaf (see fig. 1). So, even though flowering did not induce larvae to move away from the leaf and onto the stem significantly more often, larvae on flowering plants did move more locally, away from leaflets and usually towards tendrils (but staying within the leaf). It follows that there may be an interaction between floral odour emitted and certain architectural features of the plant. This seems likely since we have already shown that substrate angle and floral odour interact to influence larval movement (Perkins et al., Reference Perkins, Cribb, Hanan, Glaze, Beveridge and Zalucki2008). Furthermore, in separate experiments larval movement (kinetic activity) increased at low concentrations and decreased at high concentrations of floral odours (Perkins et al., Reference Perkins, Cribb, Hanan and Zalucki2009). The surface of leaflets is relatively flat (horizontal) over most of the plant compared to stipules and tendrils which are steeper. Movement of first instars of other insect species has been shown to be affected by interactions between plant volatiles and leaf angle (Bernays et al., Reference Bernays, Woodhead and Haines1985).
Similar to results obtained by Hassan (Reference Hassan1983), in this study many H. armigera first instars (41%) did not displace from the plant structure where they eclosed. Thus, the region where young larvae settle to feed appears to be partially dependent on where upon the plant the mother lays her eggs. The within-plant distribution of H. armigera eggs has been quantified on cotton. Hassan (Reference Hassan1983) found the distribution was clumped, and under a wide range of conditions more than 70% of eggs were located within the upper third of the plants. Leaves and bracts were the main plant components on which oviposition occurred. Where plants were spaced further apart or the cultivar had an open canopy, more eggs were laid in the lower parts of the plants than when the canopy was more impenetrable. Callahan (Reference Callahan1957) demonstrated explicitly that gravid females needed a stable grip before eggs could be deposited; for example, pubescent plant structures have consistently more eggs laid on them than smooth structures. The physical attributes of accessibility, stability and a graspable surface appear to most influence where upon a host female H. armigera lay their eggs. Plant components with these attributes are not necessarily the most suitable components for larval development; larvae that can move to more nutritious parts of the plant would grow fitter. How nectar sources and plant chemicals affect within-host oviposition is less clear, and further work is needed to resolve this.
Of those larvae that did move away from the natal leaf, the majority, 83%, moved upwards on the plant and only 17% moved downwards. This finding concurs with Hassan (Reference Hassan1983) and Johnson & Zalucki (Reference Johnson and Zalucki2005, Reference Johnson and Zalucki2007) and adds to the weight of evidence that when young Lepidoptera larvae move on a plant, in general, they display a replicable behaviour moving up on their host plant (Perkins et al., Reference Perkins, Cribb, Hanan, Glaze, Beveridge and Zalucki2008). Whether or not the pea plants were flowering did not significantly affect the likelihood or extent of intra-plant movement of 1st instars between nodes of P. sativum. This finding taken together with the findings of Johnson & Zalucki (Reference Johnson and Zalucki2005) suggests that the ‘attractiveness’ of flowers to young H. armigera larvae may be specific to pigeon pea and further supports the theory that pigeon pea might be a primary host plant of this insect (Rajapakse et al., Reference Rajapakse, Walter, Moore, Hull and Cribb2006; Rajapaske & Walter, Reference Rajapakse and Walter2007).
Larvae that hatched from eggs deposited in the lower region of pea plants moved away from their natal leaf more often and moved further than larvae that hatched in the plant's middle or upper regions. Although larvae lower in the plant have further to move upwards before reaching the apex than larvae in the upper parts of the plant, this does not account for the difference in net distances moved. Larvae hatched in the upper third of plants only moved an average of 0.4 of a node although they could have moved much further, and only 54% left their natal leaf compared to 81% of larvae hatched in the lower third.
The nitrogen and water content of food are important determinants of young caterpillar performance (Zalucki et al., Reference Zalucki, Clarke and Malcolm2002). In this study, leaves in the lower, older region of plants contained the least proportion of nitrogen and were likely to be of least nutritive value to larvae. Although larvae do not feed consistently until they are at least one day old (Perkins et al., Reference Perkins, Cribb, Hanan, Glaze, Beveridge and Zalucki2008), younger larvae do take small intermittent meals, usually starting with the leaflet upon which they eclose. These small meals could be interpreted as larvae sampling the substrate for suitability as a food source, and they could determine whether the larva moves on or not. As a larva could not predict the nutritive suitability of future samples, the decision as to whether a substrate is suitable or not must depend upon its cognitive comparison to some innate pattern of minimum suitability. Larvae that leave a nutritionally poor leaflet to move up the plant (using light and gravity as cues) should reach more nutritive leaflets or reproductive structures where they settle to feed and, therefore, should gain a fitness advantage over those that don't move. The increased fitness of ‘movers’ would result in natural selection for larvae that display this behaviour.
Contrary to nitrogen content, the water content of leaves in our study decreased with the height of the pea plants, such that lower leaves had the highest water content and upper leaves the least. Upper parts of the plants are also the first to show signs of water stress and, therefore, must be more prone to water loss. As older leaves age and senesce, they will eventually become drier but this was not the case in our study. The results of this current study can be interpreted as indicating that decreased water content was not a factor causing larvae to move from the lower nodes of the plants. In the upper nodes, the lower water content along with higher nitrogen content of leaves of the pea plants would mean these represented the most concentrated nutritive value (in terms of nitrogen per mass), and those of the lower nodes the least nutritive value. Since larvae that eclosed on the lower nodes of P. sativum moved more often and further, our findings support Hochberg's (Reference Hochberg1987) assertion that the age of the plant node upon which larvae eclose is an important determinant of their subsequent distribution. We consider that the nutritive value of its substrate is a probable mechanism underlying a larva's ‘choice’ to move.
Leaflet hardness varied considerably within plants, but with no discernable pattern. For this reason, hardness was discounted as a factor influencing the movement of 1st instars on a macro-level. However, larvae were not observed feeding on the hardest plant parts (e.g. stems) so hardness may be one factor affecting the ability of young larvae to initiate feeding. The hardness of plant components is likely to influence movement at a local (micro) scale where hard plant parts are ‘ignored’ as a potential food source, and the larva moves on until softer plant tissue is encountered.
The movement of young caterpillars on plants is assumed to be variable between Lepidoptera species and between host plants although few comparative studies exist. Our observations of 1st instar H. armigera movement on P. sativum concur with studies on other species in terms of the likelihood and direction of displacement. Perhaps more important, though, are the mechanisms underlying these observations: the ‘why’ and ‘how’ of larval movement on hosts. From these glasshouse trials, it appears that the age/nutritive value of plant tissue influences larval displacement, then light and gravity are the cues used to move up plants. Our data do not support the idea that 1st instars are directly attracted to flowers via chemotaxis, although this may happen on other hosts such as pigeon pea plants. Nevertheless, several studies have shown that larvae do not move away from flowers once they have been encountered, and our observations support this view. Larvae stopping in the vicinity of flowers are likely to encounter high quality food. Floral odour or flower structure are putative ‘arrestants’ that need further investigation.
The ecological implications of different behavioural movement strategies are best explored initially by observation and experimentation at the individual larva level on whole plants. Further studies of different caterpillar-host systems will progressively build our understanding of the mechanisms involved in movement, and patterns of response common to groups of species, or even Lepidoptera generally may emerge. The fact that only about half of 1st instars moved from the leaves they eclosed on begs the question: ‘Why do some larvae move and not others?’ More empirical studies are needed to resolve this question, although the nutritive status of the substrate looks likely to play a role. Not moving also has advantages; the larva will conserve energy and may be less exposed to predators and parasitoids.
Feeding site selection by young H. armigera larvae appears to be a result of each larva's innate response to certain plant and environmental cues rather than as a result of an active search pattern or attraction to nutritive plant parts per se. Manipulation of behaviourally significant plant attributes or the environment through plant breeding and cultural practices has the potential to minimize economic damage from feeding herbivores by altering feeding site selection. At the very least, understanding the movement behaviour of insect pests on plants assists those interpreting field data and making pest management decisions. In addition, a conceptual and methodological framework is emerging which links individual-level behavioural mechanisms to population-level movements (Nathan et al., Reference Nathan, Getz, Revilla, Holyoak, Kadmon, Saltz and Smouse2008). This should lead to a better understanding of the ecology of any species so studied. Global challenges like the effects of climate change and invasive pests cannot be addressed without such an understanding.
Acknowledgements
Thanks go to Associate Prof. Christine Beveridge and Kerry Condon, The University of Queensland, ARC Center of Excellence for Integrative Legume Research for providing P. sativum seed and advice on plant matters and Dr Simon Blomberg, The University of Queensland, School of Biological Sciences for statistical advice. Dr Dave Murray and Sue McLean, Queensland Department of Primary Industries and Fisheries, Toowoomba, Australia provided H. armigera eggs. This research was supported under the Australian Research Council's Discovery Projects funding scheme (DP0666109).







