Introduction
Phenoloxidases (POs) are a group of multicopper proteins that include tyrosinase (monophenol oxygenase, EC1.14.18.1), catecholase (EC1.10.3.1, o-benzenediol: oxygen oxidoreductase) and laccase (EC1.10.3.2, p-benzenediol:oxygen oxidoreductase) (Barrett, Reference Barrett1987). Insect prophenoloxidase (PPO) possesses the classical structural features of type-3 copper-containing proteins, including three histidine (H) residues and two copper atoms inside each active site pocket (Aguilera et al., Reference Aguilera, McDougall and Degnan2013). To date, this class of proteins has been detected in organisms such as invertebrates, vertebrates, microbes, and plants (Sánchez-Ferrer et al., Reference Sánchez-Ferrer, Rodríguez-López, García-Cánovas and García-Carmona1995; Chase et al., Reference Chase, Raina, Bruno and Sugumaran2000). The type-3 copper proteins from all the different types of organisms vary in function, and therefore, these proteins are named differently, e.g., haemocyanin in arthropods, tyrosinase in microbes and mammals, prophenoloxidase (PPO) in crabs and insects, and polyphenoloxidase (also known as PPO) in plants (Cerenius et al., Reference Cerenius, Lee and Söderhäll2008). In insects, two types of POs, namely, laccase and tyrosinase, co-exist; tyrosinase plays a vital role in the melanisation of microorganisms, wound healing and immune response and is primarily responsible for catalyzing two consecutively occurring oxygen-dependent reactions: the first step (cresolase activity) involves the hydroxylation of tyrosine to dopamine, which is followed by the subsequent oxidation step (catecholase activity), which involves the conversion of dopamine to dopaquinone. In contrast, some gene silencing studies on Tribolium castaneum, Locusta migratoria, Monochamus alternatus, and Bombyx mori have demonstrated that laccase (rather than the tyrosinase) plays an important role in cuticular pigmentation and hardening during insect development (Arakane et al., Reference Arakane, Muthukrishnan, Beeman, Kanost and Kramer2005; Elias-Neto et al., Reference Elias-Neto, Soares, Simões, Hartfelder and Bitondi2010).
As critical innate humoural proteins closely associated with defence and due to the potential physiological importance of these proteins, researchers worldwide have done a large amount of work on POs, including molecular cloning, characterization of PO properties, PPO activation and regulation, PO purification and enzymatic activity determination of stable POs, for over a century. The activation of insect PO cascades is a complex process, and work done over the past few decades has further elucidated the activation pathway and regulatory mechanisms of these cascades (Kanost & Gorman, Reference Kanost, Gorman and Beckage2008). Briefly, PO exists constitutively in the zymogen form, PPO, in certain types of haemocytes or haemolymph and is readily activated upon microbial challenge via a combination of pattern-recognition proteins and the polysaccharides on the surfaces of invading microorganisms; subsequently, inactive PPO is cleaved to yield active PO by PPO-activating enzymes (PPAE, PAP, or PPAF). In addition, expression level measurements and RNAi experiments have also been performed in different insect species from various insect orders, including B. mori, Manduca sexta, Drosophila melanogaster, Holotrichia diomphalia, and mosquitoes, which are used as model systems; in particular, analysis of the crystal structures of MsPPO and DmPPO provided important molecular data for understanding the functions of these proteins (Li et al., Reference Li, Wang, Jiang and Deng2009; Chen et al., Reference Chen, Liu, Yang, Lu, Wang, Wang, Ling, Li, Beerntsen and Ling2012).
It is known that insect PPO not only plays an important role in shielding hosts from invasion (immediate activation in vivo) but also is responsible for wound healing (Hillyer et al., Reference Hillyer, Schmidt and Christensen2003, Reference Hillyer, Schmidt and Christensen2004; Binggeli et al., Reference Binggeli, Neyen, Poidevin and Lemaitre2014). However, several recent studies have shown that the functions of insect PPO are not limited to immunity. For example, when phenylthiourea (PTU, a specific PO inhibitor) was added to inhibit the activity of BmPPO, the phytophagous insects excreted green faeces; in contrast, black faeces was excreted in the absence of PTU despite consumption of green leaves (Shao et al., Reference Shao, Yang, Xu, Li, Lu and Wang2012). In addition, the melanization caused by PO may also affect insect longevity. Wild-type D. melanogaster had significantly longer longevity than the DmPPO1/2 double mutant (half-life = 24 days) or the DmPPO1 and DmPPO2 single mutants (Binggeli et al., Reference Binggeli, Neyen, Poidevin and Lemaitre2014). PPO is also associated with insect development; inhibition of PO activity can lead to delayed egg tanning in mosquitoes (Li & Christensen, Reference Li and Christensen1993; Marinotti et al., Reference Marinotti, Ngo, Kojin, Chou, Nguyen and Juhn2014).
Due to the potential functions of PO in different aspects of physiology, potent inhibition of PO activity is likely to trigger the dysfunction of insect PO-based defence systems or lead to incomplete and soft body cuticles, thereby resulting in pest control. Kojic acid (KA), a fungal metabolite, is a type of natural compound, and quercetin is one of the most widely distributed flavonoids in plants (Kinosita & Shikata, Reference Kinosita, Shikata and Wogan1965; Parrish et al., Reference Parrish, Wiley, Simmons and Long1966). According to several studies on the effects of KA and/or quercetin on the activity of POs from Plutella xylostella, Bactrocera dorsalis, shrimp and crabs, both the abovementioned compounds can significantly inhibit PO activity (Owusu-Yaw et al., Reference Owusu-Yaw, Cohen, Fernando and Wei1990; Niwa & Akamatsu, Reference Niwa and Akamatsu1991). Meanwhile, both ecdysone and juvenile hormone (JH) are reported to regulate colour change and morphogenesis in insects, which are necessary for insect cuticular tanning (Nakamura et al., Reference Nakamura, Stiebler, Fantappié, Fialho, Masuda and Oliveira2007; Hiruma & Riddiford, Reference Hiruma and Riddiford2009; Genta et al., Reference Genta, Souza, Garcia and Azambuja2010). However, it remains unknown whether KA can also significantly suppress PO activity and whether 20E can influence the normal development of Zeugodacus tau.
Z. tau (Walker) is an important horticultural pest that can infest various vegetables and fruits, leading to substantial economic losses for farming households. As a potential insecticide target, the critical enzyme PPO1 from Z. tau was cloned and also bioinformatically characterized. In addition, the spatiotemporal expression patterns of ZtPPO1 from different developmental stages and tissues were determined. Furthermore, to explore the biological functions of ZtPPO1, the effects of 20E and RNAi on the normal development of Z. tau were studied. Most importantly, we investigated the inhibitory effects of KA on PO activity. Hopefully, these studies will contribute to a better understanding of the physiological functions of PO in Z. tau and will help us design novel PO-induced biological target inhibitors.
Materials and methods
Experimental insects
Z. tau were obtained from a lab-established colony in Fujian Agriculture and Forestry University, Fuzhou City, Fujian, China. Throughout their life cycle, Z. tau were maintained under 14L:10D conditions at 25 ± 2°C and in 65 ± 5% relative humidity.
mRNA isolation and cDNA synthesis
Total RNA was isolated from the 3rd instar larvae by using an RN28-EASYspin Plus RNA Mini Kit (Dnase1 included; Aidlab Biotech, Beijing, China) following the manufacturer's protocol. A HiScript II 1st Strand cDNA Synthesis Kit (Vazyme Biotech, Nanjing, China) was used to synthesize the first-strand cDNA.
Cloning and RACE reaction
Degenerate primers were designed according to the conserved regions of dipteran PPO1 genes. The forward and reverse primers were 5′-GCGCGTGTGGTACTTCcgngargayth and 5′-GCGGAAGGTGCGCtcrwanggrat, respectively. Polymerase chain reaction (PCR) was performed on a Dongsheng ETC 811 instrument (Beijing, China), and the 35-cycle procedure was as follows: 94°C for 1 min, 58°C for 1.5 min, 72°C for 1 min, and, finally, 7 min at 72°C. A RACE (rapid amplification of cDNA ends) Kit (Takara Biotech., Dalian, China) was used to amplify the 3′ and 5′ ends of ZtPPO1. The sequences of the primers 5′-GSP and 3′-GSP were 5′-GCCGAAAGATTCCAAATGG CGAT-3′ and 5′-GCGGAATGGACTTTGTGCCTCGTG-3′, respectively. The PCR products were separated by 1.5% agarose gel electrophoresis, purified with an EasyPure PCR Purification Kit (Transgen Biotech, China), and cloned into the pMD18-T vector (Takara Biotech., Dalian, China) for sequencing by Boshang Biotech. Co., Ltd. (Shanghai, China).
Sequence analysis of ZtPPO1
The NCBI ORF Finder and database (http://www.ncbi.nlm.nih.gov/) were used to analyze the full-length cDNA translations (open reading frames, ORFs) and sequence identities of the gene. SignalP and pI/MW (http://us.expasy.org/tools/) were used to obtain information regarding signal peptides, pI and molecular weight. Self-optimized prediction method (SOPM) (http://npsa-pbil.ibcp.fr/) was used to predict the secondary structure. Multiple sequence alignment of ZtPPO1 with published PPO sequences was carried out using ClustalX (1.81), and phylogenetic analysis was performed using MEGA software (version 5.1). The sequences used from the NCBI database were from the following organisms (the gene IDs are provided in parentheses): Heliothis virescens (DQ841706.2), Spodoptera frugiperda (DQ289581.1), Hyphantria cunea (U86875.1), B. mori (AF178462.1), M. sexta (AF003253.1), Choristoneura fumiferana (EU046570.1), Galleria mellonella (AF336289.1), Pieris rapae (ADG65539.1), B. dorsalis (AFP81887.1), Drosophila busckii (ALC40784.1), Neobellieria bullata (AAD45526.1), Ceratitis capitata (XP_004526317), Bactrocera cucurbitae (AFP81886.1), P. xylostella (GQ149238.1), Spodoptera exigua (EF684939.1), Spodoptera litura (AY703825.1), H. diomphalia (AB079664.1), T. castaneum (AY884063.1), Tenebrio molitor (ETN57874.1), S. frugiperda (DQ289581.1), L. migratoria (FJ771025.1), Diabolocatantops pinguis (FJ598047.1), D. melanogaster (AAF59001.1), Lucilia cuprina (KNC27622.1), Anopheles gambiae (AF004915.1), Armigeres subalbatus (AAF70320.1), Aedes aegypti (AF292114.1), and Scylla serrata (DQ435606.2). The neighbour-joining method was used to construct the phylogenetic tree, and the confidence value of the branch topology was measured from 1000 bootstrap replicates.
Real-time PCR
qRT-PCR was carried out in a QuantStudio™ 6 Flex Real-Time PCR System (ABI, Life Technologies, CA, USA) in a final reaction volume of 20 µl: 2 µl of cDNA, 10 µl of 2× TransStart Top Green qPCR SuperMix, 1 µl of each primer (10 µM), 0.5 µl of the passive reference dye (50×), and 5.5 µl of ddH2O. The 40-cycle amplification procedure was as follows: 94°C, 5 s; 58°C, 30 s and 72°C, 35 s. β-Actin or EF-α was used as the internal reference gene, and the 2−ΔΔCt method was employed to calculate the relative expression levels of three repeats of each independent experiment.
Temporal and spatial expression of ZtPPO1 transcripts
qRT-PCR was carried out to detect ZtPPO1 expression during different developmental and tissue-specific stages in the lifecycle of Z. tau. Life-stage-specific samples were collected from egg to adult stages, and tissues such as the head, cuticle, midgut, Malpighian tubules, fat bodies, and haemolymph were dissected from the 3rd instar larvae. One microgram of total RNA from the Z. tau samples was used in the 20-μl reactions for first-strand cDNA synthesis using the HiScript II 1st Strand cDNA Synthesis Kit (gDNA wiper) (Vazyme Biotech, Nanjing, China). β-Actin and EF-α were used as life-stage- and tissue-specific internal controls for normalization to ensure equal sample loading (the primers used for qRT-PCR are listed in table S1 in the supporting information).
Optimal mass ratio of KA and the effect of KA on the development of Z. tau
To determine the appropriate KA content to use in the assay without having an adverse effect on the normal growth of Z. tau, the 1st instars were fed artificial diets containing different mass ratios of KA (0.33, 0.99, 1.66, 2.33 and 2.99%), and the relative mortality of the 30 larvae was observed until pupation. Based on the results obtained by monitoring mortality with respect to KA dose, the larval food containing 1.66% KA was finally chosen for subsequent experiments. The physiological indices of larval and pupal duration (days), pupation rate (fraction of larvae that pupated), pupal weights (1-day-old) and emergence rate (pupae that did not emerge after 15 days were considered dead) were recorded. An artificial diet lacking KA was used as the control. Each experiment was repeated three times, and each treatment contained 30 larvae or pupae.
The effects of KA on the expression levels of ZtPPO1 and on PO activity
Newly hatched 1st instar larvae were reared on a 1.66% KA-containing larval diet. The surviving 3rd instar larvae and the cuticles dissected from these were sampled and snap frozen in liquid nitrogen followed by storage at −80°C for further analysis of the expression levels of ZtPPO1 and of PO activity. Controls were fed only an artificial diet.
Enzyme preparation and inhibition of PO activity by KA
In vitro enzyme inhibition assays of whole larvae and cuticles were performed in the presence of various concentrations of KA (0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.5 and 2 mM). The samples were ground in an ice-cold mortar with 1:8 phosphate-buffered saline (0.2 M PBS, pH = 6.8) to extract the crude PO enzyme according to the method described by Hu et al. (Reference Hu, Dou, Wang, Jia and Wang2011) and Chen et al. (Reference Chen, Liu, Yang, Lu, Wang, Wang, Ling, Li, Beerntsen and Ling2012) with slight modifications. The homogenate was centrifuged at 17,500g for 25 min at 4°C after a 30 min incubation and the supernatant was filtered through a 0.45-μm nylon filter (Navigator, Tianjin, Lab instrument Co., Ltd.) to obtain the final crude enzyme extract. Total protein concentration was determined by treating the samples with Coomassie brilliant blue G-250 dye, using bovine serum albumin (BSA) as a standard, and measuring the absorbance at 595 nm on a Synergy H1 Hybrid microplate reader (BioTek, USA). The PO activity was tested in a 96-well microplate in a total reaction volume of 260 µl per well using 0.01 M catechol as the substrate. The mixture containing 100 µl of catechol and PBS (0.2 M, pH 6.8), 30 µl of 10× diluted enzyme extract and an equal volume of KA solution was incubated at 37°C for 10 min. Changes in absorbance were measured spectrophotometrically at 420 nm every minute for 10 min. The control was processed similarly, substituting KA with PBS. One unit of enzymatic activity (U) was defined as a change of 0.001 in the absorbance value per minute. Each assay had three replicates, and each experiment was repeated three times. The results were expressed as units of enzymatic activity per mg of protein. The median inhibitory concentration (I 50) of KA was calculated from a regression analysis of log concentration vs probit (% inhibition). For inhibition kinetic analyses, the PO activity assay system was used as described above with the exception that the inhibitor concentration (0, 0.2, 0.4, 0.8, and 1.2 mM) was held at a constant value and the substrate concentrations (0.625, 1.25, 2.5, 5, and 20 mM) as well as the enzyme concentrations (10, 25, 50, 75, and 100 µl) were varied for determination of the initial reaction rate.
Functional analysis of Z. tau
Effect of 20E on ZtPPO1 expression levels and development in Z. tau
As hormones play a key role in the development of insects, the effect of 20E on the expression of ZtPPO1 and the development of Z. tau was investigated. A 1 mg ml−1 stock solution of 20E (Sigma Aldrich, St. Louis, MO, USA) was added to the artificial food (diet:water = 4 g:13 ml), different volumes (0.5, 1 and 1.5 ml) of which were fed to the 1-day-old 3rd instar larvae. The 20E was replaced with an equivalent volume of water in the control group. Five larvae were randomly selected from each group for total RNA isolation to analyse relative expression levels by using qRT-PCR as described above. The net pupal weights and larval duration time of Z. tau in the 1 mg ml−1 20E treatment were recorded after pupation. Each group contained 30 individual larvae, and the experiment was repeated three times.
Synthesis and injection of double-stranded RNA
RNAi was used here to further explore the biological functions of ZtPPO1. The most unique nucleotide region of ZtPPO1 was selected for specific dsRNA synthesis (the dsRNA primers are listed in table 1). All of the reagents and enzymes used for dsRNA synthesis were from the T7 RiboMAXTM Express RNAi System (Promega, Madison, WI, USA). The sizes of the dsRNA products were confirmed by electrophoresis on a 1.2% agarose gel. Approximately 1 µg µl−1 of dsRNA was slowly injected into the antepenultimate abdominal segments of 2-day-old 3rd instar larvae with a homemade microinjector. Control larvae were injected with equivalent volumes of dsGFP. Injected larvae were reared on an artificial diet under the conditions described above until pupation, and morphological changes were observed. Thirty larvae from each group were treated three times. Total RNA was isolated from four randomly selected insects from each (treated and control) group after injection, and the transcript levels were measured using qRT-PCR as described above.
Table 1. Primers used for the qRT-PCR assay (2.5.1) and to amplify ZtPPO1.

Statistical analysis
All the data were analyzed by one-way analysis of variance (ANOVA) using SPSS17.0 software (Chicago, IL, USA). Tukey's multiple range test was used for pairwise comparison of the differences between treatments for mean separation (P < 0.05).
Results
Sequence analysis of PPO1
The full-length cDNA for ZtPPO1 was 2310 bp long and contained a 110-bp 5′ untranslated region (UTR), a 2046-bp ORF encoding 682 amino acids (aa), and a 615-bp 3′ UTR with a poly-A tail. The predicted protein of ZtPPO1 had a molecular weight of 78.70 KDa and a theoretical pI of 5.99. The positions with conserved aa, arginine (R) at position 49 and phenylalanine (F) at position 50, are marked with a box and arrow (fig. S1 in supporting information) and constitute the predicted proteolytic cleavage site. No signal sequence was found at the N-terminus, which is similar to what has been observed for a majority of PPOs, for example, PPO1 from Pieris rapae (Lu et al., Reference Lu, Li, Gong, Gao, Zhang and Xue2015).
Multiple sequence comparison and construction of the phylogenetic tree
Multiple aa sequence alignment of ZtPPO1 with insect PPOs was carried out using the ClustalX (1.8) program, and the results showed that the ZtPPO1 aa sequence shared a high percentage of similarity with published dipterous PPO1 sequences, sharing highest identity with B. cucurbitae PPO1 (96%), Neobellieria bullata PPO (86%), and B. dorsalis (78%). Typical features of all PPOs, including the thiol ester site, six histidine residues and the copper-binding sites CuA and CuB, were also found in the ZtPPO1 aa sequence (fig. S2 in supporting information). The phylogenetic tree showed that insect PPOs were grouped in a family and were relatively evolutionarily distant from the PPO of S. serrata (fig. S3 in supporting information). The entire phylogenetic tree was established by six main PPO groups. Dipteran PPOs consisted of two distinct groups, one of which includes a majority of the reported PPOs. PPOs from Coleoptera and Orthoptera were clustered in each corresponding group. The lepidopteran PPOs were also separated into two groups. ZtPPO1 is closely related to B. cucurbitae PPO1 and B. dorsalis PPO1.
Temporal and spatial expression of ZtPPO1 transcripts
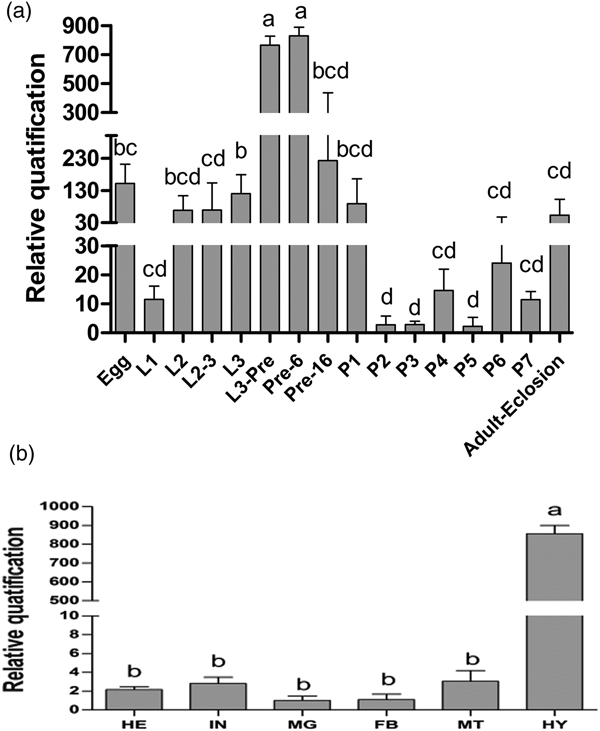
Transcripts of ZtPPO1 were detected at all tested life stages (from egg to adult) and were predominantly expressed in early-prepupae stage, before the expression suddenly decreased to relatively low levels within 1 day after pupation until the emergence of the adult. Interestingly, this gene seems to be abruptly expressed in every moulting stage when the formation of the new cuticle begins and the cuticle starts to harden (fig. 1a). The mRNA of ZtPPO1 was abundant in the haemolymph; in contrast, fewer transcripts of ZtPPO1 were detected in the other tested tissues (fig. 1b).

Fig. 1. (a) Relative expression levels of PPO1 during different developmental stages (Egg; L, larvae; L2-3, the transition period from 2nd to 3rd instar larvae; L3-Pre, the transition period from 3rd instar larvae to prepupae; Pre-6/16, 6/16 h of the prepupal stage; P, pupae; Adult eclosion, newly emerged adult) of Zeugodacus tau. (b) Relative expression levels of PPO1 in HE (head), IN (integument), MG (midgut), FB (fat body), MT (Malpighian tubules), and HY (haemolymph) of the 3rd instar larvae of Zeugodacus tau. All the data are plotted as the mean ± SE (n = 3). The different small letters above each bar indicate the significant differences at P < 0.05.
Effect of KA on the development of Z. tau
The relative mortality increased from 22.2 to 86.7% after newly hatched 1st instar larvae were reared on various mass ratios of KA (0.03, 0.99, 1.66, 2.33 and 2.99%) for 7 days (fig. 2). Under normal rearing conditions, the larval developmental time of Z. tau lasts for 5 days. However, the larval developmental time was prolonged to 7 days after the larvae were fed a 1.66% KA-containing diet. The pupal duration after KA treatment was also lengthened from 8 to 10 days (fig. 3a). Similarly, KA treatment had a significant detrimental effect on pupation and adult eclosion rates. The normal pupation and emergence rates were 96 and 89.6%, respectively (fig. 3b), and after rearing on a 1.66% KA-containing diet, the rates decreased to 70 and 39.6%, respectively. Pupal weight decreased from 12.04 mg per pupa to 11.11 mg per pupa after treatment with KA (fig. 3c).

Fig. 2. The effect of various kojic acid concentrations on relative larval mortality. All the data are plotted as the mean ± SE (n = 3). The letters a, b and c on each bar indicate the significant differences at P < 0.05, n = 30.

Fig. 3. (a) Duration of larval and pupal development. (b) Pupation and adult emergence rates. (c) Pupal weight. (d) Relative expression of ZtPPO1 in whole bodies and cuticles of 3rd instar larvae of Zeugodacus tau fed an artificial diet only or a diet containing 1.66% kojic acid. All the data are plotted as the mean ± SE (n = 3). ‘*’ indicate significant differences at P < 0.05, n = 30.
Effect of KA on the expression levels of ZtPPO1 and on PO activity
The relative expression of ZtPPO1 increased significantly after treatment with KA: 2.79-fold and 3.39-fold in the whole larvae and cuticles, respectively (P < 0.05) (fig. 3d). Furthermore, the original PO activity of the whole larvae and cuticles from Z. tau were 289.83 and 392.22 U mg−1, respectively. After KA treatment, the PO activities in both whole larvae and cuticle extracts significantly decreased to 100.17 and 147.27 U mg−1, respectively (P < 0.05) (fig. 4; the protein concentrations are shown in table S2 in the supporting information), which corresponds to the respective inhibition rates of 65.5 and 62.5%. These results proved that KA can significantly inhibit PO activity.

Fig. 4. PO activity in the whole bodies and cuticles of 3rd instar larvae of Zeugodacus tau fed artificial diets only or diets containing 1.66% kojic acid. All the data are plotted as the mean ± SE (n = 3). ‘**’ indicate significant differences at P < 0.01.
In vitro inhibition of PO activity
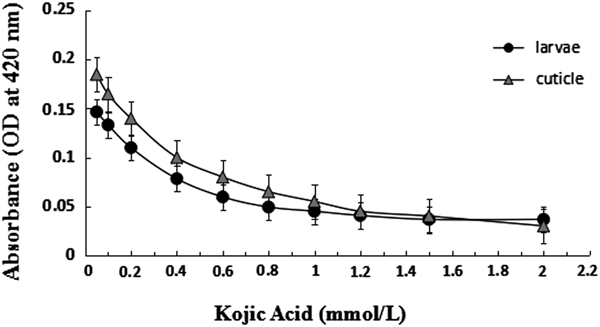
We first determined the I 50 values (the concentration required to inhibit 50% of PO activity) of KA at final concentrations of 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.5, and 2 mM in the 3rd instar larvae and cuticles of Z. tau. The I 50 value of KA in the 3rd instar larvae and cuticles was 0.42 and 0.35 mmol l−1, respectively. At these concentrations, KA significantly inhibited PO activity in whole larval enzyme extract by 22–78%, and by 16–86% in cuticles (fig. 5). These results further indicated that KA is an effective inhibitor of Z. tau PO activity.

Fig. 5. Inhibition of PO activity by kojic acid in the whole bodies (I 50 = 0.42 mmol l−1) and cuticles (I 50 = 0.35 mmol l−1) of 3rd instar larvae of Zeugodacus tau.
Mechanism of KA-induced inhibition of PO
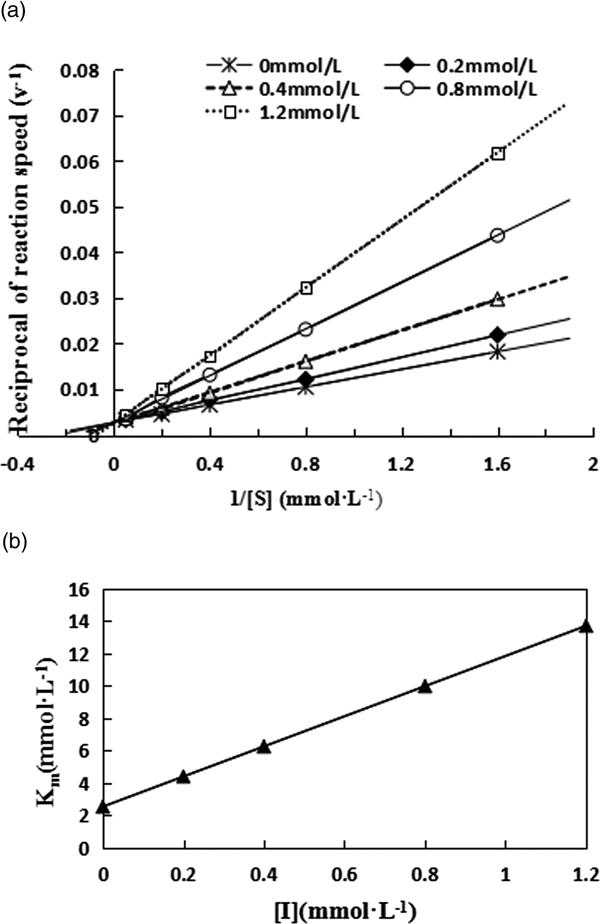
The relationship between PO activity and enzyme concentration in the presence of different concentrations of inhibitor was also studied (fig. 6). Plotting of the data yielded straight lines passing through the origin; higher inhibitory concentrations were associated with lower slopes, demonstrating that the inhibition of PO by KA was reversible. KA did not decrease the amount of enzyme, but rather, KA decreased the enzymatic activity.

Fig. 6. Relationship between PO activity and enzyme concentration in the presence of different concentrations of kojic acid.
Determination of the inhibition type of KA on PO
To determine the inhibition type of KA, a Lineweaver-Burk plot was constructed (fig. 7a). The results show that KA is a competitive inhibitor, since increasing the KA concentration results in a family of lines with the same intercept on the 1/v axis but with different slopes, indicating that the inhibitor did not change the maximum velocity (V max) but did change the Michaelis-Menten constant (K m). These results show that KA is a competitive inhibitor. The equilibrium constant for the binding of the inhibitor with the free enzyme (K I) was obtained from the linear plot of the apparent Michaelis-Menten constant (K m) vs. the concentration of KA (fig. 7b). The obtained kinetic indices are summarized in table S3 in the supporting information.

Fig. 7. Determination of the inhibition type and inhibition constant of the inhibition by kojic acid of PO activity.
Effect of 20E on ZtPPO1 transcripts and on the development of Z. tau
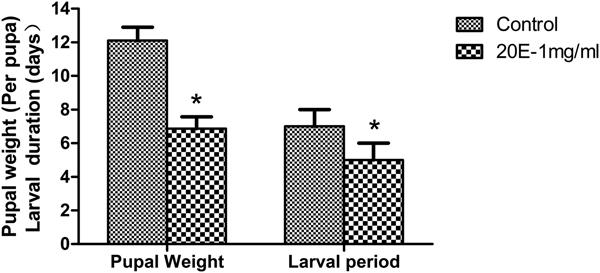
The relative expression levels of ZtPPO1 were determined by qRT-PCR after feeding the larvae 20E-containing food. The results showed that the expression of ZtPPO1 was significantly up-regulated by 20E at 0.5, 1 and 1.5 mg ml−1, with the highest expression level observed at 1 mg ml−1 (P < 0.05) (fig. 8). A shorter larval developmental period and early pupation were observed after 20E treatment at 1 mg ml−1; in addition, the net pupal weights of the tested group were significantly lighter than those of the control (P < 0.05) (fig. 9).

Fig. 8. Effect of 20E on the expression of ZtPPO1 at different concentrations. All the data are plotted as the mean ± SE (n = 3). ‘**’ indicate significant differences at P < 0.01.

Fig. 9. Pupal weight (mg per pupa) and larval duration time (days) of the tested groups (20E treatment compared with the control) of Zeugodacus tau. All the data are plotted as the mean ± SE (n = 3). ‘*’ indicates significant difference compared to the corresponding control (P < 0.05).
Gene silencing of ZtPPO1
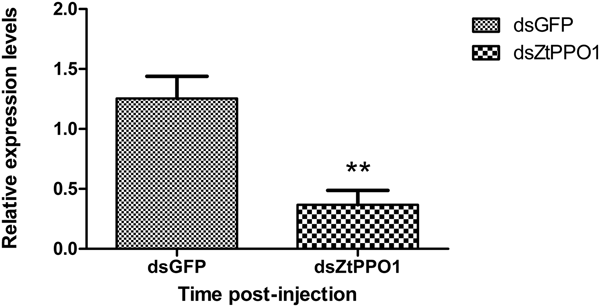
To determine whether knockdown of the gene ZtPPO1 by RNAi would impact the normal development of Z. tau, qRT-PCR was carried out using templates prepared from insects injected with dsRNA (tested and control groups). The ZtPPO1 mRNA level was significantly decreased at 24 h after injection with dsPPO1 (P < 0.01) (fig. 10). The pupation rate of the tested group was significantly reduced to 50% after injection compared to the rate of the control (P < 0.05) (fig. 11), and the emergence time of the dsPPO1 group was delayed by 3 days. The insects failed to develop into normal pupae, and the bodies of the insects slowly decayed, turning black with abnormal pigmentation and empty puparia, followed ultimately by death (fig. 12).

Fig. 10. Relative expression levels of ZtPPO1 in the 3rd instar larvae after RNAi for 24 h. All the data are plotted as the mean ± SE (n = 3).’**’ indicates significant difference compared to the corresponding control (P < 0.01).
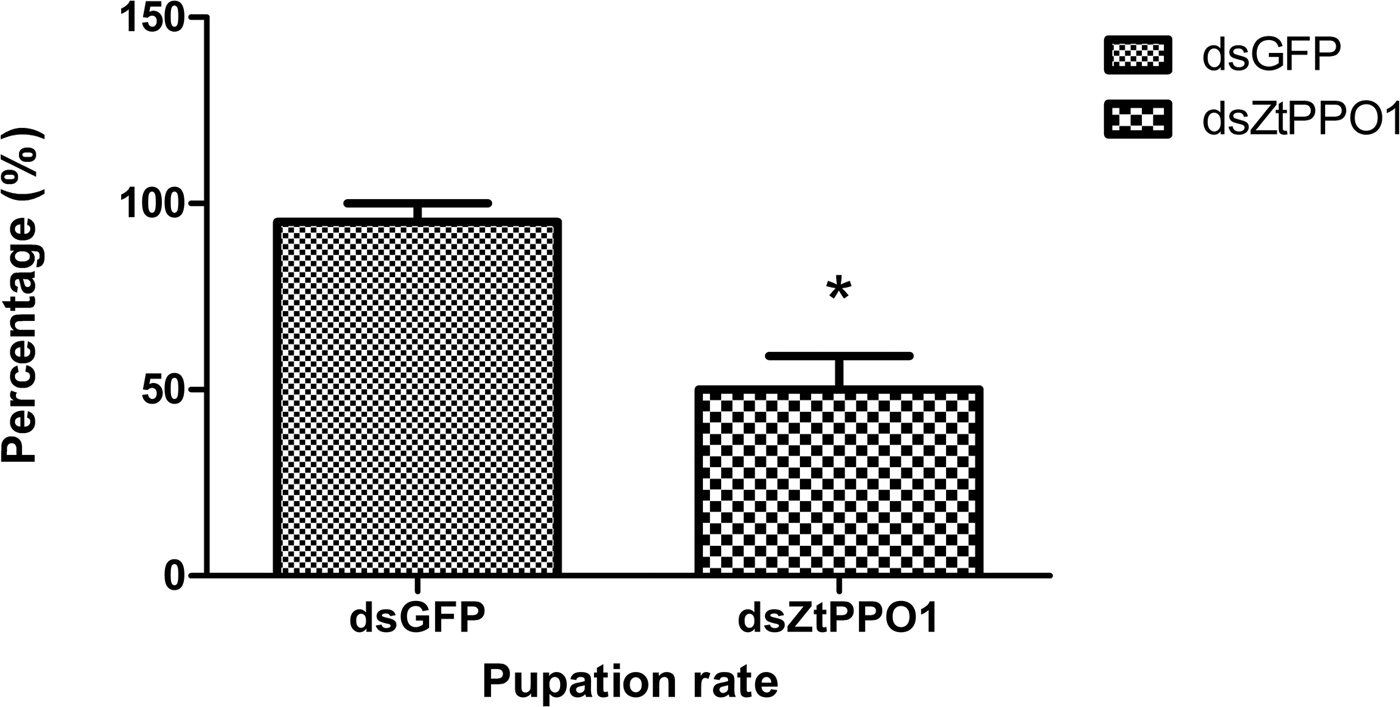
Fig. 11. Pupation rate of Zeugodacus tau after injection with ZtPPO1 dsRNA in 2-day-old 3rd instar larvae. All the data are plotted as the mean ± SE (n = 3). ‘*’ indicates significant difference compared with the corresponding control (P < 0.05).

Fig. 12. Morphological phenotypes of Zeugodacus tau after injection of corresponding dsRNA (dsGFP: control groups (a and c); dsZtPPO1, tested groups (b and d)) in day-2-old 3rd instar larvae.
Discussion
POs are widespread at the phylogenetic scale and are found in organisms such as bacteria, mammals, microbes, insects, shrimp, and plants (Mayer, Reference Mayer2006; Olivares & Solano, Reference Olivares and Solano2009; Phonpala et al., Reference Phonpala, Benjakul, Visessanguan and Eun2009; Fairhead & Thöny-Meyer, Reference Fairhead and Thöny-Meyer2012). In insects, PO is an important multifunctional enzyme in normal developmental processes that plays roles in numerous crucial physiological events, such as wound healing, cuticular hardening and melanisation of foreign microorganisms (Ashida & Brey, Reference Ashida, Brey, Brey and Hultmark1997). Despite the significance of PO in the moulting of older epidermises and tanning of new cuticles, the molecular and biochemical properties of PO as well as the effect of KA on PO activity and development in Z. tau have been poorly characterized.
In this study, the full-length cDNA of ZtPPO1, containing a 2049-bp ORF that encodes a 682-aa protein, was cloned. No signal sequence was found at the N-terminus, which is similar to what has been observed for a majority of PPOs. ZtPPO1 has two conserved regions, CuA and CuB, which correspond to Regions I and II, respectively. Another typical conserved structure (Region III, GCGWPQH) of PPO is the thio-ester motif, which is characteristic of C3 and C4 complement proteins and α2-macroglobulin and is mainly responsible for macromolecule binding (Aspán et al., Reference Aspán, Huang, Cerenius and Söderhäll1995; Hall et al., Reference Hall, Scott, Sugumaran, Söderhäll and Law1995). The Region III present in PPOs may be associated with the role of PPOs in the immobilization of invading microbes during an immune response (Sritunyalucksana et al., Reference Sritunyalucksana, Cerenius and Söderhäll1999; Lourenço et al., Reference Lourenço, Zufelato, Bitondi and Simões2005). In addition, cleavage at Arg49-Phe50 to obtain active PO from the inactive PPO form was also predicted and is associated with the PO activation cascade response (Söderhäll et al., Reference Söderhäll, Cerenius and Johansson1994).
We constructed a phylogenetic tree based on the sequences of other known PPOs present in the NCBI database. Lepidopteran and dipteran PPOs were divided into two groups as were coleopteran and orthopteran PPOs, which were separated into distinct groups. PPO1 from Z. tau was in the dipteran group that is closely related to B. cucurbitae. However, PPO1 from Z. tau is not in the other dipteran group, which includes D. melanogaster and A. gambiae, which might explain the different numbers of PPOs in insects. Two PPOs have been found in Z. tau to date, which is similar to the number of PPOs in most insects; this observation might explain why PPO1 from Z. tau was in a different group from D. melanogaster, A. gambiae (Hartzer et al., Reference Hartzer, Zhu and Baker2005), which have more than two PPOs that may function in the developmental stage. The fact that the same types of PPOs from different species share much higher identity than distinct types of PPOs from the same species implied that dipteran speciation occurred prior to PO gene divergence and duplication (Cui et al., Reference Cui, Luckhart and Rosenberg2000).
Though expression levels of PPO were tested for temporal and spatial patterns among many different species, the transcripts of ZtPPO1 remain unknown; furthermore, PPO genes in different insects were not expressed uniformly, not to mention the expression of different types of PPOs. For example, PxPPO2, PrPPO1, and AgPPO were continuously expressed during all life stages from egg to adult (Cui et al., Reference Cui, Luckhart and Rosenberg2000). PPO transcripts were detected in all the developmental stages except pupae of Hyphantria cunea (Park et al., Reference Park, Shin, Kim, Park, Lee, Brey and Park1997). Transcripts of PPO2 from P. xylostella were abundant in eggs and mature larval stages but exhibited low expression levels in the other stages; in contrast, the expression levels of AmPPO were higher in adult and older pupae than in larval-prepupae stages (Lourenço et al., Reference Lourenço, Zufelato, Bitondi and Simões2005; Du et al., Reference Du, Li, Gao, Xue, Lin and Luo2010). In the present study, ZtPPO1 expression was also detected in all life stages, with higher levels in prepupae than in other stages; interestingly, from the egg to late-prepupae stages, the transcript levels appear to first increase and then suddenly decrease to relatively low levels when the larvae undergo moulting for new cuticle formation. This unusual expression may be associated with metamorphosis development of Z. tau. Meanwhile, the results obtained from tissue-specific quantification showed that ZtPPO1 was mainly expressed in the haemolymph instead of in the insect integument and in other tissues such as head, fat bodies, midgut and Malphigian tubules, which exhibited low transcript levels and no significant difference among them. This observation accurately demonstrated that ZtPPO1 is produced predominantly in the haemolymph and can be effectively involved in immune defence against foreign organisms. In B. dorsalis, cuticle, fat body and midgut tissues were tested, and PPO1 was seen to be principally transcribed in the cuticles; even though the haemolymph tissue was not included, this result does show a significant difference between the integument and other tested tissues (Bai et al., Reference Bai, Xie, Shen, Wei and Wang2014), which is inconsistent with what was observed for Z. tau. However, the study performed in silkworm verified that cuticular PPO can be transported from the haemolymph (Yatsu & Asano, Reference Yatsu and Asano2009). Further study is required to determine why PPO genes from different species exhibit distinct transcriptional patterns.
Interestingly, few studies have been conducted on the effects of inhibitors on expression levels and enzyme activities of PO in insects; a majority of the studies have focused on the regulation of PO in microorganisms (Sugumaran & Kanost, Reference Sugumaran, Kanost, Beckage, Thompson and Fredric1993; Söderhäll & Cerenius, Reference Söderhäll and Cerenius1998; Franssens et al., Reference Franssens, Simonet, Breugelmans, Van Soest, Van Hoef and Broeck2008). If PO plays a vital role in the normal development of insects, effective inhibitors of PO activity could possibly be used for pest control; therefore, we performed a detailed investigation of the effects of KA on the development of Z. tau. In addition, the effect of a 1.66% KA-containing diet on PO transcriptional levels and enzymatic activity of Z. tau was also determined. The results showed that KA could significantly increase the PO expression levels and inhibit enzyme activity. The results are similar to those observed for POs from P. xylostella and B. dorsalis (Du et al., Reference Du, Li, Gao, Xue, Lin and Luo2010; Bai et al., Reference Bai, Xie, Shen, Wei and Wang2014); in addition, KA has been used to control the browning of some fruits and vegetables and to prevent melanosis in shrimp and crabs (Owusu-Yaw et al., Reference Owusu-Yaw, Cohen, Fernando and Wei1990; Niwa & Akamatsu, Reference Niwa and Akamatsu1991). To confirm the inhibition efficiency of KA, the results obtained from the in vitro inhibition experiment indicate that 0.42 and 0.35 mmol l−1 KA could decrease the PO activity by 50% in whole larvae and cuticles, respectively. Furthermore, the inhibition rate gradually increased, and with increasing KA concentrations, the enzyme activity of PO was even completely suppressed. The above experiments all revealed that KA is an effective inhibitor of PO activity.
JH and 20E are reportedly involved in many physiological events that control moulting and morphogenesis. Previous studies on the effects of 20E on PO expression levels from B. dorsalis, Apis mellifera, and B. mori indicate that PO transcription could be up-regulated by treatment with 20E (Zufelato et al., Reference Zufelato, Lourenco, Simoes, Jorge and Bitondi2004; Wang et al., Reference Wang, Lu, Cai, Liang, Niu and Miao2013; Bai et al., Reference Bai, Xie, Shen, Wei and Wang2014). Similar up-regulation of ZtPPO1 mRNA levels was observed. Generally, PPO mRNA is highly expressed and PO activity in insects increases rapidly during metamorphosis in order to adapt for melanization. Analysis of the expression levels of ZtPPO1 in all developmental stages showed that this gene was primarily expressed during larval–pupal transition, and the results suggested that the expression was associated with the pupation of Z. tau. It has been reported that hormone-driven mechanisms play a key role in the regulation of the PO necessary for insect cuticular melanization (Hiruma & Riddiford, Reference Hiruma and Riddiford2009). At the onset of metamorphosis in Lepidoptera, in particular, in M. sexta, ecdysone acting in the absence of JH causes larval-pupal commitment of the epidermis (Riddiford, Reference Riddiford1976, Reference Riddiford1978), cessation of feeding, and the onset of wandering behaviour (Dominick and Truman, Reference Dominick and Truman1985). As the granular PPO is synthesized in the epidermis and is independent of ecdysone, and the cuticular pro-PO is a modified form that is trans-epithelially transported from the haemolymph to the cuticle (Hiruma & Riddiford, Reference Hiruma and Riddiford1985; Yatsu & Asano, Reference Yatsu and Asano2009), it is possible that the high PO activity observed in this study was caused by the up-regulation of ZtPPO1 mRNAs after treatment with 20E, and it is expected that an increased amount of transcripts would result in intensification of cuticular melanization, which would be accompanied by the cessation of feeding and early pupation, followed by substantial reduction in net pupal weight.
The knockdown of ZtPPO1 by gene silencing in 3rd instar larvae induced abnormal puparia and low pupation rates, which is important for the normal transition from the larvae to pupae. The absence of normal pupation and malformed larvae in B. dorsalis after injection with dsRNA-BdPPO1 (Bai et al., Reference Bai, Xie, Shen, Wei and Wang2014), the incomplete moth pupation phenotype and abnormal larvae of B. mori with PPO1 dsRNA treatment (Wang et al., Reference Wang, Lu, Cai, Liang, Niu and Miao2013), and the lower pupal survival rate and rapid death of deformed adults following emergence all show that the RNAi technique will be useful for pest control. The results obtained from the study of the effect of 20E and RNAi all revealed that PO is important for the normal development of Z. tau. This result further confirmed the significance and necessity of studying the effects of KA on the enzyme activity and development of Z. tau.
In conclusion, temporal and spatial expression of ZtPPO1 showed that this gene was mainly expressed in the haemolymph during the larval–prepupal transition, which is important for normal moulting and metamorphosis. Analysis of the PO transcripts indicated that PO expression could be significantly up-regulated by KA, and KA is an effective inhibitor of PO activity, has significant detrimental effects on normal growth, and induces an abnormal phenotype. The functional investigation of 20E and gene silencing of ZtPPO1 by RNAi revealed that 20E was able to not only significantly up-regulate the expression of ZtPPO1 but also cause early pupation and lower pupal weight. In addition, the knockdown of ZtPPO1 induced abnormal pupation and low pupation rates, disrupting the eventual survival and emergence of Z. tau. In summary, PO plays a critical role in the normal development of Z. tau, and KA is a potent inhibitor that can significantly suppress PO activity, which could lead to pest control. Hopefully, this work can contribute to a better understanding of PO and of the biological functions of PO in Z. tau and will provide reliable data for developing novel PO-induced biological target inhibitors.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318000470.
Acknowledgement
This work was supported by Grant from the National Natural Science Foundation of China (No. 0203c6).