INTRODUCTION
Species of the apicomplexan genus Besnoitia parasitise cattle, goats, equids, reindeer, caribou, opossums, rabbits, rodents, and lizards (Leighton and Gajadhar, Reference Leighton, Gajadhar, Samuel, Pybus and Kocan2001; Dubey et al. Reference Dubey, Sreekumar, Lindsay, Hill, Rosenthal, Venturini, Venturini and Greiner2003a). To date, 9 species in the genus have been named: Besnoitia bennetti, Besnoitia jellisoni, Besnoitia wallacei, Besnoitia tarandi, Besnoitia darlingi, Besnoitia caprae, Besnoitia besnoiti (type species), Besnoitia oryctofelisi, and Besnoitia akodoni (Dubey et al. Reference Dubey, Sreekumar, Lindsay, Hill, Rosenthal, Venturini, Venturini and Greiner2003a, Reference Dubey, Sreekumar, Rosenthal, Lindsay, Grisard and Vitorb). However, considerable uncertainty exists regarding the identity of some of these species because the life cycles of only 3 (B. darlingi, B. wallacei, and B. oryctofelisi) of these species are known, and morphological differences among the remaining species are poorly defined (Dubey et al. Reference Dubey, Sreekumar, Lindsay, Hill, Rosenthal, Venturini, Venturini and Greiner2003a).
Among all species of Besnoitia, B. besnoiti is the most pathogenic to domestic animals; it causes economic losses in cattle in Africa, and the parasite is now spreading in Europe (Leighton and Gajadhar, Reference Leighton, Gajadhar, Samuel, Pybus and Kocan2001; Cortes et al. Reference Cortes, Reis, Waap, Vidal, Soares, Marques, Pereira da Fonseca, Fazendeiro, Ferreira, Caeiro, Shkap, Hemphill and Leitão2006; Fernández-García et al. Reference Fernández-García, Risco-Castillo, Pedraza-Díaz, Aguado-Martínez, Álvarez-García and Gómez-Bautista2009; Mehlhorn et al. Reference Mehlhorn, Klimpel and Schein2009; Schares et al. Reference Schares, Basso, Majzoub, Cortes, Rostaher, Selmair, Hermanns, Conraths and Gollnick2009). Transmission of B. besnoiti is unknown because its definitive host has not been identified. Cats are the only known definitive hosts for Besnoitia species but the role of the cat in transmission is not well understood because only a few oocysts are typically excreted. In the present paper we report the isolation and characterization of a novel species of Besnoitia that might be useful in studying the biology of Besnoitia species.
MATERIALS AND METHODS
Naturally infected woodrats
Tissues (tongue, heart, and brain) from 38 woodrats had been collected at 2 sites during a Trypanosoma cruzi and Toxoplasma gondii epidemiological study in Uvalde County, TX, USA during August 2008. Rats were trapped with small squirrel cage traps (Havahart, Lititz, PA, USA) or large Sherman traps (H.B. Sherman Traps, Tallahassee, FL, USA) baited with sunflower seeds, dried fruit, dried oatmeal or dried dog food. Animals were anaesthetized with 100 mg/kg ketamine (Fort Dodge Laboratories, Inc., Fort Dodge, IA, USA) and 10 mg/kg xylazine (Mobay Corporation, Shawnee, KS, USA) and euthanized by an intracardiac injection of sodium pentobarbital (1 ml/kg; Butler Company, Columbus, OH, USA).
Infection of laboratory animals
Laboratory-raised mice, rats and cats were infected with B. neotomofelis in 5 experiments as described below. Two strains of mice were utilized; 20–25 g female Swiss Webster (SW) out-bred mice obtained from the National Cancer Institute (NCI, Frederick Cancer Research and Developmental Center, Frederick, MD, USA) and 5 to 10-week-old interferon-gamma gene knockout (KO) mice obtained from the Jackson Laboratories (Bar Harbor, ME, USA). Interferon-gamma KO mice are highly susceptible to intracellular parasites because they lack the capacity to produce the cytokine interferon-gamma necessary for intracellular immunity (Dubey and Lindsay, Reference Dubey and Lindsay1998). The cats were from a parasite-free colony (Dubey, Reference Dubey1995).
Experiments were performed in animals according to Animal Care Protocols of the U.S. Department of Agriculture, Beltsville, Maryland or University of Georgia, Athens, Georgia. Although controls were not included in each experiment, the distinctive Besnoitia cysts have never been detected in hundreds of cats, mice, and numerous rabbits and gerbils used in the senior author's laboratory at Beltsville.
Serological examination for Toxoplasma gondii
Sera were diluted 1:25 in phosphate-buffered saline and examined by the modified agglutination test (MAT) as described by Dubey and Desmonts (Reference Dubey and Desmonts1987).
In vitro cultivation
An infected spleen from a KO mouse inoculated subcutaneously (s.c.) with tachyzoites from the first subpassage was homogenized and seeded onto a monolayer of African Green Monkey (CV-1) cells with RPMI 1640 medium supplemented with 3% bovine fetal calf serum (Dubey et al. Reference Dubey, Lindsay, Rosenthal, Sreekumar, Hill, Shen, Kwok, Rickard, Black and Rashmir-Raven2002). The parasite grew rapidly, destroying the monolayer in 4 days; this culture was cryopreserved for future studies.
Transmission electron microscopy
For transmission electron microscopy (TEM), tissues were fixed in Karnovsky’s fixative, or in 10% buffered neutral formalin. They were subsequently post-fixed in osmium and processed for TEM. For the study of in vitro-grown tachyzoites, tachyzoites were collected from a flask of CV-1 cells that was seeded with tachyzoites 10 days earlier by removing the medium, scraping cells and re-suspending them in medium, centrifuging and finally suspending the pellet in Karnovsky's fixative. The cells were then centrifuged again and the supernatant replaced with new fresh fixative. For in vivo-derived specimens, pieces of spleen of a KO mouse, 11 days post-inoculation (p.i.) with tachyzoites, were fixed in Karnovsky's fixative. For TEM of tissue cysts, the hearts of 2 SW mice inoculated 66 (with tachyzoites) or 89 (with oocysts) days p.i. were fixed in Karnovsky's.
Necropsy and histopathological examination
Samples of brain, lung, heart, tongue, liver, kidney, intestine, mesenteric lymph node, urinary bladder and limb muscle of mice and cats were fixed in 10% neutral buffered formalin. Paraffin-embedded sections were cut at 5 μm, and examined after staining with haematoxylin and eosin (H and E) or periodic acid Schiff (PAS) reaction and counter stained with haematoxylin (PASH) or a silver impregnation (Gomorie's) stain.
Immunohistochemical staining
Two types of antibodies were used for immunohistochemical staining. Polyclonal anti-B. oryctofelisi antibody (rabbit No. 1, Dubey et al. Reference Dubey, Sreekumar, Lindsay, Hill, Rosenthal, Venturini, Venturini and Greiner2003a) was diluted 1:10 000. Bradyzoite-specific rabbit antibody (BAG-1, also called BAG-5) directed against a heat-shock protein from T. gondii was supplied by McAllister et al. (Reference McAllister, Parmley, Weiss, Welch and McGuire1996) and was used at 1:1000 dilution. Staining was performed as described previously (Dubey and Sreekumar, Reference Dubey and Sreekumar2003).
Bradyzoite and tissue cyst formation
Tissues of SW mice fed oocysts or tissue cysts and KO and SW mice inoculated s.c. with tachyzoites were examined for bradyzoite and tissue cyst formation. The mice fed oocysts or tissue cysts were examined between 8 and 89 days p.i. as shown in Table 2. Numerous KO and SW mice inoculated with tachyzoites were examined at 9–25 days p.i.; 1 mouse was examined at 66 days p.i.
Molecular biology
For PCR, DNA was extracted from 10 μl of culture using the DNeasy blood and tissue kit (Qiagen, Inc., Valencia, CA, USA) following the manufacturer's protocol. Amplification for the 18S rRNA gene was conducted as described (Yabsley et al. Reference Yabsley, Work and Rameyer2006). Briefly, 5 μl of DNA was added to 20 μl of a master mix containing 10 mm Tris-Cl (pH 8·3), 50 mm KCl, 1·5 mm MgCl2, 0·2 mm each dNTP (Promega, Madison, WI, USA), 2·5 units Taq DNA Polymerase (Promega), and 0·8 μ m of primers 5.1 and B. The ITS-1 region of the B. neotomofelis was amplified using primers 15C and 13B (Bostrom et al. Reference Bostrom, Wolf, Greene and Peterson2008). Amplified products were separated in 2% agarose gels, stained with ethidium bromide, and visualized with UV light. Amplicons were purified with a Gel Extraction Kit (Qiagen) and independently bi-directionally sequenced at The University of Georgia sequencing facilty. Sequences obtained from this study and from other Besnoitia spp. stored in GenBank were aligned and phylogenetic analyses were conducted using the MEGA (Molecular Evolutionary Genetics Analysis) version 3 program (Kumar et al. Reference Kumar, Tamura and Nei2004). A Neighbor-joining algorithm using the Kimura 2-parameter model was used for analyses.
Measurements
Besnoitia stages were photographed with an Olympus AX 70 photomicroscope fitted with a digital DP 70 digital camera. Measurements were made from printed images.
Experiment 1
In this experiment, the brain, heart and tongue from each of the 38 woodrats were pooled, homogenized in 50 ml of normal saline (0·85% NaCl), and digested in acidic pepsin for 1 h as previously described (Dubey, Reference Dubey2009). The digests were centrifuged, neutralized and, after adding antibiotics (penicillin 1000 units, streptomycin 100 μg/ml of saline), the digests were each inoculated subcutaneously (s.c.) into 2 SW mice and 2 KO mice in an attempt to isolate T. gondii. All of the mice inoculated with woodrat tissues from 37 animals remained clinically normal and all were serologically negative for T. gondii when bled at 42 days p.i.
All 4 mice inoculated with tissues of woodrat no. 38 died; the KO mice died 9 days p.i. and the SW mice died 11 days p.i. All 4 mice were necropsied and their tissues were fixed in formalin, retained for bioassay, and impression smears of lungs were examined microscopically for tachyzoites. These procedures were followed because T. gondii strains from USA are rarely lethal for mice. First, it was thought that we had isolated a virulent strain of T. gondii from the woodrat. Tissues of all mice (KO and SW) were fed to a cat (no. 10) to obtain oocysts, and homogenates of mouse lung were inoculated s.c. into another group of 4 SW and 2 KO mice.
Experiment 2
Sixteen cats were fed tissues of Besnoitia experimentally infected laboratory rodents. Fourteen of these cats were fed tissues with demonstrable tissue cysts and these cats were killed 2–69 days p.i. (Table 1). Two cats were fed tissues of acutely-infected mice; 1 cat (no. 10) was fed tissues of KO and SW mice that died 9–11 days p.i., as mentioned in Exp. 1. Another cat (no. 46) was fed tissues of KO mice that had died on day 20 p.i. Faeces of all cats were examined for coccidian oocysts throughout the observation period indicated in Table 1, or for a maximum of 30 days.
Table 1. Experimental infection in cats fed Besnoitia neotomofelis tissue cysts

a Rat tissues, the remaining were Swiss Webster mice.
b Tachyzoites in mesenteric lymph nodes.
c Tissue cysts also in intestinal muscles.
For study of intestinal stages, the entire small intestine was divided into 5 equal parts and fixed in 10% neutral buffered formalin. From each intestinal region, 6–8 sections were embedded in paraffin for histological sections; thus 30–40 sections were examined from the intestine of each cat. Sections of mesenteric lymph nodes, liver, spleen, kidneys, adrenals, heart, lungs, skeletal muscle, eyes, and brain of each cat were also fixed in formalin for histological examination.
Extra-intestinal tissues (mesenteric lymph nodes, spleen, liver, lungs,) of 5 cats (nos 16, 17, 75, 18 and 19) killed on days 2–11 p.i. (Table 1) were pooled (total weight approximately 20 g), homogenized in 100 ml of saline, centrifuged at 400 g for 10 min. After discarding the supernatant, the sediment was suspended in approximately 10–20 ml of saline (depending on the volume of sediment) and 1 ml of homogenate was inoculated s.c. into 4 SW mice for each cat sample.
Experiment 3
Numerous (16 KO, 198 SW) mice were inoculated s.c. with tachyzoites or tissue cysts. Additionally, mice were infected orally with sporulated oocysts or tissue cysts (Table 2). To determine whether Besnoitia tissue stages are infectious orally to animals, the heart and liver of an SW mouse containing microscopically confirmed tissue cysts were homogenized in saline in a pestle and mortar and an aliquot was fed to 4 KO and 4 SW mice. The number of tissue cysts or bradyzoites in the inocula was unknown. Portions of all organs of the donor mouse were examined immunohistochemically using both the BAG1 and polyclonal sera; only tissue cysts and no tachyzoites were demonstable in sections of all tissues examined.
Table 2. Experimental infection in out-bred Swiss Webster mice fed Besnoitia oocysts or tissue cysts

a Lesions/parasites.
b Positively reacted with BAG 1 anti-T. gondii rabbit polyclonal antibodies.
c A, adrenal; B, brain; E, eye; H, heart; I, intestine; K, kidney; Li, liver; Lu, lung; M, skeletal muscle, Me, Mesentery; ML, mestenteric lymph nodes; O, ovary; Sp, spleen; T, tongue.
d Starting day p.i. (day post-inoculation) for sulfadiazine medication until necropsy or stated otherwise.
e Killed, the remaining mice died or killed when comatose.
f Single bradyzoites.
Experiment 4
To determine the dose-related pathogenicity of the woodrat Besnoitia oocysts and tachyzoites, oocysts from cat no. 91 and cat no. 99 (Table 1) were pooled, and six 10-fold dilutions were made; only a few oocysts (<1000/ml, using a haemocytometer) were present in the undiluted suspension. Aliquots from each dilution were inoculated orally in to SW mice (Table 3). For tachyzoite titration, cell-culture derived tachyzoites were passed through a 27-gauge needle and the tachyzoites were filtered through a 5 μm microfilter (PALL, Gelman Laboratories, Ann Arbor, MI, USA). Filtered tachyzoites were counted in a haemocytometer, and diluted 10-fold until the last 2 dilutions contained no tachyzoites. Aliquots from each dilution were inoculated s.c. into 5 SW mice. Mice that died were examined microscopically for Besnoitia organisms. Mice were observed for a period of 60 days p.i.
Table 3. Infectivity of Besnoitia neotomofelis to SW mice

a Aliquots from 10-fold dilutions were fed to 5 mice.
b Estimated viable organisms, based on infectivity data and the assumption that 1 organism is infective.
c Oocysts were inoculated orally.
d Tachyzoites were inoculated subcutaneously.
e No. of mice inoculated/ No. of mice infected. Data in parentheses are the day of death or euthanasia.
Experiment 5
Two Norwegian CD-1 rats (Rattus norvegicus) obtained from Charles River Laboratory International Inc., Wilmington, MA, USA were each inoculated orally with oocysts (unknown number) at the University of Georgia, Athens, Georgia; 1/20th aliquot of the same inoculum fed to each rat was lethal for SW mice. The rats were killed on day 32 p.i.
RESULTS
Isolation of B. neotomofelis in cell culture
Plaques were recognized in CV-1 monolayers 3 days after inoculation with spleen homogenate from an infected KO mouse. Tachyzoites were observed in cells adjacent to the plaques. Tachyzoites from this culture were preserved in liquid nitrogen 4 days after this culture had been originally seeded. Tachyzoites were also grown in M67 bovine monocytes.
Immunohistochemistry
All stages of B. neotomofelis reacted positively with B. oryctofelisi polyclonal rabbit antibodies (Fig. 1D). Only bradyzoites were stained with the BAG-1-antibodies.

Fig. 1. Different stages of Besnoitia neotomofelis. (A and B) Impression smear, Giemsa stain; (C–I) histological sections of cat small intestine. (C, E, F) H and E stain, (D) immunohistochemical staining with Besnoitia polyclonal rabbit antibody, (H) PAS counter-stained with haematoxylin, (J) unstained. (C and D) Cat no. 26, 14 days p.i., (E–I), cat no. 27, 24 days p.i. Scale bar = 10 μm and applies to all figures. (A) Tachyzoite (arrow), mouse lung. (B) Bradyzoites released from a tissue cyst from the heart of SW mouse, 66 days p.i.. (C) Young tissue cyst in submucosa. Note thick cyst wall enclosing host cell nuclei (arrowheads), and bradyzoites enclosed in the parasitophorous vacuole (arrow). (D) Several developing first-generation schizonts (arrows) in the lamina propria. (E) Two first-generation schizonts in the lamina propria. Intracellular immature schizont with 2 nuclei (black arrowheads) and the host cell nucleus (white arrow). A mature schizont within a blood vessel. The merozoites are arranged in several distinct groups (black arrows).White arrowhead points to 2 intravascular erythrocytes. (F) Second-generation schizont in surface epithelium. Six merozoites (arrows) are arranged around a residual body (arrowhead). (G) Immature microgamont (arrow) apparently in a goblet cell of surface epithelium. (H) Mature microgamont with microgametes (white arrows) with a large residual mass (black arrow) in a goblet cell. PASH. (I) Intracellular unsporulated oocyst (long arrows) with a central nucleus (short arrow) in epithelium. (J) Unsporulated oocyst in cat faeces with the sporont occupying most of the oocyst. Arrow points to the nucleus.
Bradyzoite and tissue cyst formation
BAG 1-positive zoites were seen in tissues of mice at 13–25 days p.i. Most of these reacted strongly with BAG 1 antibodies, but did not stain with PAS- or silver-impregnated stains. Tissue cysts with thick cyst walls were observed in tissues of mice from 30 to 89 days p.i.
Experiment 1
Of the 2 KO mice and the 4 SW mice inoculated s.c. with mouse lung homogenate, the KO mice died at 11 and 15 days p.i. and tachyzoites were found in their lungs. The 4 SW mice became ill, and were medicated with sulfadiazine sodium in drinking water (1 mg/ml) from days 4–7 p.i. and 14–19 p.i. and 1 of these mice died on day 13 p.i. despite medication. Of the 3 mice that survived, 2 mice were killed on day 51 p.i. and thick-walled Besnoitia tissue cysts were found in their hearts, and their tissues were fed to 2 cats (cat nos 91, 99, Table 1). The fourth mouse was killed on day 42 p.i. and small Besnoitia tissue cysts were found in its lungs, heart, and spleen.
Experiment 2
All cats fed Besnoitia-infected tissues remained asymptomatic. Seven of the 13 cats fed tissue cysts shed unsporulated oocysts (Fig. 1J) with a minimum pre-patent period of 11 days (Table 1). Only a few oocysts were found in spite of prolonged searches; only a single oocyst was found on 1 day in 2 cats fed infected rat tissues (Table 1). The 2 cats fed acutely infected mice (10 and 20 days p.i.) did not shed oocysts.
Mice inoculated with extra-intestinal tissues of 5 cats died on day 21 p.i. and numerous Besnoitia tachyzoites were found in tissues of mice as revealed by immunohistochemical examination.
Experiment 3
All KO and SW mice fed oocysts or tissue cysts died of acute besnoitiosis, starting on day 6 p.i. (Fig. 2). Representative data from orally inoculated mice are shown in Table 2. Medication with sulfadiazine sodium (1 mg/1 ml in drinking water) was not very effective in preventing mortality after clinical signs were observed. Besnoitia tachyzoites initially caused necrosis of the cells of the lamina propria of small intestines and tachyzoites were seen in lesions (Figs. 2B). Tachyzoites multiplied in virtually all cells of the intestinal lamina propria, but rarely in enterocytes. Infection extended from the lamina propria to the serosal layer of intestines. Large numbers of parasites led to the formation of ulcers (Fig. 2A). Spread to other organs was noted with extra-intestinal stages being found most commonly in the liver, spleen, mesenteric lymph nodes, and heart. Enteritis was the primary lesion observed in mice that died 6–10 days p.i. Pneumonitis and hepatitis were the predominant lesions observed in mice that died after day 12 p.i. Parasites were not detected in sections of brain until the fourth week.

Fig. 2. Lesions in small intestine of SW mice, 8 days after feeding Besnoitia neotomofelis. (A) Ulcerative transmural necrosis (a) with lamina propria contents exuding in the lumen (arrows). The villus (b) on the left of the ulcer is partially necrotic. The surface epithelium on the villus on the right (c) is intact and apparently unaffected. There are numerous tachyzoites in the lesion but they are not visible at this magnification. Eight days after feeding oocysts. H and E stain. Scale bar=100 μm. (B) Numerous tachyzoites (arrow heads, all red areas) are destroying the lamina propria cells a mouse 8 days after feeding tissue cysts. Stained with anti-Besnoitia rabbit polyclonal antibody. Scale bar=20 μm.
Experiment 4
The SW mice inoculated with graded doses of oocysts died 13–18 days p.i., mainly due to hepatitis and pneumonitis, while those inoculated with tachyzoites lived for a few days longer (Table 3).
Experiment 5
The 2 rats fed oocysts remained asymptomatic and 2 tissue cysts were found in histological sections of lungs of 1 of them. Both cats fed tissues from these rats shed oocysts (Table 1).
Description of Besnoitia neotomofelis n. sp. (Figs 1–9)
Tachyzoites and tissue cysts were present in tissues of rodents and cats. Tachyzoites were lunate, approximately 1×5 μm in size (Fig. 1A). Tachyzoites were located in a parasitophorous vacuole containing tubular network, in the host cell cytoplasm (Fig. 3). They contained a conoid, micronemes, rhoptries, a large mitochondrion, and a centrally or subterminally located nucleus (Fig. 3). The micronemes were located anterior to the nucleus and their number varied; none are visible in Fig. 3B. The rhoptries were few (<6 in any plane of section) in number, often convoluted, and their contents were electron dense (Fig. 3C). Rhoptries extended posterior to the nucleus. An unidentified membraned body was present at the conoidal end (Fig. 3B). Tachyzoites divided by endodyogeny (Fig. 3D–F).

Fig. 3. TEM of Besnoitia neotomofelis tachyzoites in cell culture (A, B, D–F) or mouse spleen (C). The conoidal ends are orientated towards the top of the figure. Scale bar =1 μm in all figures. (A, B, C) Note high variability in the presence of organelles; micronemes (Mi) are not visible in B and arranged differently in A and C. Rhoptries (Ro) appear to be more numerous in B than A and C. The nucleus (Nu) is located in the posterior half of the tachyzoite. Note that the tachyzoite in A is located near the host cell nucleus (hcn). (D, E, F) Dividing tachyzoites located in a parasitophorous vacuole limited by the parasitophorous vacuolar membrane (arrowheads). (D) Parent nucleus has divided into 2 daughter zoite nuclei (Nu). (E) Note conoidal ends of 2 daughter cells (Dc). The conoidal end of the mother cell is smaller in size than that in Fig. 3D. (F) Separation (arrow) of the 2 daughter cells. Note a conoid (Co), micronemes (Mi), rhoptries (Ro), a large mitochondrion (Mt), and a membrane-bound body (Mb).
Tissue cysts were located in several tissues of mice and in intestines of cats (Figs 1C and 4A–D). In SW mice that survived after sulfadiazine treatment, tissue cysts were found in many organs with most being found in the heart and spleen. Most tissue cysts were not associated with inflammation; however, mononuclear cell infiltrations were observed around a few intact tissue cysts and a few degenerating ones (Fig. 4E, F).

Fig. 4. Tissue cysts of Besnoitia neotomofelis in sections of cat or mouse tissues. (A–C, E and F) Stained with H and E, (D and G) stained with Toluidine blue. (A and B) Cat no. 27, 24 days p.i., (C–E) SW mouse no. 66 days p.i., (F) SW mouse, 89 days p.i., (G) SW mouse, 80 days p.i.. (A) Unizoite tissue cyst, incorporating the host cell nucleus (arrowhead) and a bradyzoite (arrow) in submucosa. (B) Tissue cyst with a few bradyzoites enclosed in thin parasitophorous vacuolar membrane (arrows) and 4 host cell nuclei (arrowheads). (C) Two tissue cysts (arrows) in Purkinje cells of the brain. (D) Two tissue cysts (arrows) in the heart. (E) Degenerating tissue cyst (arrow) within an inflammatory focus around cyst wall in the brain. (F) Degenerating tissue cyst (arrow) within an inflammatory focus in the lung. The cyst wall has disappeared. (G) Part of a tissue cyst incorporating host cell nucleus (hcn) and numerous bradyzoites. Arrows point to longitudinally cut bradyzoites,
Tissue cysts were microscopic, up to 210 μm in diameter, and embedded in host tissue. The tissue cyst wall enclosed host cell nuclei, even in young cysts containing only few bradyzoites (Figs 1C, 4A, B and 5A–D). Bradyzoites were slender and divided by endodyogeny. Bradyzoites mechanically released from a tissue from the heart of a mouse killed on day 66 p.i. measured 1·0–1·5×7·6–9·8 μm (n=54) in smears. Longitudinally cut bradyzoites in 1 μm Toluidine blue-stained sections were 1·4–1·6×7·7–9·3 (n=32) μm in size. Tissue cyst walls were up to 15 μm thick (Fig. 4C). The thickness of the tissue cyst wall varied. The cyst wall was PAS-negative, but silver-positive. The bradyzoites in mature cysts were PAS-positive.
By TEM, the tissue cyst wall consisted of 3 layers (Figs 5 and 6). The outer layer consisted of connective tissue. The middle layer contained host cell nuclei and an accumulation of endoplasmic reticula (Fig. 6A). The inner most layer consisted of a thin parasitophorous vacuolar membrane (pvm). Mycelia-like tubular structures were present beneath the pvm; these structures extended into the interior of the tissue cyst (Fig. 5B). Five longitudinally cut bradyzoites were 1·6–2·0×7·8–9·7 μm in size on TEM sections. Bradyzoites contained a conoid, micronemes, rhoptries, a nucleus, amylopectin, a mitochondrion, a micropore, enigmatic bodies, and dense granules (Fig. 7). Micronemes (up to 200 nm long) were present throughout the bradyzoite but mostly located at the conoidal end. The rhoptries extended up to the posterior end. A maximum of 3 rhoptries were seen in a given section. The contents of the rhoptries were electron dense. The position of the nucleus in bradyzoites was subterminal (Fig. 7). Enigmatic bodies were located mostly post-nuclear and measured 70×500 nm in size (Fig. 7A, C).
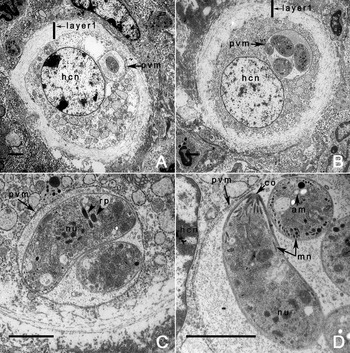
Fig. 5. TEM of young tissue cysts of Besnoitia neotomofelis in the liver of a KO mouse, 21 days p.i.. Scale bar = 2 μm in all figures. Note 1–4 bradyzoites enclosed in a thin parasitophorous vacular membrane (pvm). The outer layer (layer 1) consists of connective tissue. The middle layer (layer 2) is electron dense and contains the host cell nuclei. Note rarity of amylopectin granules, and absence of enigmatic bodies. Note conoid (co), micronemes (mn), nucleus (nu), rhoptries (rp), and pvm.
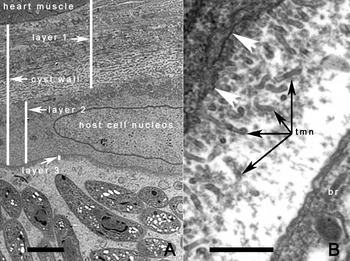
Fig. 6. TEM of tissue cysts of Besnoitia neotomofelis, 66 days p.i.. (A) Note 3 tissue cyst wall layers (1–3). Layer 1 consists of connective tissue, closely applied to the heart muscle. Layer 2 contains the host cell nuclei, and layer 3, the true parasitophorous vacuolar membrane enclosing bradyzoites. Scale bar = 2 μm. (B) Higher magnification of layer 3. Note the presence of numerous tubular structures (tmn) and a bradyzoite (br). The arrowheads point to the junction of layers 2 and 3. Scale bar = 1 μm.

Fig. 7. TEM of Besnoitia neotomofelis bradyzoites. Scale bar = 1 μm and applies to all figures. (A) Bradyzoite with numerous micronemes (Mi) that are present throughout the length of the bradyzoite, 2 rhoptries (R), a mitochonrion (Mt). (B) Post-conoidal end of a bradyzoite showing numerous enigmatic bodies (Eb) and amylopectin (Am). (C) Higher magnification of post-conoidal end of bradyzoite showing enigmatic bodies (Eb) with a membrane and the central core. Also note amylopectin (Am), conoid (C), rhoptries (R), rhoptrie neck (Rn), micronemes (Mi), micropore (Mo), mitochondrion (Mt), and nucleus (Nu).
Schizonts and oocysts were present in the definitive host, the domestic cat. The jejunum was the most heavily parasitized region of the small intestine. Individual zoites, seen at 2–7 days after feeding tissue cysts were small (2–4 μm long), often globular, and had a vesicular nucleus; these were considered tachyzoites (Fig. 8A, B). Two generations of schizonts were recognized. First generation schizonts were present in the intestinal lamina propria of cats (Fig. 8C–E). Most schizonts were located towards the villar tips (Fig. 1D). The youngest identified schizont seen in the cat killed on day 8 p.i. was approximately 8 μm long and had a prominent nucleolus (Fig. 8C). The host cell parasitized was not definitively indentified but appeared to be a vascular endothelial cell (Fig. 1E). The host cell nucleus was often hypertrophied and indented (Fig. 8C). The nucleus of the schizont divided into numerous nuclei that were arranged in separate groups or whorls (Fig. 8D). Merozoites were arranged in separate groups, sometimes with residual bodies (Fig. 8D). Merozoites were approximately 1×5 μm in size, slender, and appeared to have terminal nucleus (Fig. 8E). Some schizonts ruptured in the lamina propria, leading to local infiltration by neutrophils around the free merozoites. Schizonts and merozoites were PAS-negative. Schizonts varied in size; the largest schizont measured 40×50 μm and contained numerous merozoites (Fig. 8E).

Fig. 8. Enteric stages of Besnoitia neotomofelis in sections of small intestines of cats. (A) Stained with Besnoitia polyclonal rabbit antibody, B–I, stained with H and E. The villar brush border of the intestine is oriented up. Arrowheads point to host cell nuclei that are pushed towards one side. Scale bar applies to all parts. Sections of small intestine of cat no.16, 2 days p.i. (A, B) or cat no. 27, 24 days p.i. (C–I) or cat no. 26, 14 days p.i. (D). (A) Organism (arrow) in the lamina propria. Note the organism is swollen. (B) Five zoites (arrow) in the lamina propria. (C) Two immature schizonts in the lamina propria. Arrows point to schizont nuclei. (D) Immature schizont with numerous nuclei arranged in groups (arrows). (E) Mature schizont with numerous merozoites apparently arranged around residual bodies (arrows) in the lamina propria. White arrows point to longitudinally cut merozoites. (F) Single zoite in a goblet cell of surface intestinal epithelium. (G) Immature microgamonts (arrows) apparently in goblet cells at surface of intestinal epithelium. (H) Young macrogamont (arrow) in surface intestinal epithelium. (I) Intracellular oocyst (arrow).
A second generation of small schizonts was seen in the epithelium of the small intestine of 2 cats (nos 26 and 27, Table 1, days 14 and 24 p.i.). These schizonts were <8 μm in diameter and contained 4–8 banana-shaped merozoites; they were located above the enterocyte nucleus (Fig. 1F). Only a few schizonts were found after an intensive search of numerous sections.
Individual zoites (Fig. 8F) and gamonts (Fig. 8G, I) were observed in goblet cells. Only a few gamonts were seen. The micorgamonts were above the host cell nucleus and appeared to contain <20 microgametes in 5 μm sections (Fig. 1H).
Unsporulated oocysts were 13·2×13·9 μm (13–15×12·7–14·1 μm; n=20) in size with a length-width ratio of 1:1·14. (Fig. 1J). Micropyle and polar granules were absent. Oocysts sporulated within 2 days at room temperature (22°C). In oocysts removed from rectal contents of a cat that was killed, the sporont completely filled the oocyst (Fig. 1J). During sporulation, the sporont shrank, and separated into globular sporoblasts (Fig. 9). The sporoblasts then became elongated and lighter areas (interpreted as nuclei) were seen at the polar ends (Fig. 9). A residual body was left after formation of sporozoites. Each oocyst contained 2 sporocysts, and each sporocyst contained 4 sporozoites and a dispersed or compact sporocyst residuum. Stieda body and oocyst residuum were absent.

Fig. 9. Phototypes of unstained Besnoitia neotomofelis oocysts. (A) Three sporulating oocysts. (a) The sporont has irregular edges. Arrow points to the central nucleus. (b, c) The sporont has divided into 2 sporoblasts, and a nucleus is visible towards the pole of a developing sporocyst (arrow). (B) Oocyst with 2 elongated sporocysts with terminal nuclei (arrows). (C) Sporulated oocyst with partially collapsed oocyst wall (ow). Note 2 sporocysts (st) with sporozoites (sp). Arrowheads point to a longitudinally orientated sporozoite. The scale bar applies to all figures.
Molecular biology
A 1723 bp amplicon of the 18S rRNA gene was amplified and sequenced from the B. neotomofelis. For the overlapping region of species in GenBank, the woodrat Besnoitia was most similar to B. jellisoni (99·8%, 3 of 1593 bp, AY291426) and B. akodoni (99·7%, 2 of 791 bp, AY623624). The ITS-1 region was 270 bp and was most similar to B. jellisoni (97·8%, AF076860) and B. akodoni (94·1%, AY545987). Alignment of the ITS-1 sequence of the B. neotomofelis with other Besnoitia and N. caninum (as outgroup, NCU16159) resulted in an alignment 288 bp in length, of which, 55 of 148 variable characters were parsimony informative. Neighbor-joining analysis (Fig. 10) placed the B. neotomofelis in a clade with B. jellisoni, which was a sister clade to one containing B. akodoni, B. darlingi (AF489696), and B. oryctofelisi (AY182000).

Fig. 10. Phylogenetic tree based on internal transcribed spacer (ITS)-1 region sequences of Besnoitia neotomofelis and related organisms. Percentages of 1000 bootstrap samplings that supported clades are shown on branches for Neighbor-joining analysis.
TAXONOMIC SUMMARY
Intermediate type host: southern plains woodrat (Neotoma micropus)
Experimental definitive host: domestic cat (Felis domesticus)
Locality: Texas, USA
Etymology: The species is named combining the genus of intermediate host (Neotoma) and the definitive host, cat (Felis domesticus)
Remarks
Differences among the 4 Besnoitia species that utilize domestic cats as definitive hosts are summarized in Table 4.
Table 4. Salient characters of the four Besnoitia species with cats as definitive hosts

a Oocysts were measured in sucrose solution diluted 50:50 with distilled water.
Specimens deposited
Phototypes of oocysts depicted in Fig. 9 and permanently stained specimens (hapantotypes) were deposited in the United States National Parasite Collection (USNPC, nos.1026897–102712) United States Department of Agriculture, Beltsville, MD, USA; details of each specimen are given in Table 5.
Table 5. Details of specimens deposited in USNPC museum
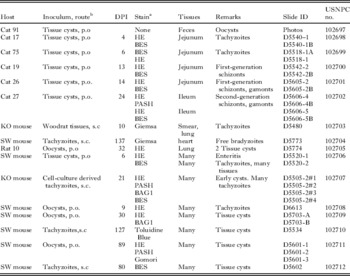
a BES, immunostaining with Besnoitia polyclonal antibody; BAG1, immunostaining with BAG1 (bradyzoite specific) antibody; PAS, periodic acid Schiff reaction, counter-stained with haematoxylin.
b p.o., per os; s.c., subcutaneous.
DISCUSSION
The woodrat Besnoitia described in the present study was considered a new species based on biological and structural differences from other known species, especially the rodent Besnoitia species. Besnoitia jellisoni, initially described from the white-footed deer mouse (Peromyscus maniculatus), forms macroscopic tissue cysts in connective tissue; its life cycle is unknown and cats are not the definitive host (Frenkel, Reference Frenkel1953, Reference Frenkel1977). Besnoitia akodoni, described from the rodent, Akodon montensis, is not pathogenic to SW mice; its life cycle is unknown and cats are not the definitive host (Dubey et al. Reference Dubey, Sreekumar, Rosenthal, Lindsay, Grisard and Vitor2003b). Besnoitia neotomofelis most closely resembles B. wallacei, first identified in the faeces of a naturally infected cat (Wallace and Frenkel, Reference Wallace and Frenkel1975). Unlike other species of Besnoitia, B. wallacei has an obligatory 2-host life cycle; tissue cysts were infective to cats but not to intermediate hosts. Additionally, B. wallacei oocysts and schizonts are much larger in size than those of B. neotomofelis. Furthermore, a second generation of schizonts found in the present study in B. neotomofelis has not been reported previously for other species.
Staining tissues with polyclonal Besnoitia rabbit antibodies facilitated the search for entro-epithelial coccidian stages of B. neotomofelis in the intestine of cats. All stages of this Besnoitia, including the enteric stages reacted with this antibody which is not species specific; B neotomofelis stages reacted strongly with B. oryctofelisi antibodies which were isolated from the rabbit. Organisms swell during the immunohistochemical staining procedure and appear larger than those in H and E-stained sections (Fig. 8A), and thus are recognized easily.
Among closely related tissue cyst-forming apicomplexans (Sarcocystis, Neospora, Toxoplasma, Hammondia, Besnoitia) tissue cysts of Besnoitia species are distinctive because they enclose host cell nuclei. The structure and life cycle of Besnoitia is similar to Toxoplasma and they share common antigens (Lunde and Jacobs, Reference Lunde and Jacobs1965). For many pathogenesis and immunological studies, the murine B. jellisoni has been used as a model for T. gondii because of its biological and pathological characteristics infecting adrenals and eyes (Frenkel, Reference Frenkel1956, Reference Frenkel1977; Frenkel and Lunde, Reference Frenkel and Lunde1966; Frenkel and Wilson, Reference Frenkel and Wilson1972; Chinchilla and Frenkel, Reference Chinchilla and Frenkel1978). To our knowledge, the isolate of B. jellisoni obtained by Frenkel has been lost and would have to be re-isolated from Peromyscus maniculatus from northern Idaho. Tachyzoites of Besnoitia and Toxoplasma are morphologically similar but biologically different; T. gondii tachyzoites are non-motile at room temperature whereas Besnoitia spp. tachyzoites (present study with B. neotomofelis) and B. jellisoni (personal oral communication from Dr J. K. Frenkel to J.P.D. in 1970) retain their motility at room temperature (22°C). To our knowledge Besnoitia species are not anthropozoonotic.
Coccidian bradyzoites are PAS-positive because they contain numerous amylopectin granules that are rare or absent in the tachyzoite stage. The stage conversion of tachyzoite to bradyzoite has been studied in detail in T. gondii (reviewed by Dubey et al. Reference Dubey, Lindsay and Speer1998) but little is known of this stage conversion in Besnoitia. In T. gondii, tissue cysts can be formed in mice as early as day 3 p.i. in vivo and in vitro and these early tissue cysts are infective to cats (Dubey and Frenkel, Reference Dubey and Frenkel1976). The availability of the BAG 1 antibodies that are specific for the bradyzoite stage makes it easier to follow conversion from tachyzoite to bradyzoite. In the present study, the earliest BAG 1-positive zoites were detected on day 13 p.i.; however, these zoites did not have time to accumulate amylopectin. Dubey and Lindsay (Reference Dubey and Lindsay2003) reported that zoites in young B. oryctofelisi tissue cysts at day 12 p.i. were PAS-negative, lacked amylopectin and enigmatic bodies, which is characteristic of bradyzoites in older tissue cysts. In this respect the tissue cyst formation in B. neotomofelis resembled that described for B. oryctofelisi (Dubey and Lindsay, Reference Dubey and Lindsay2003). In the present study, tissues of mice heavily infected with tachyzoites (day 10 p.i.) or early tissue cysts (day 20 p.i.) were not infective to cats as revealed by the lack of oocyst shedding, although the 20-day infected mice contained numerous organisms that were BAG 1-positive.
There were few genetic differences observed between B. neotomofelis and other Besnoitia species (>99% for the 18S rRNA gene). This finding supports other studies that have shown that limited genetic diversity has been observed among the genus Besnoitia based on 18S and 28S rRNA genes (Dubey et al. Reference Dubey, Lindsay, Rosenthal, Sreekumar, Hill, Shen, Kwok, Rickard, Black and Rashmir-Raven2002). Phylogenetic analysis of the ITS-1 region produced a tree that was similar in morphology to another study (El Sheikha et al. Reference El Sheikha, Hussein, Monib Mel and Mansfield2007) and indicated that B. neotomofelis was most similar to B. jellisoni of rodents. Although there was only 2·2% nucleotide divergence between B. neotomofelis and B. jellisoni, previous studies have shown that B. darlingi from opossums only differed from B. oryctofelisi by <1% while most other species only differ from each other by 2–4·4% (Dubey et al. Reference Dubey, Sreekumar, Lindsay, Hill, Rosenthal, Venturini, Venturini and Greiner2003a; El Sheikha et al. Reference El Sheikha, Hussein, Monib Mel and Mansfield2007). Further studies are needed to identify additional gene targets that might be more useful for species designation.
Unlike T. gondii, only a few oocysts are shed by domestic cats infected with B. neotomofelis (present study), B. oryctofelisi (Dubey et al. Reference Dubey, Sreekumar, Lindsay, Hill, Rosenthal, Venturini, Venturini and Greiner2003a), and B. darlingi (Dubey et al. Reference Dubey, Lindsay, Rosenthal, Sreekumar, Hill, Shen, Kwok, Rickard, Black and Rashmir-Raven2002), even when large numbers of infected tissues are fed to cats. These data indicate that transmission of Besnoitia via oocysts is inefficient and an intensive search may be needed to identify the few oocysts shed by the definitive host. Other members of the Felidae (e.g. bob cats, cougars) might be more efficient definitive hosts for Besnoitia species, but have not been tried for any species of Besnoitia.
ACKNOWLEDGEMENTS
The authors would like to thank Jack Frenkel for advice and sharing sections of different Besnoitia species from his collection for comparison with B. neotomofelis, Eric Hoberg and Patricia Pilitt for advice on taxonomy, John Jenkins for electronmicroscopy, O. C. H. Kwok for cell culture, Leandra Ferreira for help with illustrations, Milton McAllister for the gift of BAG1 antibodies, S. Kjos, D. M. Roellig, and E. L. Blizzard with field assistance, and J. Barnes for permission to trap woodrats. This paper is dedicated to Dr J. K. Frenkel who contributed immensely to our knowledge of Toxoplasma and Besnoitia.

















