INTRODUCTION
Microsporidia are obligate intracellular pathogens once believed to be early-diverged eukaryotes, now known to be a sister group to Eumycota (true fungi) designated in the superphylum Opisthosporidia (Karpov et al. Reference Karpov, Mamkaeva, Aleoshin, Nassonova, Lilje and Gleason2014). Being highly reduced parasites, they have adapted to a parasitic intracellular life, losing the genes for metabolic processes and depending almost entirely on their infected host cell for energy requirements (Williams et al. Reference Williams, Haferkamp and Keeling2008). Microsporidia are especially prevalent in aquatic organisms, which are hosts for over half of the described species (Stentiford et al. Reference Stentiford, Feist, Stone, Bateman and Dunn2013), and known to cause disease in various finfish species (Kent et al. Reference Kent, Shaw, Sanders, Weiss and Becnel2014). Species in the genus Pleistophora have emerged as important pathogens of fish, most frequently associated with disease in the skeletal muscle, such as Pleistophora hyphessobryconis associated with muscular disease in ornamental and farmed fishes (Sanders et al. Reference Sanders, Lawrence, Nichols, Brubaker, Peterson, Murray and Kent2010; Li et al. Reference Li, Chang, Wang, Liu, Liang and Wu2012; Winters et al. Reference Winters, Langohr, Souza, Colodel, Soares and Faisal2016). The genus Ovipleistophora, previously assigned to Pleistophora, are fish microsporidia that infect primarily ovarian tissue and are associated with reduced fecundity in fish (Summerfelt and Goodwin, Reference Summerfelt and Goodwin2010). Microsporidia have also been reported as parasites infecting other parasites. Perhaps as a result of the large diversity of microsporidia in aquatic organisms, hyperparasitic species are particularly well known in fish-infecting parasites, such as Unikaryon nomimoscolexi infecting a gut nematode of the Spiny Catfish, Clarotes laticeps (Sene et al. Reference Sene, Ba, Marchand and Toguebaye1997), Nosema ceratomyxae infecting a myxozoan parasite from rabbitfish, Parapristipoma octolineatum (Diamant and Paperna, Reference Diamant and Paperna1985) and Paranucleospora theridion, synonymous with Desmozoon lepeophtherii (Freeman and Sommerville, Reference Freeman and Sommerville2011), which is a hyperparasite of the salmon louse Lepeophtheirus salmonis and also causes gill disease in Atlantic salmon, Salmo salar (Nylund et al. Reference Nylund, Anderson, Saevareid, Plarre, Watanabe, Arnesen, Karlsbakk and Nylund2011).
There is a growing number of hyperparasitic microsporidians in trematodes with several known species in digeneans that infect molluscs or fish. Nearly all of these described species belong to the genera Nosema or Unikaryon. Microsporidian infection by a Nosema spp. was reported by Cort et al. (Reference Cort, Hussey and Ameel1960a ) in 12 different trematode species from snails, which were shown to be infected when spores were ingested by the snail (Cort et al. Reference Cort, Hussey and Ameel1960b ). Since then, several other Nosema species have been described as hyperparasites of digeneans, which themselves infect fish or molluscs; some of these include N. dollfusi (Sprague, Reference Sprague1964), N. strigeoideae (Hussey, Reference Hussey1971), N. eurytremae (Colley et al. Reference Colley, Joe, Zaman and Canning1975), N. diphterostomi (Levron et al. Reference Levron, Ternengo, Toguebaye and Marchand2004), N. monorchis (Levron et al. Reference Levron, Ternengo, Toguebaye and Marchand2005) and N. podocotyloidis (Toguebaye et al. Reference Toguebaye, Quilichini, Diagne and Marchand2014). The genus Unikaryon was established with the type species Unikaryon piriformis described from two snail trematodes, Echninoparyphium dunni and Echinostoma audyi (Canning et al. Reference Canning, Foon and Joe1974). Following this, several Unikaryon species have been described, including U. legeri from metacercariae infecting the common cockle, Cerastoderma edule (Canning and Nicholas, Reference Canning and Nicholas1974), U. allocreadii from an adult trematode of the freshwater fish Aplocheilus melastigma from India (Canning and Madhavi, Reference Canning and Madhavi1977) and U. slaptonelyi from a larval trematode in the snail Lymnaea peregra (Canning et al. Reference Canning, Barker, Hammond and Nicholas1983). Microsporidia in the genus Pleistophora and in the collective group Microsporidium were recorded from trematodes by Sprague (Reference Sprague, Bulla and Cheng1977), though the species were inadequately described, thus little is known of any Pleistophora species from trematode hosts. In the present study, a microsporidium consistent with the genus Ovipleistophora was discovered within the metacercarial cyst wall of the digenean parasite Posthodiplostomum minimum infecting bluegill sunfish, Lepomis macrochirus.
Posthodiplostomum minimum is a digenean parasite within the family Diplostomatidae with an indirect life cycle including physid snails and fish as intermediate hosts and birds as definitive hosts (Miller, Reference Miller1954; Paperna and Dzikowski, Reference Paperna, Dzikowski and Woo2006). The encysted metacercariae in fish, also known as ‘white grub’, are one of the most common and widespread parasites found in cyprinid and centrarchid fishes. Once believed to be multiple species due to naming by locality and host species, P. minimum is now known to be one species with a wide host range. Further field and laboratory work distinguished two subspecies, including P. minimum minimum, which only infects cyprinid fishes and P. minimum centrarchi, which infects only centrarchid fishes (Klak, Reference Klak1940; Ferguson, Reference Ferguson1943). Posthodiplostomum minimum centrarchi had been considered a ‘generalist’ parasite infecting all centrarchids, although evidence suggests that the parasite is in fact a ‘specialist’ for sunfish species in the genus Lepomis (Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015), while other centrarchids may become infected opportunistically and to a lesser degree. This is further supported by prevalence studies, which indicate that Lepomis spp. are most likely to be infected with the parasite (Palmieri, Reference Palmieri1975). Heavy infections with white grub at times were believed to negatively impact the health of some fish (Mitchell et al. Reference Mitchell, Smith and Hoffman1982), though most studies on wild centrarchids indicated that minimal health impacts occurred from infection by this common parasite (Lewis and Nickum, Reference Lewis and Nickum1964; Grizzle and Goldsby, Reference Grizzle and Goldsby1996). The widespread nature of the parasite in wild bluegill sunfish, often found at 100% prevalence, and the minimal health impacts to the host represents a highly successful host–parasite relationship.
Posthodiplostomum minimum is one of the most widely reported fish parasites and they have been subject to evaluation as measures of environmental health (Chapman et al. Reference Chapman, Marcogliese, Suski and Cooke2015) and parasite ecology studies (Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015). The present study describes the first microsporidian parasite associated with P. minimum and the first described Ovipleistophora species associated with a trematode. In contrast to the majority of hyperparasitic microsporidia described, including Nosema and Unikaryon spp., which directly infect the trematode tissues, the Ovipleistophora described herein infects the cyst wall of the trematode in a location at the interface of the fish host and the digenean parasite. Herein, this unique infection was characterized by histology and transmission electron microscopy (TEM) to better understand the parasite developmental cycle. Further, the first molecular data was provided for this unique species to better understand its relationship to other microsporidia.
MATERIALS AND METHODS
Fish collection and sampling
All fish were collected by electrofishing using a 13·2′ electrofishing boat (Smith-Root, Vancouver, WA, USA) from Assunpink Lake, located in the Assunpink Wildlife Management Area, Monmouth County, New Jersey, USA (40°13′07·4″N, 74°31′01·6″W). A total of 60 fish were collected from two sampling dates, including 30 fish from early spring, March 22nd, 2016, and 30 fish during the early fall taken on September 13th, 2016. Following collection, fish were maintained alive in an aerated livewell and transported to the Pequest Fish Health Laboratory within a transport tank. Fish were maintained alive and sampled within 48 h of collection. For sampling, fish were euthanized with an overdose of tricaine methanesulphonate (MS-222). The 30 fish sampled in March were dissected and organs, including gill, liver, spleen, anterior and posterior kidney, gastrointestinal tract, heart and reproductive tissue were fixed in 10% neutral buffered formalin (NBF). In September, half of the liver was dissected from each of 30 fish and screened on a Zeiss Stemi-dissecting microscope (Carl Zeiss, Jena, Germany) for opaque cysts consistent with microsporidia. Cysts were dissected from the liver and wet mounts were prepared to examine for microsporidian spores using a Zeiss Axio-plan 2 research microscope (Carl Zeiss) using differential interference contrast (DIC) microscopy. Microsporidian spores were photographed with a microscope mounted Jenoptik ProgRes Speed XT core 3 digital camera (Jenoptik AG, Jena, Germany). Measurements of microsporidian spores were made using the ProgRes (Version 9·1) program from digital images taken directly from wet mounts using a 100× oil immersion objective. Preparations of microsporidian spores were transferred to 1·2 ml centrifuge tubes and immediately frozen at −80 °C for PCR analysis. Microsporidian cysts from infected fish were transferred to 2% phosphate-buffered glutaraldehyde for TEM and the other half of the liver along with the remaining internal organs, as reported above, were transferred to 10% NBF for histology.
Histology, high-resolution microscopy (HRLM) and TEM
Organs from all 60 fish were processed for routine histology. Briefly, the tissues were fixed in 10% NBF for 48 h, dehydrated through an ascending series of ethanol, cleared in Shandon xylene substitute and embedded in paraffin wax. The 5 µm sections were cut, mounted on glass slides, and stained with haematoxylin and eosin (H&E) and McDonald's Gram stain (Mastertech Stain Kits, Lodi, CA). The total number of digeneans was counted within a single tissue section plane per fish. The prevalence of microsporidian infection was noted by the number of infected P. minimum cysts out of all cysts within a single histological section per fish.
For HRLM and TEM, the P. minimum cysts infected with microsporidia were fixed in 2% phosphate-buffered glutaraldehyde for 48 h at 4 °C, followed by washing twice with phosphate buffer and post-fixation in 1% osmium tetroxide for 1–2 h at room temperature. The samples were then dehydrated through an ascending series of ethanol's, including two changes of 50, 70 and 95% at 10 min each and two changes of 100% ethanol at 15 min each. The samples were cleared with two changes of propylene oxide (PO), each for 10 min, followed by infiltration with resin (EMBED 812 resin, Electron Microscopy Sciences, Hatfield, PA), which included two changes of each, resin to PO mixed at a 1 : 1 ratio, followed by resin to PO at a 3 : 1 ratio for 2 h each. The samples were then infiltrated in pure resin overnight, followed by embedding into flat capsules, and polymerized at 60 °C for about 24 h. Samples were trimmed and sections were cut on a Leica Ultracut-E ultramicrotome. For HRLM, semi-thin sections (0·5 µm) were mounted onto glass slides and stained with epoxy tissue stain containing toluidine blue and basic fuchsin in water and ethyl alcohol (Electron Microscopy Sciences). Sections were viewed and photographed with the above mentioned light microscope. For TEM, ultrathin sections (90 nm) were cut, mounted on 100 mesh copper grids, and stained with 1% uranyl acetate in 50% ethanol for 30 min, washed in distilled water, stained in Sato's lead stain for 2 min, and washed with distilled water. Samples were viewed and photographed using a Philips CM12 TEM with an AMT-XR11 digital camera housed at the Department of Pathology, Robert Wood Johnson Medical School, Rutgers University.
Polymerase chain reaction, sequencing and phylogenetic analysis
Microsporidia samples were digested for 3 h at 56 °C with 20 µL proteinase K in 180 µL of lysis buffer, followed by DNA extraction using the DNeasy Blood and Tissue Kit automated on the QIAcube (Qiagen), according to manufacturer's instructions for purification of total DNA from animal tissues. For amplification of microsporidian DNA, PCR amplification of overlapping DNA fragments containing the SSU 16S rDNA, internal transcribed spacer (ITS) and partial large subunit (LSU) rDNA was done using the following primer pairs: V1F (5′-CAC CAG GTT GAT TCT GCC-3′)–1492R (5′-GGT TAC CTT GTT ACG ACT T-3′) and 530F (5′GTG CCA GCA GCC GCG G-3′)–580R (5′-GGT CCG TGT TTC AAG ACG G-3′) (Vossbrinck et al. Reference Vossbrinck, Baker, Didier, Debrunner-Vossbrinck and Shadduck1993, Reference Vossbrinck, Andreadis, Vavra and Becnel2004). Additionally to amplify the digenean parasite, the SSU 18S ribosomal RNA gene was amplified using universal eukaryotic primers ERIB1 (5′-ACC TGG TTG ATC CTG CCA G)–ERIB10 (5′-CTT CCG CAG GTT CAC CTA CGG-3′) (Barta et al. Reference Barta, Martin, Liberator, Dashkevicz, Anderson, Feighner, Elbrecht, Perkins-Barrow, Jenkins, Danforth, Ruff and Profous-Juchelka1997) in the same samples. PCRs were run in 50 µL reaction volumes containing 6 µL of extracted DNA, 1× PCR Buffer, 1·5 mm MgCl2, 0·2 mm dNTPs, 0·5 µ m of each Primer, 2·5 U Taq polymerase (Invitrogen), and molecular grade water. DNA amplification was performed on a Veriti thermocycler (Applied Biosystems) with initial denaturation at 95 °C for 5 min, followed by 35 cycles of 95 °C for 50 s, 56 °C for 50 s, and 72 °C for 1 min, followed by a final extension of 72 °C for 10 min. Amplified products were electrophoresed on 1·2% agarose E-gels (Invitrogen) containing ethidium bromide and imaged under ultraviolet light. Sequencing was performed in both directions on amplified PCR products using the PCR primers. Sequencing reactions were prepared by purification of PCR products with ExoSAP-IT (Affymetrix), dilution to approximately 4 ng of DNA µL−1 with molecular-grade water, and addition of 5 µ m of the PCR primer. DNA sequencing was completed by GENEWIZ, Inc. (South Plainfield, NJ, USA) using ABI BigDye version 3.1 (Applied Biosystems) and run on an ABI 3730xl DNA analyser (Applied Biosystems).
DNA sequences were visually inspected and edited using Chromas Lite Version 2.1., followed by DNA sequence alignment using BioEdit Sequence Alignment Editor V7.2.5 (Hall, Reference Hall1999). DNA sequences were assembled by aligning overlapping DNA sequences for each sample. The resulting assembled sequences were checked using the National Center for Biotechnology Information's (NCBI) Basic Local Alignment Search Tool (BLAST) to confirm that the sequences were related to their respective organisms, either microsporidia or the digenean P. minimum. Identities to individual microsporidia species was done by BLAST. Pairwise genetic distances were estimated from the microsporidian sequence compared with other related microsporidia using the Kimura2-parameter model (Kimura, Reference Kimura1980) in MEGA7, Version 7.0.14 (Kumar et al. Reference Kumar, Stecher and Tamura2016). Estimation of pairwise genetic distances was done for 14 species within the main clade, including Heterosporis, Pleistophora and Ovipleistophora and then for five of the closest related Ovipleistophora and Pleistophora species.
Phylogenetic analysis was completed on the microsporidian sequence using MEGA7 by both maximum likelihood and maximum parsimony analyses. Chosen microsporidian sequences had at least 70% sequence coverage and those that did not align well with our available sequence were eliminated to include the longest sequence possible for analysis. The analysis involved a total of 33 microsporidian nucleotide sequences and the species Spraguea lophii and Dictyocoela berillonum were used as outgroups to root the tree. All positions containing gaps and missing data were eliminated. There were a total of 1257 positions in the final dataset. For maximum likelihood analysis all combinations of the evolutionary model and rates among sites were tested using MEGA7 and the model that best matched the dataset was used. The General Time Reversible model (Nei and Kumar, Reference Nei and Kumar2000) was used to infer evolutionary history and a discrete Gamma distribution was used to model evolutionary rate differences among sites [five categories (+G, parameter = 0·2712)]. The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 34·3321% sites). The tree with the highest log likelihood (−4236·4153) is shown. The percentage of trees in which the associated taxa clustered together (1000 replicates) was shown next to the branches. Initial trees for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. For maximum parsimony analysis, tree #1 out of the six most parsimonious trees (length = 1074) was examined. The consistency index was (0·549139), the retention index was (0·804310), and the composite index was 0·471053 (0·441678) for all sites. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) was shown next to the branches (Felsenstein, Reference Felsenstein1985). The tree was obtained using the Subtree–Pruning–Regrafting (SPR) algorithm (Nei and Kumar, Reference Nei and Kumar2000) with search level 1 in which the initial trees were obtained by the random addition of sequences (10 replicates).
RESULTS
Based on histology, two microsporidian species were detected from the tissues of the sunfish. One species rarely occurred and formed xenomas near the renal tubular epithelium in one fish and around the connective tissue of the kidney of another fish. Spores within the xenomas stained heavily Gram-positive and all were monomorphic (Fig. 1). Due to the rare occurrence and light infection of this microsporidium, no fresh tissue was available for analysis. A second microsporidian species occurred frequently and was associated with the metacercarial cyst wall of P. minimum.

Fig. 1. An uncharacterized microsporidium forming xenomas in the kidney of bluegill sunfish. (A) Xenoma in close association with a renal tubule, stained with H&E. (B) Gram-stained xenoma containing monomorphic spores. Scale 20 µm.
The digenean parasite P. minimum was present in all sixty fish (Fig. 2). A mean of 37 (±27·61, range 2–98) and 62 (±26·78, range 28–164) P. minimum cysts occurred per fish tissue section sampled from the spring and fall, respectively. Based on analysis of a total of 2967 P. minimum cysts from both sampling times, 351 cysts were associated with microsporidian infection within the metacercarial cyst wall. Prevalence of the parasite was similar between the two seasons. In spring, the microsporidium was present in digeneans from 16 out of 30 bluegill sunfish; from infected fish a total of 189 digeneans had microsporidium infections out of 720. An average of 26% (±20%) of digeneans were infected by the microsporidium within individual fish with a range of 1–60%. In fall, the microsporidium was present in digeneans from 17 out of 30 bluegill sunfish; 161 digeneans were infected out of 1184. An average of 14% (±13%) of the digeneans were infected in individual fish with a range of 1–41%.

Fig. 2. Bluegill sunfish liver heavily infected with Posthodiplostomum minimum metacercariae. Scale 1 mm.
Spore morphology
Opaque cysts were filled with dimorphic microsporidian spores with microspores being most numerous (Fig. 3A) and macrospores in smaller numbers (Fig. 3B). Microspores had a length of 4·3 ± 0·3 (3·7–5·2) μm and width of 2·5 ± 0·2 (2·1–2·9) μm (n = 125); macrospores had a length of 7·5 ± 0·6 (6·4–9·4) μm and a width of 4·7 ± 0·3 (3·9–5·6) (n = 125). Microspores and macrospores developed in separate sporophorous vesicles (SPV) with up to 22 microspores and six macrospores within respective SPVs (Fig. 3C). Spores were monokaryotic with typical microsporidian features, including a prominent posterior vacuole, anchoring disk, thick electron-lucent endospore, which is thinner adjacent to anchoring disk, and thin electron-dense exospore (Fig. 3D). Abundant ribosomes often arranged in layers of polysomes frequently occurred adjacent to the polaroplast (Fig. 3D). Microspores had six to nine coils of polar tube arranged in a single row (n = 25; Fig. 3E) and macrospores had 19–43 coils of polar tube arranged in one to four rows (n = 7; Fig. 3F).

Fig. 3. Microsporidian spores of Ovipleistophora diplostomuri n. sp. (A) Large number of mainly microspores from fresh microscopic preparation of a cyst. Scale 10 µm. (B) A mixture of microspores and macrospores in fresh spore preparation. Scale 10 µm. (C) High-resolution light micrograph showing macrospores and microspores developing separately within sporophorous vesicles. Scale 10 µm. (D) A mature microspore showing anchoring disk and polysomes adjacent to the polaroplast. Scale 500 nm. (E) Microspores with seven to eight coils in the polar filament. Scale 500 nm. (F) Macrospore with polar filament arranged in several layers. Scale 500 nm.
Parasite tropism and pathology
Infection was only associated with the outer metacercarial cyst capsule of P. minimum and not observed in any of the internal tissues of the metacercariae itself or within other internal tissues of the fish host. The normal uninfected cyst capsule of P. minimum was about 6·5 µm thick and composed of a primary cyst wall measuring about 2·5 µm that had basophilic staining along the inner surface, otherwise staining eosinophilic. The outer portion of the capsule was composed of a fibroblast layer measuring roughly 4 µm wide, made up of two to three layers of overlapping elongated fibroblasts (Fig. 4A and B). Microsporidian infection was observed in two forms, one causing severe hypertrophy of the metacercarial cyst capsule often leading to degeneration of metacercariae (Fig. 4C–H) and a second form with large microsporidian spore aggregates forming from the metacercarial cyst capsule (Fig. 5).

Fig. 4. Histology of fish organs with Posthodiplostomum minimum affected by the microsporidium Ovipleistophora diplostomuri n. sp. stained with H&E unless otherwise stated. (A) P. minimum metacercariae (arrow) within a normal metacercarial cyst (arrowhead). Scale 40 µm. (B) Structure of the normal metacercarial cyst wall of P. minimum made up of an inner layer (arrowhead) surrounded by host fibroblasts. Scale 10 µm. (C) Microsporidian-infected metacercarial wall with increased thickness of wall adjacent to inner layer of cyst wall (arrowheads). Scale 40 µm. (D) High magnification showing microsporidian spores (arrow) developing between the inner parasite wall (arrowhead) and the host fibroblast layer. Scale 10 µm. (E) Microsporidian infection (arrow) directly adjacent to inner metacercarial wall (arrowheads), notice the Gram-positive spores in the infected region (arrow). Scale 40 µm, Gram stain. (F) Degenerating P. minimum metacercaria within a collapsed and hypertrophic metacercarial cyst. Scale 40 µm. (G) Severely infected and hypertrophied metacercarial cyst wall which has collapsed and obstructed the cyst lumen. Scale 40 µm. (H) Higher magnification of metacercarial cyst wall from (G) showing the collapsed inner cyst wall (arrowheads) and microsporidian spores within the hypertrophied cyst wall. Scale 10 µm.

Fig. 5. Aggregates of Ovipleistophora diplostomuri n. sp. within internal organs of bluegill sunfish associated with Posthodiplostomum minimum stained with H&E unless otherwise stated. (A) Liver heavily infected with P. minimum and microsporidian aggregates (arrows) surrounded by a thin host macrophage response. Scale 200 µm; (B) P. minimum with large aggregation of microsporidian spores (*) within the metacercarial cyst wall. Scale 50 µm. (C) Higher magnification of boxed in area from (B) showing microsporidian aggregate (*) directly beneath the inner metacercarial cyst wall (arrowheads). Scale 50 µm. (D) Microsporidian microspores and macrospores (*) released directly into liver of bluegill sunfish with minimal macrophage response (arrow). Scale 50 µm. (E) P. minimum metacercariae with large microsporidian aggregate (*) directly beneath the metacercarial cyst wall (arrowheads), notice the Gram-positive staining of microsporidian spores. Scale 100 µm, Gram stain. (F) Higher magnification of microsporidian aggregate from (E) showing Gram-positive mature micro and macrospores. Scale 10 µm, Gram stain.
During early infection, microsporidian spores were apparent between the primary cyst wall and the fibrous capsule, associated with a thickening of the fibrous layer which stained highly eosinophilic and had a thin surrounding of macrophages (Fig. 4C and D). Proliferation of spores within cells between the cyst wall and fibrous capsule led to a severe thickening of the capsule which contained microsporidian developmental stages and mature spores (Fig. 4E). Later infection was characterized by increased hypertrophy of the eosinophilic fibrous layer containing microsporidian spores, which was associated with an irregularly-shaped and collapsed metacercarial cyst wall, losing the normal rounded shape and leading to degeneration of the P. minimum metacercariae (Fig. 4F). The severe hypertrophy of the cyst wall, proliferation of microsporidia, and collapse of the rounded structure of the cyst led to the metacercarial cyst containing mainly the hypertrophic cyst wall folded on itself in numerous layers and remnants of the parasite (Fig. 4G). Microsporidian spores were evident throughout the hypertrophic cyst wall (Fig. 4H).
In a second form of infection, large microsporidian aggregates were surrounded by several layers of macrophages particularly in liver (Fig. 5A) and more rarely in the epicardium, kidney and spleen. Though not always obvious due to the tissue section plane, these large cysts were in continuum with the primary metacercarial cyst wall (Fig. 5B and C). In two cases, full breakdown of the metacercarial cyst wall occurred in the liver of the fish and microsporidian spores occurred freely in the liver tissue of the fish host. Little to no fish-host response occurred to the presence of spores, with only a thin surrounding of host macrophages (Fig. 5D). Gram-staining of the microsporidian aggregates associated with the metacercarial wall showed that mature microspores and macrospores stained Gram-positive, while others did not (Fig. 5E and F), likely dependent on their stage of development. When microsporidian spores were aggregated focally within a region of the cyst wall and the cyst wall was not hypertrophic then intact metacercariae were observed (Fig. 5E). In other cases, the metacercariae cysts were replaced with microsporidian spores and remnants of degenerated metacercariae.
Developmental stages of the microsporidium
The earliest observed stages of development were large multi-nucleated merogonial plasmodia, which were rich in ribosomes (Fig. 6A) and occurred directly in the host cell cytoplasm surrounded by two membranes. These developed into sporogonial plasmodia, which underwent cytokinesis, giving rise to individual sporonts within a newly formed SPV that separated them from the host cell cytoplasm (Fig. 6B). The SPV had a finely granular material that was electron-lucent compared with other components of the cell cytoplasm. Sporonts were rich in ribosomes and rough endoplasmic reticulum and had a thickened parasite wall that was composed of two layers that had increased electron density. Following sporogony, sporoblasts were formed and characterized by an increased electron dense cytoplasm and first appearance of a primordial polar tube (Fig. 6C). Late sporoblasts had an organized polar tube and a wavy spore wall in which the endospore and exospore have not yet been fully formed (Fig. 6D). Finally groups of up to 22 mature microspores within a single section plane formed within the SPV (Fig. 6E). It was common to see small electron dense tubules throughout the granular matrix within the SPV during development from sporoblasts to mature spores. The complete developmental progression of macrospores could not be confirmed due to the smaller numbers of these spores compared with microspores. In two instances, mature spores with fully formed, though disorganized polar tubes appeared connected by the endospore and exospore (Fig. 6F). The large number of coils in the polar tube would suggest these to be macrospores. The significance of the apparent connection of the spores is unknown and may be either a deformed spore or an artefact, as mature spore division is not common in microsporidium development.

Fig. 6. Developmental stages of Oviplesitophora diplostomuri n. sp. as seen with TEM. (A) Merogonial plasmodium with multiple nuclei (n) directly in the host cell cytoplasm. (B) Division by cytokinesis to form sporonts within an SPV (*). (C) Early sporoblasts within an SPV (*), sporoblasts have an irregular shape and are starting to form a primordial polar tube. (D) Late sporoblasts with a formed polar tube within a SPV (*). (E) Mature microspores enclosed within a SPV. (F) Mature spores with disorganized polar tube with apparent connection of the endospore and exospore (arrow). Scales 1 µm.
Genetics and phylogenetic analysis
To confirm the identity of P. minimum associated with the microsporidium, the 18S rRNA gene was sequenced from the microsporidian cyst and the resultant 1874 bp long sequence was deposited to GenBank under accession number KY809062. BLAST analysis of this sequence demonstrated 99·3% identity (1862/1874) with P. minimum (AY245767).
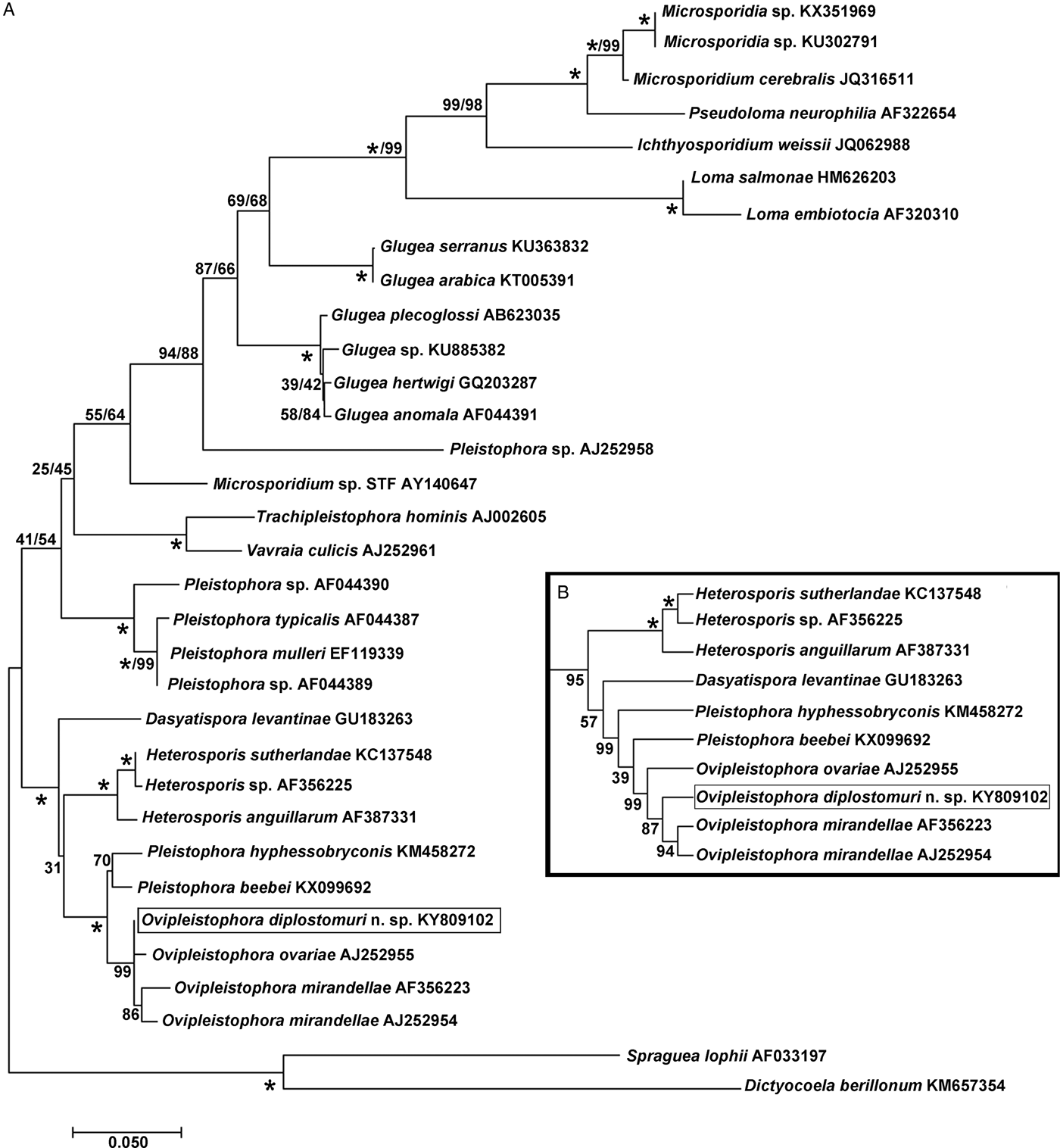
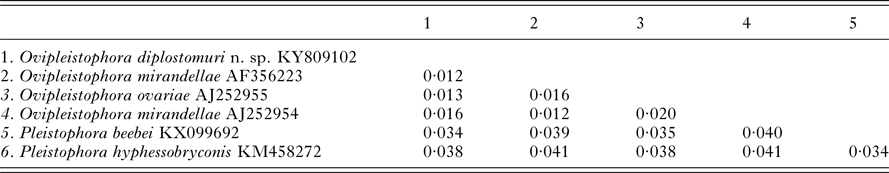
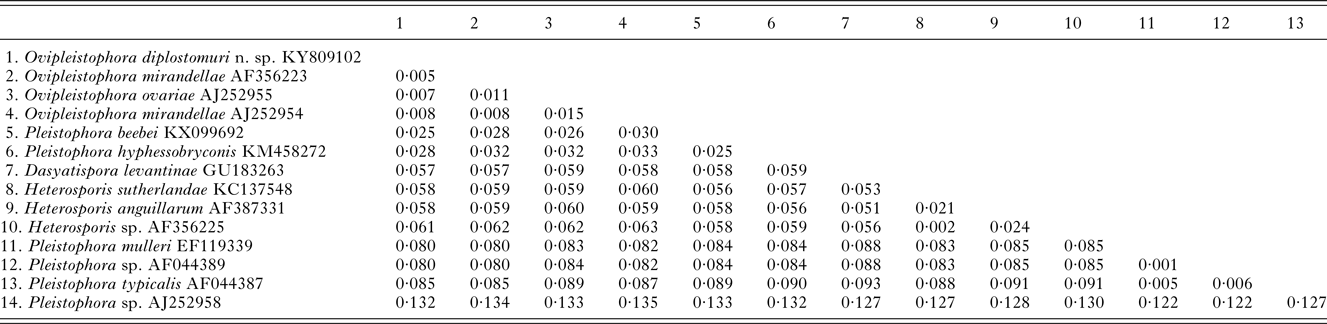
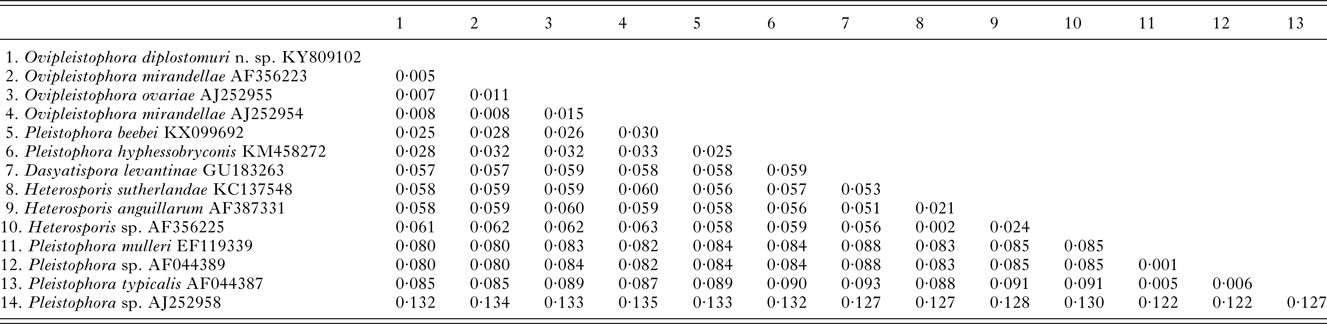
The 1832 bp long sequence for the microsporidium was deposited to GenBank under accession number KY809102. Genetic identities supported that this species grouped closest to the genus Ovipleistophora. A BLAST search showed closest identity of this species to several isolates of Ovipleistophora mirandellae, with AJ295327 having 96·9% identity (1395/1440), AJ252954 with 98·2% (1320/1344 identities), AF356223 with 98·4% (1298/1319 identities) and AF104085 with 98·8% (1271/1287 identities). The species also had nearly equally close identity to O. ovariae AJ252955 with 97·8% (1319/1348 identities). The next closest identities were to P. beebei (KX099692)at 95% and P. hyphessobryconis (KM458272) at 94%. Pairwise genetic distance estimates varied based on the size of the sequences included in the analysis. When compared with five of the closest related species, estimates indicated that the current microsporidian is between 98·4–98·9% similar to O. mirandellae (AF356223 and AJ252954) and 98·7% similar to O. ovariae (AJ252955) (Table 1). When pairwise genetic estimates were done on the entire clade including Heterosporis, Pleistophora and Ovipleistophora close identity of the microsporidium to Ovipleistophora was confirmed (99·2–99·5%) (Table 2). Both maximum likelihood and maximum parsimony produced similar phylogenetic trees supporting the grouping within Ovipleistophora spp. and both analyses showed that Pleistophora is polyphyletic (Fig. 7). Maximum-likelihood analysis showed Ovipleistophora spp., P. beebei, P. hyphessobryconis, several species of Heterosporis and Dasyatispora levantinae within a main clade. At least two subclades occurred with P. hyphessobryconis, P. beebei and Ovipleistophora spp. forming a sister group to the Heterosporis spp. group. The microsporidium from the current study grouped closely with Ovipleistophora spp. and separate from P. hyphessobryconis and P. beebei, with strong bootstrap support (99%) (Fig. 7A). Maximum parsimony provided similar topology, except that D. levantinae was grouped within the Pleistophora/Ovipleistophora node, though with less bootstrap support (57%) (Fig. 7B). A cropped maximum parsimony tree showing the main differences in the Ovipleistophora/Pleistophora/Heterosporis node is shown in Fig. 7B.
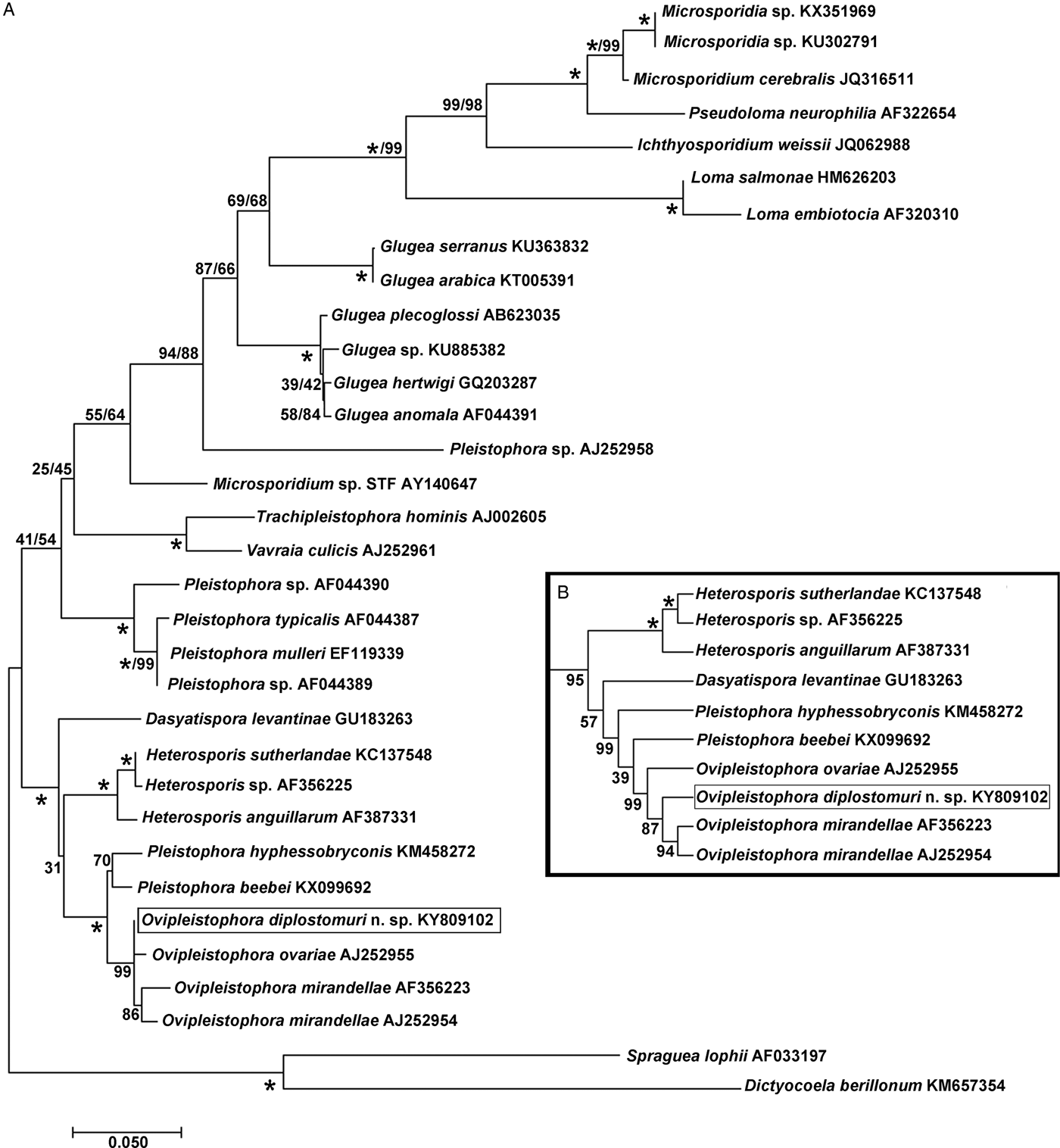
Fig. 7. Phylogenetic analysis by maximum likelihood and maximum parsimony of Ovipleistophora diplostomuri n. sp. (boxed) and 32 other microsporidians; % bootstrap support (based on 1000 replicates) appears next to each node for maximum likelihood/maximum parsimony; 100% bootstrap support is denoted with (*). (A) Maximum likelihood tree. (B) A cropped maximum parsimony tree showing the different topology in the Ovipleistophora/Pleistophora/Heterosporis node, otherwise topology of the two trees was similar.
Table 1. Pairwise genetic distance table between Ovipleistophora diplostomuri n. sp. and closely related Ovipleistophora and Pleistophora species based on 1303 nucleotides
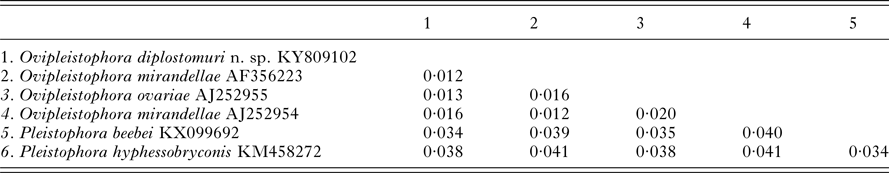
Table 2. Pairwise genetic distance tables between Ovipleistophora diplostomuri n. sp. and related microsporidia within the main clade containing Heterosporis, Pleistophora and Ovipleistophora based on 1230 nucleotides
DISCUSSION
To date there have been no other formal descriptions of Pleistophora or Ovipleistophora species associated with trematodes. While other microsporidian species described from trematodes (Nosema and Unikaryon) directly infect the trematode tissues, the species herein had a unique tropism for fibroblasts surrounding the metacercarial cyst wall. Paperna et al. (Reference Paperna, Sabnai and Castel1978) reported a similar microsporidian infection within fibroblasts of the outer metacercarial capsule of the digenean Heterophyes heterophyes (Siebold) infecting the grey mullet Liza ramada (Risso) from the Mediterranean Sea, though a complete species description was not possible. A single spore type was described, which measured 3·7 µm by 1·7 µm with 7–19 coils in the polar tube. Although inconclusive it was suggested that the species belonged to the genus Pleistophora (Paperna et al. Reference Paperna, Sabnai and Castel1978). An apparent difference in the species reported by Paperna et al. (Reference Paperna, Sabnai and Castel1978) is that spores were monomorphic, whereas they are dimorphic in the species described in the present study. This should be carefully interpreted since Paperna et al. (Reference Paperna, Sabnai and Castel1978) indicated that the electron microscopy was not suitable for an accurate description. Nonetheless these two microsporidia are most likely separate species based on the host type, sunfish vs. mullet, and locality, seawater habitat of the Mediterranean Sea vs. an inland freshwater lake in the USA.
The molecular results from this study demonstrated that the microsporidium groups within the genus Ovipleistophora. The developmental cycle including formation of multiple spores within a SPV and dimorphism in spores are common features of Pleistophora and Ovipleistophora (Lom, Reference Lom2002; Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002). The genus Pleistophora contains species mainly known for causing infections in the skeletal muscle (Dyková, Reference Dyková and Woo2006) with P. typicalis as the type species (Canning and Nicholas, Reference Canning and Nicholas1980), while Ovipleistophora is known for causing infections of the ovaries with O. mirandellae as the type species (Maurand et al. Reference Maurand, Loubes, Gasc, Pelletier and Barral1988; Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002). Genetic and morphological features supported inclusion of the presently described microsporidium with other Ovipleistophora spp., though several differences were apparent, including neither having a tropism for fish ovary or a thick envelope during merogony, which are considered definitions of the genus (Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002). Based on the current description, the genus Ovipleistophora should not be defined by having a tropism for ovarian tissue. Additionally, the thick envelope surrounding meronts should be excluded as an obligatory trait for the genus. Lom and Nilsen (Reference Lom and Nilsen2003) suggested that the thick merogonial envelope may either be a product of the meront plasmalemma or induced by the oocyte. If in fact the envelope is a product of the oocyte then it is not surprising that it is not present in the microsporidium herein which develops in non-ovarian tissue. Spore morphology and size was most similar to O. mirandellae, which have macrospores ranging from 8 to 12 µm in length and microspores 3 to 7·5 µm in length (Maurand et al. 1988); the spores described herein are on the smaller scale of this range. In O. mirandellae, macrospores were reported to outnumber microspores (Maurand et al. Reference Maurand, Loubes, Gasc, Pelletier and Barral1988), which is the reverse for that described for the species herein. In contrast to the dimorphic spores described above, spores of O. ovariae are monomorphic measuring about 6·5 µm long by 3·6 µm wide. Based on the morphological characteristics, genetics and unique tropism of the microsporidium described herein, it belongs to a new species for which we propose the name Ovipleistophora diplostomuri n. sp.
A remarkable finding from this study was the unique tropism of this microsporidium, and the important remaining question: Is this microsporidium infecting the fish, the digenean parasite or does it require both as hosts? Previous studies on P. minimum demonstrated that the composition of the metacercarial cyst wall originates from parasite secretions, making an inner wall which is tightly adhered by several layers of fish-host endothelial-like cells or fibroblasts (Mitchell, Reference Mitchell1974; Crider and Meade, Reference Crider and Meade1975). Based on the findings herein it is likely that O. diplostomuri n. sp. infects a fish-host cell, presumably a fibroblast that forms the outer metacercarial capsule. Though, despite extensive searching, the microsporidium did not occur in host tissues that were free of metacercarial cysts, where fibroblasts were also abundant. It is possible that the close apposition of the inner parasite-derived cyst wall and the host fibroblasts results in the mixing of parasite and host proteins, which has been suggested to occur based on immunological studies (Crider and Meade, Reference Crider and Meade1975). Considering that microsporidia are known to acquire their nutrients from their host cells, perhaps this association can be explained by the microsporidian requiring both parasite and host proteins for development. Another possible explanation for this unique tropism may reflect the opportunistic nature of the microsporidium infecting an immune privileged site, with immunosuppression of fibroblasts likely induced by the P. minimum metacercariae. Ovipleistophora mirandellae has a tropism for gonads, particularly the oocytes, but can also infect fibrous connective stroma of ovaries, and connective tissue between seminiferous tubules in testes (Maurand et al. Reference Maurand, Loubes, Gasc, Pelletier and Barral1988; Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002). The ovary is also considered an immune privileged site, so the tropism for connective tissue cells in immune privileged sites appears to be shared between O. mirandellae and O. diplostomuri n. sp.
The unique tropism of O. diplostomuri n. sp. seemingly involving both host (fish) and parasite (digenean) factors distinguish this microsporidium from other described species. Genetic results from this study suggest that the microsporidium is closely related to and likely evolved from a fish microsporidium and not a hyperparasitic microsporidian of a digenean. This is also supported by the fact that infection seemed to occur in a fish-host cell and not in the digenean tissue, which is distinct from previously reported hyperparasitic microsporidians in digeneans primarily belonging to the genera Nosema and Unikaryon, in which microsporidia occur directly in the digenean tissues. To explore the evolutionary origins of O. diplostomuri n. sp. other fish microsporidia in sunfish species should be investigated. The current study did detect very light infections of a different, monomorphic microsporidian species within the kidney that formed xenomas, though its rare occurrence did not allow for full morphological and molecular characterization to determine its relationship to O. diplostomuri n. sp. Perhaps to best understand the evolution of this parasite, other Ovipleistophora species in the fish should be investigated. Ovipleistophora ovariae causes reduced fecundity in golden shiners and fathead minnows throughout the USA (Ruehl-Fehlert et al. Reference Ruehl-Fehlert, Bomke, Dorgerloh, Palazzi and Rosenbruch2005; Summerfelt and Goodwin, Reference Summerfelt and Goodwin2010), though this species is not known to infect sunfish. The close genetic identity and similar morphology of O. diplostomuri n. sp. to O. mirandellae warrants further investigation to understand the evolution of this microsporidium. To date O. mirandellae has only been described from cyprinid fish in Europe (Maurand et al. Reference Maurand, Loubes, Gasc, Pelletier and Barral1988; Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002), though with the close genetic identity to O. diplostomuri n. sp. it would be interesting to determine if O. mirandellae is present in North America. It is possible that the common and long-lasting infections of P. minimum metacercariae in the internal organs of sunfish, which are often detected at 100% prevalence (Palmieri, Reference Palmieri1975; current study), may have created a niche for the evolution of this opportunistic microsporidian parasite. The unnamed microsporidium described by Paperna et al. (Reference Paperna, Sabnai and Castel1978) showed a similar pattern in mullet with a tropism to the metacercarial wall of H. heterophyes, a digenean with nearly 100% prevalence in that host species (Paperna et al. Reference Paperna, Sabnai and Castel1978). To better understand the evolution of these microsporidia it will be important to determine if they phylogenetically cluster together or if they group separately from each other and have closer relationships with fish microsporidia from their respective hosts. Genetic analysis of the species described by Paperna et al. (Reference Paperna, Sabnai and Castel1978) could aid in revealing this information, as currently only genetic information for O. diplostomuri n. sp. is known.
Posthodiplostomum minimum centrarchi infects a broad range of centrarchids, though it is considered a specialist for Lepomis sunfish (Lane et al. Reference Lane, Spier, Wiederholt and Meagher2015). The present study only examined bluegill sunfish and detected microsporidium infection at a relatively high frequency in this species. Further studies should target all centrarchid species to determine if microsporidium infection is species-specific to sunfish or if other species hosting P. minimum can be affected. Previous observations from Paperna et al. (Reference Paperna, Sabnai and Castel1978) suggest that these infections may be highly host-specific. Heterophyasis occurred in various mullet and sea bass species, though the microsporidium was only detected in the fish host L. ramada (Paperna et al. Reference Paperna, Sabnai and Castel1978). Infections of O. diplostomuri n. sp. in bluegill sunfish did not appear to have a seasonal pattern, since infection was detected in both the spring and fall seasons at relatively high levels. In contrast to this, the species reported by Paperna et al. (Reference Paperna, Sabnai and Castel1978) showed a highly seasonal pattern with infection most common in February, while rarely detected during other times of year. Microsporidian infection associated with P. minimum occurred at a relatively high prevalence in the sunfish population described in the current study. The resulting infection caused hypertrophy and collapse of the metacercarial wall resulting in degeneration of the metacercariae, thus having greatest impact to the digenean parasite itself. Further research should aid in determining if these microsporidian infections impact the ecology of the affected digenean parasites.
Understanding the benefits to the parasite ecology for an apparent fish microsporidium to have tropism for P. minimum may shed light into the biology of this species. Other microsporidia have been shown to have varied tropisms aiding in transmission to different hosts. For example, P. theridion, synonymous with D. lepeophtherii (Freeman and Sommerville, Reference Freeman and Sommerville2011), infects and causes disease in salmonids and it hyperparasitizes sea lice, which may aid in the dissemination and transmission of the parasite between fish hosts (Nylund et al. Reference Nylund, Nylund, Watanabe, Arnesen and Karlsbakk2010, Reference Nylund, Anderson, Saevareid, Plarre, Watanabe, Arnesen, Karlsbakk and Nylund2011; Sveen et al. Reference Sveen, Overland, Karlsbakk and Nylund2012). Further, the microsporidium Hyperspora aquatica is hyperparasitic in Marteilia cochillia, which is a paramyxid parasite of European cockles, and it groups closely with other microsporidia that infect aquatic crustaceans, suggesting that M. cochillia may vector the microsporidium between molluscan and crustacean hosts (Stentiford et al. Reference Stentiford, Ramilo, Abollo, Kerr, Bateman, Feist, Bass and Villalba2017). Further understanding the biology of the microsporidium from the current study in other hosts for P. minimum may aid in understanding this interesting host–parasite relationship. The first intermediate host for P. minimum is a physid snail which produces cercariae after infection. The cercariae are shed from the snail and infect fish through the skin, migrate to the internal organs, and form metacercariae (Paperna and Dzikowski, Reference Paperna, Dzikowski and Woo2006). The possibility for snails or cercariae to be vectors in the transmission of this microsporidium should be further explored. Though many microsporidia infect hosts through the gut, it is possible that cercarial invasion into fish tissues may provide a means for the current microsporidium to gain entry to fish-host tissues. This could also explain the association with only the metacercariae and not other fish tissues. Similarly a further understanding of the fate of the microsporidium in the digestive tract of birds, which are the definitive host for P. minimum may help to understand its biology and transmission dynamics.
Taxonomic summary for Ovipleistophora diplostomuri n. sp.
Phylum: Microsporidia Balbiani, 1882.
Family: Pleistophoridae Doflein, 1901.
Genus: Ovipleistophora Pekkarinen et al. Reference Pekkarinen, Lom and Nilsen2002.
Type Species: Ovipleistophora diplostomuri n. sp.
Type host: Bluegill sunfish, L. macrochirus infected with P. minimum metacercariae.
Type locality: Assunpink Lake, Assunpink Wildlife Management Area, Monmouth County, New Jersey, USA (40°13′07·4″N, 74°31′01·6″W).
Site of infection: Internal organs, most commonly in liver, but also in spleen, kidney and epicardium. Microsporidium associated with P. minimum metacercarial cyst wall, most likely infecting a fish-host fibroblast surrounding the cyst wall.
Prevalence: Found in 33/60 bluegill sunfish (55%); 351/2967 metacercariae affected (12%).
Merogony: Multinucleated merogonial plasmodia in direct contact with cell cytoplasm, often surrounded by two host cell membranes (Fig. 6A).
Sporogony: Sporogonial plasmodia with increasingly electron dense wall. A SPV is produced during sporogony and is apparent after cytokinesis of sporogonial plasmodia to produce individual sporonts within an SPV. Sporont wall is composed of two apparent layers with increased electron density (Fig. 6B).
Spores: Dimorphic spores with microspores measuring 4·3 ± 0·3 (3·7–5·2) μm by 2·5 ± 0·2 (2·1–2·9) μm (n = 125) and 6–9 coils of polar tube arranged in a single layer and macrospores 7·5 ± 0·6 (6·4–9·4) μm by 4·7 ± 0·3 (3·9–5·6) (n = 125) with 19–43 coils of polar tube arranged in up to four rows. Microspores and macrospores developed in separate SPVs with up to 22 microspores and six macrospores within respective SPVs (Fig. 3).
Pathology: Causes hypertrophy of metacercarial wall leading to collapse of wall and degeneration of the digenean P. minimum (Fig. 4). Forms large aggregates of microsporidian spores within the metacercarial wall between the inner parasite-derived layer and the fish-host fibroblast layer. Microsporidian aggregates surrounded by host macrophages, otherwise minimal pathology induced in the fish-host tissue (Fig. 5).
Genbank accession: KY809102.
material deposited: Histology slides have been catalogued at the National Parasite Collection housed at the Smithsonian Institution, National Museum of Natural History, Department of Invertebrate Zoology, under accession numbers USNM 1422266–1422269. Frozen parasite material, tissue embedded in paraffin and resin blocks, and additional histology slides are catalogued at the N.J. Division of Fish and Wildlife, Pequest Fish Health Laboratory, Oxford, NJ, USA.
Etymology: The species name is derived from the digenean family Diplostomidae because of its strong association with P. minimum and the latin word ‘muri’ meaning wall, for its tropism for the metacercarial cyst wall.
Amended diagnosis of the genus Ovipleistophora: Tropism for ovary is not obligatory; tropism is extended to include fibroblasts within wall of encysted digenean metacercariae. Previously reported electron-dense material surrounding meronts during merogony is not always present within the genus.
ACKNOWLEDGEMENTS
We would like to thank staff from the N.J. Division of Fish and Wildlife, including Chris Smith (Bureau of Freshwater Fisheries), for his assistance in collecting fish for this study, and Josette Hutcheson (Office of Fish and Wildlife Health and Forensics) for assistance in sample processing. We are also grateful for the staff at the Animal Health Diagnostic Laboratory, N.J. Department of Agriculture, specifically Lana Castellano and Denise Dicarlo-Emery, for assistance in the processing of laboratory samples. Additionally we would like to acknowledge the peer-reviewers who have provided helpful comments to improve this manuscript.
FINANCIAL SUPPORT
Financial support was provided by the Federal Aid in Sport Fish Restoration Act, Project FW-69-R18, and the New Jersey Hunter and Anglers Fund.