Introduction
The latices of figs (Ficus spp.) and papaya (Carica papaya) are known to contain a rich supply of cysteine proteinases (CPs) (Kimmel & Smith, Reference Kimmel and Smith1954; Rawlings & Barrett, Reference Rawlings and Barrett1994) of the papain family (Family C1 in the Merops database, http://merops.sanger.ac.uk/; Rawlings et al., Reference Rawlings, Barrett and Bateman2012) whose primary function is probably protection against arthropod pests (Dussourd & Eisner, Reference Dussourd and Eisner1987) and plant-parasitic nematodes (Konno et al., Reference Konno, Hirayama, Nakamura, Tateishi, Tamura, Hattori and Kohno2004). Extracts of papaya, containing CPs have been shown to have potent anthelmintic properties, and studies to date have reported significant reductions in nematode parasite egg output and worm burdens of gastrointestinal (GI) nematodes in monogastric animals, including rodents (Stepek et al., Reference Stepek, Lowe, Buttle, Duce and Behnke2006, Reference Stepek, Lowe, Buttle, Duce and Behnke2007a, Reference Stepek, Lowe, Buttle, Duce and Behnkeb), pigs (Satrija et al., Reference Satrija, Nansen, Bjorn, Murtini and He1994), humans (Hansson et al., Reference Hansson, Veliz, Naquira, Amren, Arroyo and Arevalo1986) and even poultry (Mursof & He, Reference Mursof and He1991), as well as in multigastric ruminants such as sheep (Buttle et al., Reference Buttle, Behnke, Bartley, Elsheikha, Bartley, Garnett, Donnan, Jackson, Lowe and Duce2011). However, many other plants also synthesize CPs in their fruits and tissues but relatively little is known about their effects on nematodes and whether they also have anthelmintic properties.
Pineapple plants (Ananas comosus) contain proteolytic enzymes from the C1 family, often collectively called ‘bromelain’, although they actually comprise four distinct cysteine proteinases. The constituent enzymes can be distinguished as either stem bromelain (EC.3.4.22.32) or fruit bromelain (EC 3.4.22.33) depending on their source in the plant and physico-chemical properties. Other pineapple stem-derived CPs are ananain (EC 3.4.22.31) and comosain (Rowan et al., Reference Rowan, Buttle and Barrett1988, Reference Rowan, Buttle and Barrett1990). ‘Bromelain’ has been used traditionally as an anti-parasitic agent in the Philippines and is known to be able to digest nematodes in vitro (Berger & Asenjo, Reference Berger and Asenjo1939). It also has wider medicinal uses, having been employed in the past to reduce swelling and inflammation, bruising, healing time and pain following surgery and physical injuries by different native societies of the world, including those indigenous to South America, China and South-East Asia (Lotz-Winter, Reference Lotz-Winter1990; Gregory & Kelly, Reference Gregory and Kelly1996; Chobotova et al., Reference Chobotova, Vernallis and Majid2010), and as a wound-debriding agent (Rowan et al., Reference Rowan, Buttle and Barrett1990). ‘Bromelain’ has been demonstrated to have a variety of clinical benefits in therapeutic applications and it has been shown to be safe at high doses (LD50≥ 10 g/kg) for prolonged periods of time (Hale et al., Reference Hale, Greer and Sempowski2002). The enzyme is also sold in health food stores as a digestive aid and used as a non-prescription anti-inflammatory agent, particularly for large animals such as horses and cattle (Gregory & Kelly, Reference Gregory and Kelly1996; Hale et al., Reference Hale, Greer and Sempowski2002).
Another relatively unstudied CP is actinidain (EC 34.22.14) from the kiwi fruit (Actinidia deliciosa or A. chinensis) (McDowall, Reference McDowall1970). Although the enzyme is used widely as a meat tenderizer (Christensen et al., Reference Christensen, Tørngren, Gunvig, Rozlosnik, Lametsch, Karlsson and Ertbjerg2009), stabilizer of beer (Préstamo, Reference Préstamo1995), in milk clotting (Katsaros et al., Reference Katsaros, Tavantzis and Taoukis2010) and protein digestion ((Kaur et al., Reference Kaur, Rutherfurd, Moughan, Drummond and Boland2010), there is little published research on its medical applications. Stepek et al. (Reference Stepek, Buttle, Duce, Lowe and Behnke2005) reported this CP to have no detrimental effect on worms, even though the enzyme is very similar to the CPs that have strong anthelmintic properties, including papain, in both its structure and activity (Kamphuis et al., Reference Kamphuis, Drenth and Baker1985; Kaur et al., Reference Kaur, Rutherfurd, Moughan, Drummond and Boland2010).
Both pineapples and kiwi fruits are grown on an industrial scale in many parts of the world, and pineapples in particular constitute an important cash crop for many developing countries, such as Costa Rica, Philippines, Ivory Coast, India, Kenya and Nigeria (International Labour Rights Forum, 2008). If their CPs can be shown to have effective anthelmintic properties, then the by-products of these industries could be exploited to produce relatively cheap anthelmintics for use in the treatment of human GI nematode infections in developing countries of the world and for domestic livestock worldwide. Clearly then, since the CPs in pineapples have been used in the treatment of worm infections in the past (Berger & Asenjo, Reference Berger and Asenjo1939), it was of interest to re-evaluate their anti-parasitic efficacy using the methodologies that were successfully applied recently in re-assessing the anthelmintic properties of the CPs found in papaya (Stepek et al., Reference Stepek, Lowe, Buttle, Duce and Behnke2007a). Although to the best of our knowledge there are no reports of the usage of actinidain in the treatment of worm infections, it was pertinent to also examine the anthelmintic properties of this enzyme, due to its similarity in structure and function to bromelain and the CPs in papaya.
Materials and methods
Preparation of cysteine proteinases
The cysteine proteinases used in this study were a supernatant derived from papaya latex (PLS), pineapple stem bromelain, fruit bromelain and kiwi fruit actinidain. Since PLS has been shown recently to be a highly effective anthelmintic, with marked effects on worm motility in vitro and capable of reducing intestinal worm burdens by over 90% when given orally to mice (Luoga et al., Reference Luoga, Mansur, Buttle, Duce, Garnett and Behnke2012), this was used in the present work as our positive control. PLS was prepared by dissolving 4 kg of Carica papaya spray-dried latex purchased from Enzymase International S.A. (Brussels, Belgium) in 12 litres of water. The preparation was centrifuged at 17,700 × g at 4°C (Beckman model J2-21 centrifuge and JA10 rotor, High Wycombe, Bucks, UK) and the pellet was discarded. The supernatant was concentrated to a third of its original volume by placing it in dialysis tubing with molecular weight (MW) cut-off 3500 Da (SpectraPor 45 mm diameter; Spectrum Laboratories Inc., Rancho Dominguez, California, USA) over polyethylene glycol 20,000 Da. The concentrated PLS was aliquoted into individual vials and stored at − 80°C. At each step of the purification the molar concentration of the active cysteine proteinase was measured by active-site titration adapted from Barrett et al. (Reference Barrett, Kembhavi and Hanada1981) and Zucker et al. (Reference Zucker, Buttle, Nicklin and Barrett1985) with increasing concentrations of the cysteine proteinase specific inhibitor, l-trans-epoxysuccinyl-l-leucylamido-(4-guanidino) butane (E-64) (Apollo Scientific Ltd, Stockport, Cheshire, UK) with 4 mml-cysteine as a reducing agent and benzoyl-arginyl-p-nitroanilide (BAPNA) (Bachem Ltd, St. Helens, Merseyside, UK) as the synthetic substrate for PLS enzymes.
For preparation of fruit bromelain and actinidain we adapted the method of Rowan et al. (Reference Rowan, Buttle and Barrett1990). Ripe fruits were purchased from Sainsbury's supermarket, UK, peeled, cut into small pieces and then crushed in a laboratory juicer (Philips juicer HR 1861; Royal Philips, Amsterdam, The Netherlands) and filtered. The filtrate was then subjected to vacuum filtration to remove the remaining fine debris. The filtered juice was concentrated by dialysis over polyethylene glycol or by acetone precipitation and the concentrated juice was aliquoted into vials and stored at − 80°C for future use. The active enzyme concentrations from each stage of preparation were determined by active-site titration with E-64 using benzyloxycarbonyl–Phe–Arg–para-nitroanilide (Z–Phe–Arg–pNA) (Bachem Ltd) as the substrate.
Pineapple stem bromelain was obtained from Hong Mao Biochemical Co. Ltd, Thailand, kindly provided as a gift. Two hundred grams of stem bromelain powder were dissolved in 1 litre of distilled water and centrifuged at 17,700 × g at 4°C and the pellets were discarded while the supernatant was aliquoted into vials and stored at − 80°C. Its enzyme activity was assessed by active-site titration with E-64 using benzyloxycarbonyl–Arg–Arg–para-nitroanilide (Z–Arg–Arg–pNA) (Bachem Ltd) as its substrate. On the day of each experiment, individual vials were thawed and assessed for active enzyme concentration before use, and diluted according to the experimental requirements.
Experimental infections
For in vitro experiments, we purchased male BKW mice from B&K Universal Ltd. (Hull, UK) and for in vivo experiments male C3H mice from Charles River Ltd (Margate, Kent, UK), at 5 weeks of age in both cases. Mice were given a minimum of a week to adjust to animal house conditions before infection, and were provided throughout with food and water ad libitum.
Mice were infected by oral gavage with their natural rodent GI nematode, Heligmosomoides bakeri (previously known as Nematospiroides dubius, H. polygyrus and H. p. bakeri (Behnke & Harris, Reference Behnke and Harris2010)) at 6 weeks of age. For in vitro experiments mice were administered a suspension of 300 L3 larvae in 0.2 ml of distilled water on day 0. From day 10 post-infection the worms were mature and could be used for in vitro experiments. The mice were then killed by exposure to increasing concentrations of CO2 and dissected. The small intestines were removed in their entirety, opened longitudinally with a pair of blunt-ended dissecting scissors and were placed into a Petri dish containing pre-warmed Hanks' Balanced Salt Solution (HBSS) at 37°C to allow the worms to detach from the mucosa. Adult male and female worms were picked out with the help of fine forceps under the microscope and washed in pre-warmed HBSS before use in motility assays.
For in vivo experiments, mice were infected with a suspension of 150 L3 of H. bakeri in 0.2 ml of distilled water on day 0. At days 14, 16, 18, 21, 23 and 25 post-infection, faecal samples were collected from each mouse and the number of eggs present was counted using the McMaster technique modified from Behnke & Parish (Reference Behnke and Parish1979). Briefly, faeces were collected from individual mice, weighed and then 10 ml of saturated NaCl were added to each vial containing the faeces. The pellets were broken up for 1 h using a rotary mixer, and then washed through a sieve (diameter of 5 cm with 800 μm × 800 μm square apertures) with 50 ml of saturated NaCl. The eggs were counted using a two-chamber McMaster slide, each chamber holding a volume of 0.15 ml, and the number of eggs per gram (EPG) of faeces was calculated accordingly. For the recovery of worms, mice were culled as described above and their intestines were incubated at 37°C for 4 h while suspended in gauze in a 50 ml beaker of Hanks' saline in a water bath. The tissues were checked for any remaining worms, the supernatant was discarded carefully and the worms that had collected in the bottom of the beakers were counted with the aid of a dissecting microscope.
In vitro effects of cysteine proteinases on worm motility and ultrastructure
Worms were transferred individually and incubated in 48-well plates (1 worm/ well) containing HBSS (without phenol red), pH 7.2 and 16 mml-cysteine and one of the following enzyme preparations: 0, 20, 40, 200, 400, 2000 and 4000 μm stem bromelain; 0, 10, 30, 60, 100 and 150 μm fruit bromelain; 0, 5, 10, 20, 33 and 50 μm actinidain; and 0, 10, 30, 100, 300, 1000 and 3000 μm PLS (used as a positive control). In all cases molarity here refers to that of the functional proteinase active site as determined by active-site titration with E-64. The control wells were incubated in parallel and contained either one of the concentrations of enzyme without cysteine; no enzyme but with cysteine; or Hanks' saline alone. The worms were incubated at 37°C for 2 h and their motility was recorded every 15 min using a standard motility scale from 0–5 adapted from Stepek et al. (Reference Stepek, Buttle, Duce, Lowe and Behnke2005), where 0 was assigned to completely motionless worms not responding to manual stimulation; 1, movement only when prodded; 2, active only in the tip ends; 3, slowly spontaneously active; 4, more active; and 5, highly active.
For examination of the surface changes by scanning electron micrcoscopy, adult H. bakeri worms were incubated in well plates containing either 200 μm PLS +l-cysteine, 200 μm stem bromelain+l-cysteine, 150 μm fruit bromelain+l-cysteine and 50 μm actinidain +l-cysteine or HBSS. At 0, 30, 60 and 90 min worms were removed from the wells and placed into bijou jars containing 2.5% glutaraldehyde. After 1 h the glutaraldehyde was removed and replaced with 0.1 m phosphate buffer (pH 7.2) and left for another 1 h. The worms were then prepared for scanning electron microscopy (SEM) by fixation in 1% osmium tetrachloride for 1 h, followed by dehydration in increasing ethanol concentrations from 30 to 100%. They were next subjected to critical point drying with CO2. The dried worms were mounted on disc stubs with quick-drying silver paint and sputter coated with gold before examination by SEM.
Design of experiments in vivo
In the first in vivo experiment (Experiment 1), three groups of five male C3H mice were infected orally with 150 H. bakeri L3 larvae on day 0. The first group was treated orally with 0.2 ml containing 240 nmol PLS daily for 5 days from day 20 post-infection; the second group was treated with 0.2 ml containing 240 nmol pineapple stem bromelain; and the last group was given pure water. Faecal egg counts (FEC) were conducted on days 14, 16 and 18 before treatment and on days 21, 23 and 25 during treatment. Autopsies of mice were carried out on day 25 post-infection and the number of worms in the small intestine was counted.
In the second in vivo experiment (Experiment 2) four groups of five male C3H mice were used and the first three groups were treated as in Experiment 1, while the fourth group was treated with 60 nmol fruit bromelain. FEC and intestinal worm burdens were quantified as in Experiment 1.
Data analysis
In vitro motility data were analysed using repeated measures general linear models (rmGLM) in SPSS (version 19.0; SPSS Inc., Chicago, Illinois, USA) with TIME after introduction of experimental treatment being fitted as a within-subject factor and CONCENTRATION (concentration of fruit bromelain, PLS, actinidain and stem bromelain) as a covariate. In addition to the standard outputs of rmGLM, when significant we also provide gradients (β) for the covariates at different times after incubation and Student's t-test of departure from zero. Faecal egg counts (FEC) were log10(EPG+25) transformed and were analysed by rmGLM, fitting TIME (day on which FEC were carried out) as the within-subject factor and TREATMENT (three levels, PLS, stem bromelain and control group or four levels PLS, stem bromelain, fruit bromelain and control group) as the between-subject factor. The Huynh–Feldt adjustment to the degrees of freedom was used to interpret significance on the side of caution when the data did not meet the requirements of Mauchley's Test of Sphericity. Worm counts were analysed by one-way GLMs on log10(x+10)-transformed data. In all cases P= 0.05 was considered as the cut-off for statistical significance. All statistical models were checked for approximately normal distribution of residuals. The IC50 for the motility assays was determined using data collected after 30 min of incubation and calculated by GraphPad Prism 5 (GraphPad Software, California, USA) using sigmoidal dose–response regression.
Results
In vitro effects of cysteine proteinases on worm motility and ultrastructure
We obtained relatively poor yields of 16.5% and 4.6% for actinidain and fruit bromelain, respectively. For this reason it was not possible to assess fruit bromelain at higher than 150 μm concentration and actinidain at higher than 50 μm.
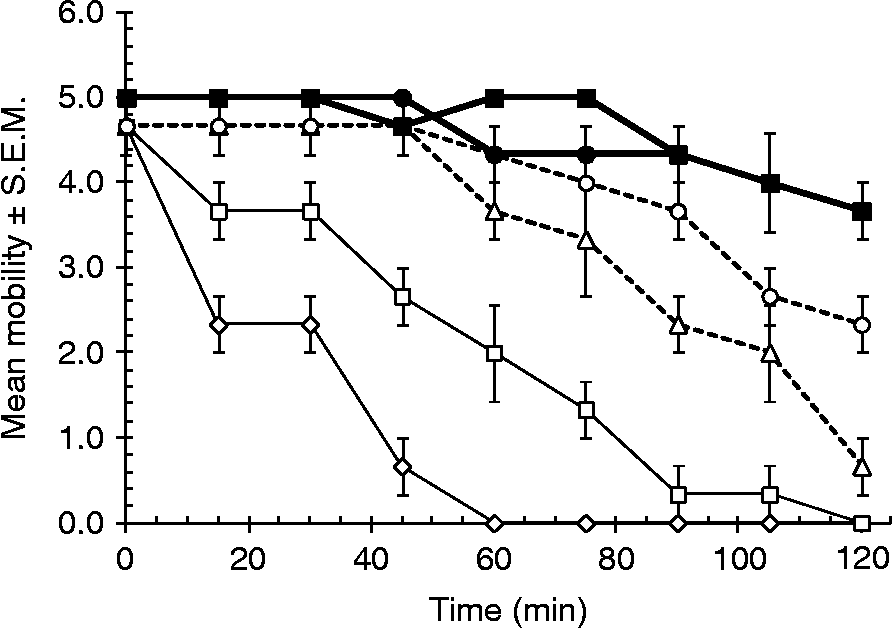
Family C1 proteinases require a reducing agent for full activity and for this reason cysteine was included in the standard incubation mixtures. Worms incubated in fruit bromelain with cysteine showed some reduction in motility after 15 min of incubation and a more rapid dose-dependent subsequent decline in motility with increasing concentrations of the enzyme (fig. 1; two-way interaction between TIME and CONCENTRATION of fruit bromelain; F 2.8,83= 7.3 P< 0.001 and the main effect of CONCENTRATION, F 1,22= 26.937, P< 0.001; at 30 min β = − 0.012 ± 0.003, t= − 4.3, P< 0.001 and significant negative gradients were recorded at all time points other than at the start of the experiment). However, importantly, there was no decline in motility of the worms incubated in fruit bromelain without cysteine, indicating that the effect of the enzyme depended on the presence of cysteine as well as fruit bromelain, and therefore, the effect on motility was entirely dependent on a functional active site on the CP (fig. 1). Very little loss of motility occurred among worms maintained in Hanks' with cysteine but without fruit bromelain.

Fig. 1 The effect of fruit bromelain on worm motility. Adult H. bakeri were incubated individually in 48-well plates (mean ± standard error of the mean (S.E.M.), n= 3) at 37°C for 2 h in the following concentrations of active enzyme: 150 μm (⋄), 100 μm (□), 60 μm (△) and 30 μm (○), each with 16 mml-cysteine; and control treatments of Hanks' saline (HBSS) +l-cysteine (●) and 100 μm fruit bromelain without cysteine (■).
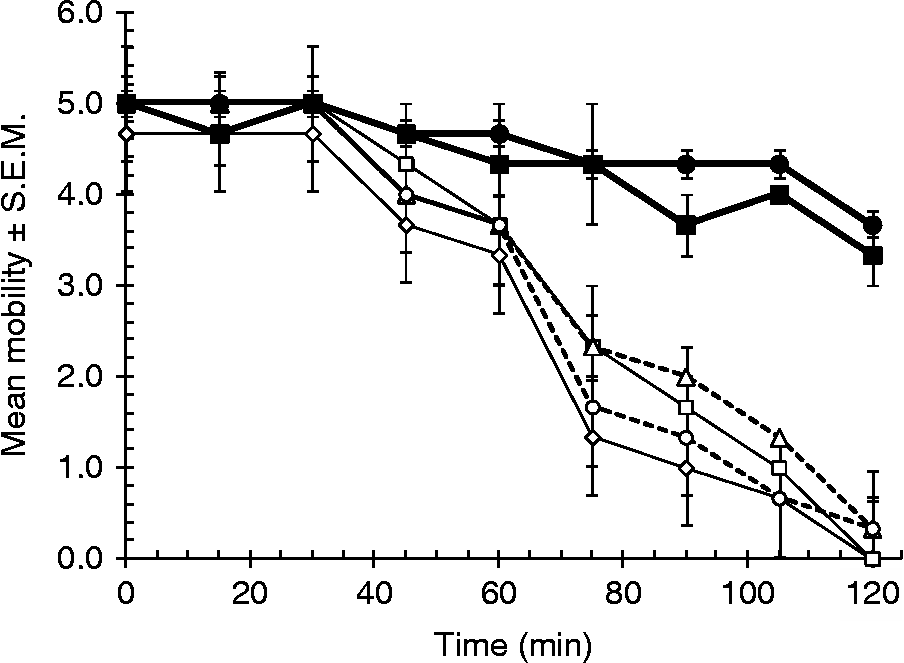
There was a significantly greater loss in motility among worms exposed to stem bromelain + cysteine compared with those in the control groups (main effect of CONCENTRATION of stem bromelain, F 1,22= 12.478, P< 0.002; at 30 and 45 min β was not significant but at 60, 75, 90, 105 and 120 min, β was negative and < 0.001, t was − 2.3, − 3.2, − 2.8, − 2.8, − 2.9, respectively, and P< 0.03 in all cases) and this was time dependent and varied with concentration of enzyme (fig. 2; main effect of TIME, as the within-subject factor, F 2,44.9= 36.9, P< 0.001 and two-way interaction between TIME and CONCENTRATION of stem bromelain F 2,44.9= 4.5, P= 0.016). As in the case of fruit bromelain, worms incubated in stem bromelain without cysteine and those in Hanks' with cysteine showed only minor loss of motility over the 2 h incubation when compared with those incubated in stem bromelain with cysteine. The inhibitory effect of stem bromelain on worm motility is dependent therefore on the presence of enzyme activated by cysteine.

Fig. 2 The effect of stem bromelain on worm motility. Adult H. bakeri worms were incubated at 37°C for 2 h in stem bromelain (mean ± S.E.M., n= 3) in the following concentrations: 4000 μm (⋄), 2000 μm (□), 400 μm (△), 200 μm (○), each with 16 mml-cysteine; and control treatments of 400 μm stem bromelain without cysteine (■) and HBSS +16 mm cysteine (●).
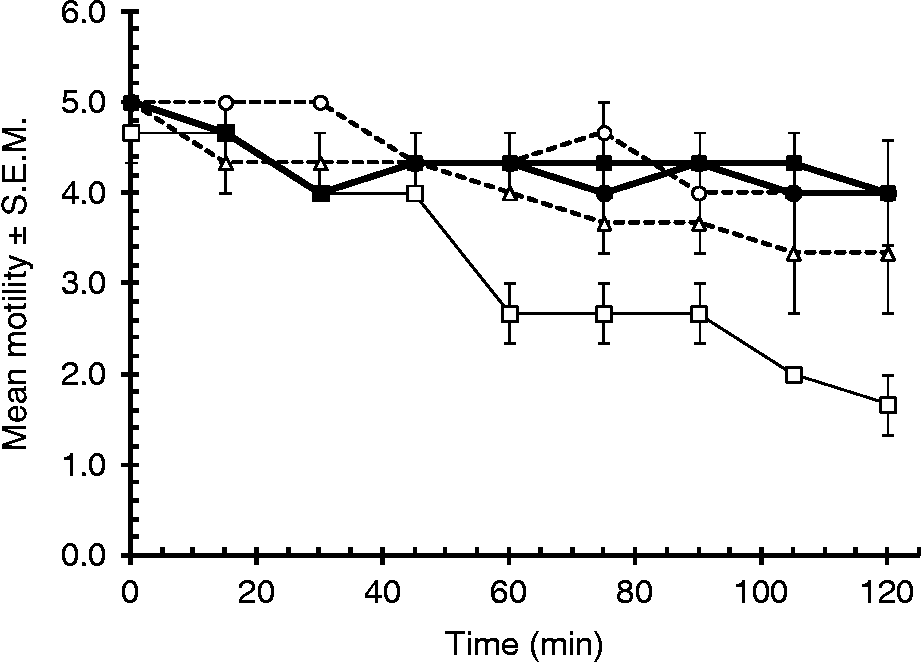
Worm motility was affected by the presence of actinidain (main effect of CONCENTRATION; F 1,19= 31.4, P< 0.001; at 30 min β = − 0.18 ± 0.007, t= − 2.7, P= 0.015, and significant negative gradients were also recorded at 60, 75, 90, 105 and 120 min of observation with t= − 3.8, − 3.9, − 4.1, − 6.2 and − 4.6, respectively, and P< 0.005 in all cases), with worms incubated in the highest concentration of actinidian (50 μm) + cysteine showing some loss of motility while worms in the lower concentrations (33 and 10 μm) and without enzyme showed very little change in motility (fig. 3). Loss of motility was time dependent (within-subject factor TIME, F 4.9,93.4= 4.03, P= 0.02) and the rate of loss over time depended on the concentration of enzyme (two-way interaction TIME and CONCENTRATION of enzyme, F 4.9,93.4= 6.004, P< 0.001). There was very little loss of motility among worms incubated in Hanks' with cysteine and in 50 μm enzyme without cysteine.

Fig. 3 Effect of actinidain on worm motility. Adult H. bakeri worms were incubated at 37°C for 2 h in actinidain (mean ± S.E.M., n= 3) in the following concentrations: 50 μm (□), 33 μm (△), 10 μm (○), all with cysteine; and in 50 μm actinidain without cysteine (■) and HBSS + cysteine without actinidain (●).
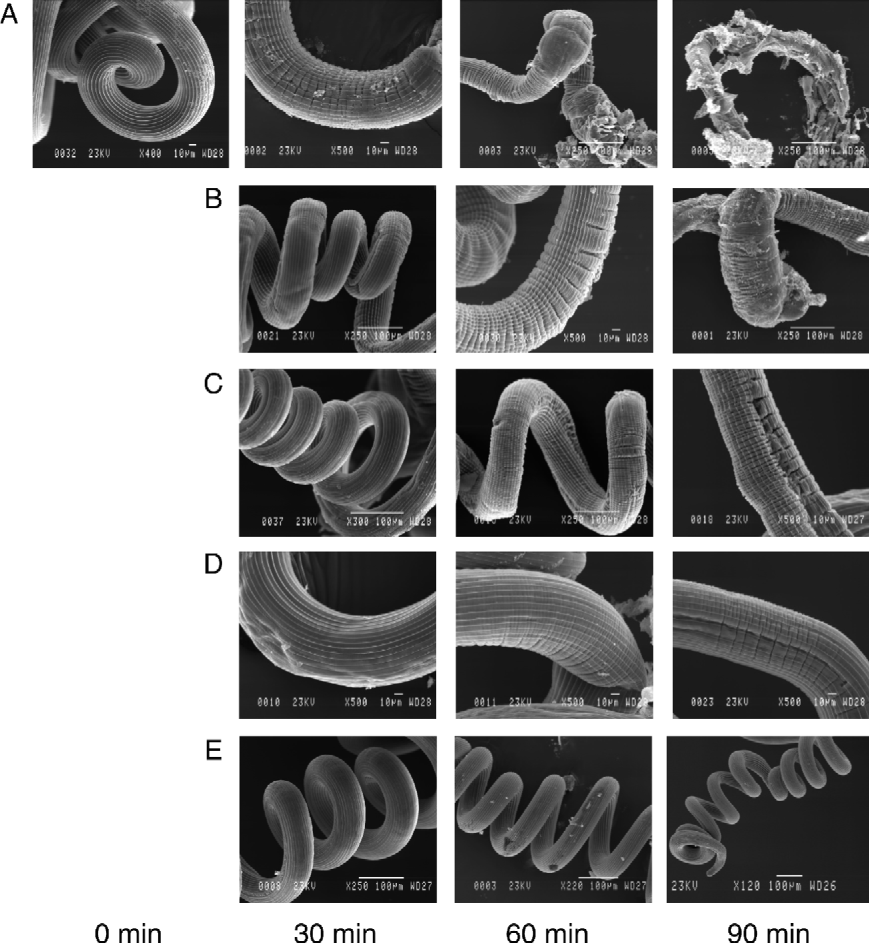
Typical cuticular changes on H. bakeri worms incubated in different CPs, as observed under SEM at equivalent points along the body of the adult worms, are illustrated in fig. 4. Progressive cuticular damage was seen within 30 min for worms incubated in PLS (fig. 4A), starting with the appearance of transverse wrinkles followed by blistering (at 60 min), and finally digestion of the cuticle with expulsion of the worms' internal structures and contents (at 90 min). The same effect was seen in the worms incubated in fruit bromelain (fig. 4B) and stem bromelain (fig. 4C) but not at the same intensity as that of PLS. However, the worms incubated in actinidain (fig. 4D) only showed a few initial signs of cuticular damage, i.e. formation of transverse wrinkles along some parts of the cuticle, after 60 min. This was probably due to the low concentration of active actinidain in the extracts used (50 μm compared with 200 μm of PLS, 200 μm of stem bromelain and 150 μm of fruit bromelain). In contrast, the cuticles of the worms incubated in HBSS alone (fig. 4E) showed no detectable signs of cuticular damage even after 90 min of incubation, with the longitudinal ridges remaining intact throughout the experimental period.

Fig. 4 Scanning electron micrographs of H. bakeri adult worms exposed to different plant CPs in vitro: (A) incubation in 200 μm PLS; (B) 150 μm fruit bromelain; (C) 200 μm stem bromelain; (D) 50 μm actinidain; (E) HBSS.
Determination of IC50 values for motility inhibition
Fruit bromelain and stem bromelain were compared with PLS in their ability to inhibit the motility of worms by estimating their half maximal inhibitory concentration (IC50), using dose–response relationships after 30 min of incubation with adult worms in vitro (fig. 5). Since the concentration of active actinidain was so low (after 30 min of incubation there was almost no loss of motility, see fig. 3), the IC50 for this enzyme was estimated after 2 h of incubation of worms in the extract (fig. 5B). The IC50 values for PLS, fruit bromelain and stem bromelain, estimated at 30 min, were 67.8 μm, 96.1 μm and 150.0 μm, respectively, while that of actinidain, estimated at 120 min, was 47.53 μm. This experiment was repeated, with almost identical values for PLS (69 μm), a lower value for fruit bromelain (33 μm) but with stem bromelain showing an even higher value (1142 μm). According to these IC50 values, PLS and fruit bromelain can be considered to have had the most potent inhibitory effects on worm motility, but stem bromelain was clearly poorer compared to the first two (fig. 5). However, the effect of actinidain cannot be compared directly to the other CPs as its value was determined after 2 h of incubation, at which time worm motility in the other CPs was reduced to almost zero and, in most cases, the worms were largely digested.
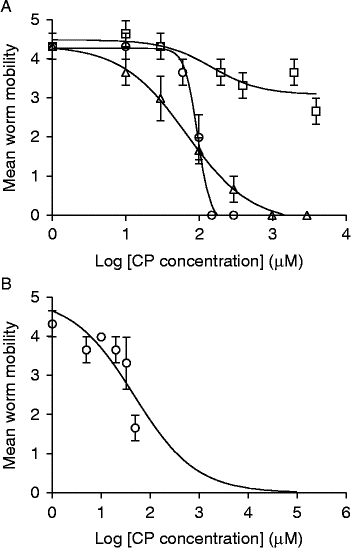
Fig. 5 The in vitro comparison of anthelmintic efficacy of four plant-derived sources of CPs as determined by their ability to inhibit the motility of the worms. (A) PLS (△), fruit bromelain (○) and stem bromelain (□) at 30 min of incubation; (B) actinidain (○) at 120 min of incubation. Sigmoidal dose–inhibition curves were fitted with an upper limit of 5 (maximum motility) and lower limit of zero (minimum motility) in GraphPad Prism (version 5); error bars are standard error of the mean (S.E.M.).
In vivo effects of cysteine proteinases on worm survival
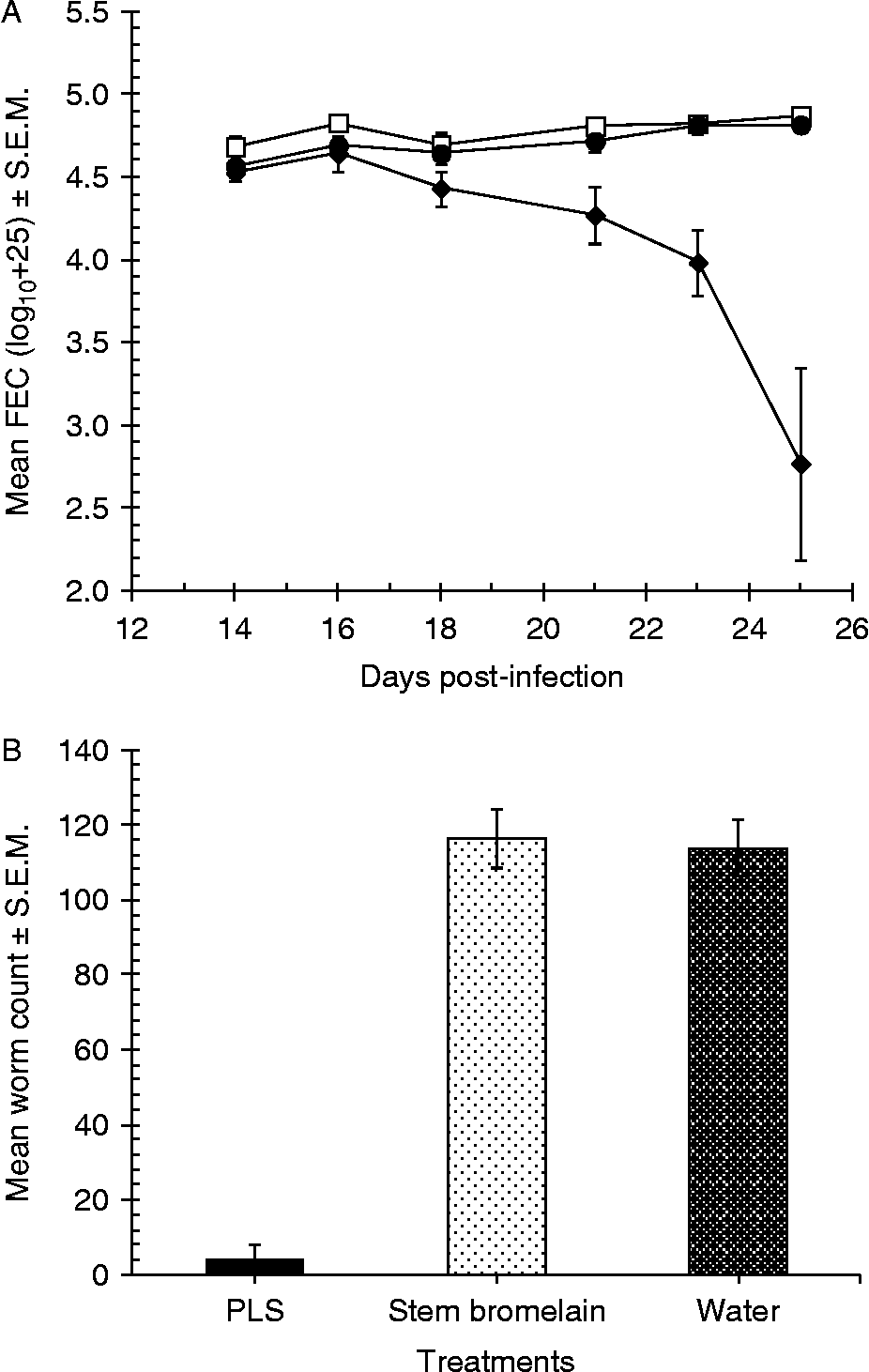
For the first in vivo trial, we used a dose of PLS that had been shown previously to be highly effective in removing worms from infected mice (240 nmol, as in Luoga et al., Reference Luoga, Mansur, Buttle, Duce, Garnett and Behnke2012) as a positive control and compared efficacy to that in mice treated with the same amount of active stem bromelain. As fig. 6 shows, FEC in the stem bromelain and the water-treated groups remained steady whereas those in the PLS-treated animals fell sharply soon after treatment began. The divergence in FEC between treatments over time was significant (two-way interaction between TIME and TREATMENT in rmGLM, with TIME as the within-subject factor, F 4,24= 7.7, P< 0.001). There was also a highly significant overall difference between treatment groups (main effect of TREATMENT F 2,12= 25.8, P< 0.001).

Fig. 6 In vivo efficacy of stem bromelain against H. bakeri worms; three groups of five male C3H mice were infected by oral gavage with 150 L3 on day 0, and were treated for five consecutive days from day 20 with CPs or water. (A) Faecal egg counts in mice treated with a standard dose of 240 nmol PLS (♦), 240 nmol of stem bromelain (□) or water (●); (B) worm recovery on day 25 post-infection; error bars are standard error of the mean (S.E.M.).
Since our key interest was in the relative efficacy of stem bromelain compared with that of PLS, we analysed post hoc the two groups, excluding the control group. The results indicated a significant main effect of TREATMENT (F 1,8= 30.9, P< 0.001), and examination of the relative efficacy in terms of percentage reduction in FEC showed that the fall in FEC was higher in mice treated with PLS (92.9%) compared with stem bromelain ( − 13.85%), which, on average, actually gained FEC. Clearly PLS was highly effective in reducing FEC and stem bromelain not at all.
Similarly, worm burdens were reduced by 95.8% in mice treated with PLS but showed no change ( − 1.9%) in mice treated with stem bromelain (main effect of TREATMENT, at two levels (PLS and stem bromelain excluding the control group) on log10(x+10)-transformed worm burdens, F 1,8= 117.6, P< 0.001).
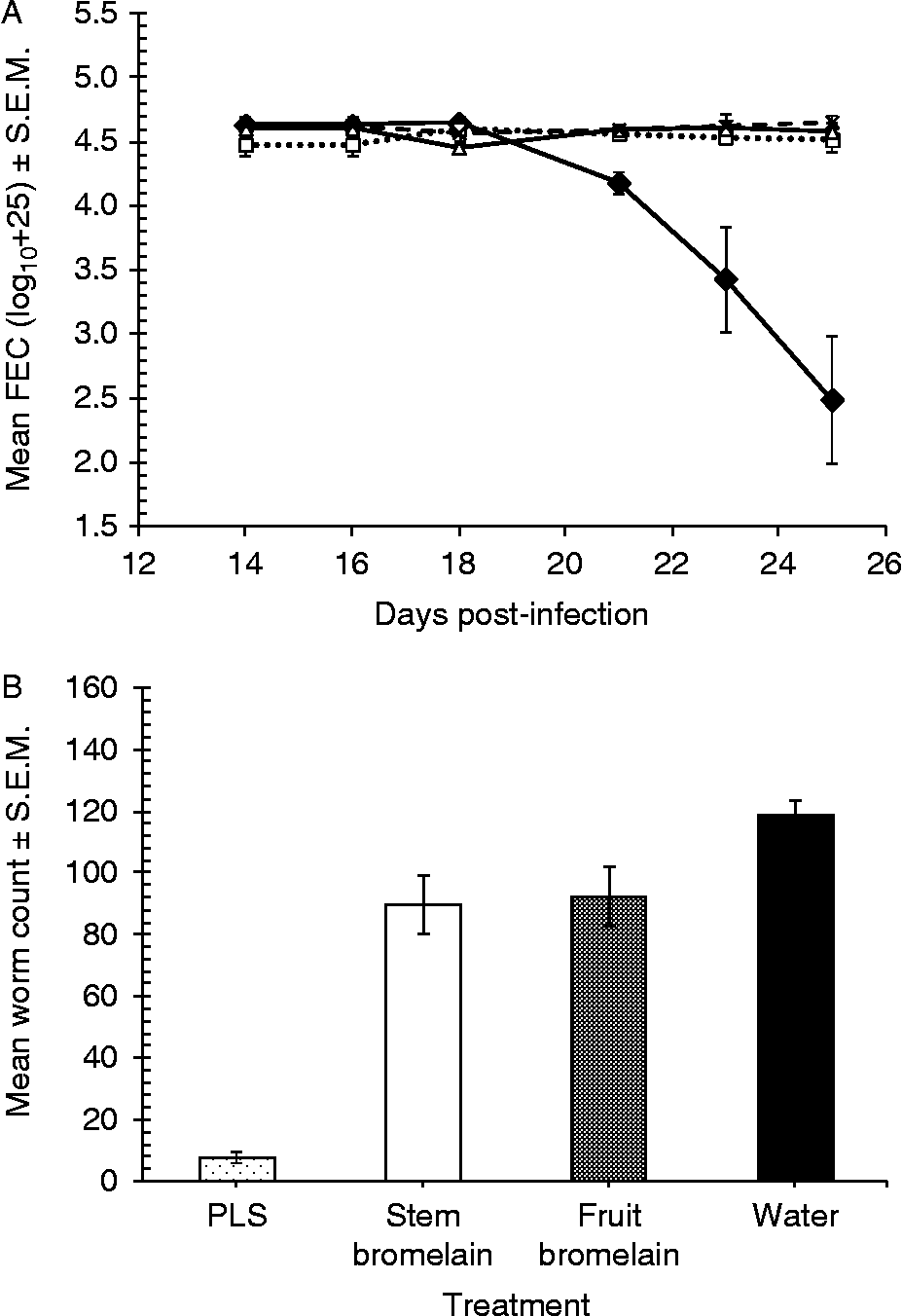
In a second in vivo trial we repeated the experimental design used for the first trial but also included a group of mice treated with fruit bromelain, but since extraction of the active enzyme had proved difficult we dosed with only a quarter of the amount of enzyme used for PLS- and stem bromelain-treated animals (60 rather than 240 nmol). Figure 7 shows that the FEC of the mice treated with fruit bromelain, stem bromelain and water (control group) remained steady throughout the experimental period, while the FEC of those treated with PLS fell sharply soon after treatment began, diverging markedly from the other three treatments (rmGLM with TIME as the within-subject factor, two-way interaction between TIME and TREATMENT on log10(x+25)-transformed data, F 6.3,39.6= 9.7, P< 0.001). There was also a highly significant overall difference between treatment groups (main effect of TREATMENT on log10(x+25)-transformed EPG, F 3,19= 19.0, P< 0.001). Arithmetically, the efficacy of PLS was considerably higher (94.5%) than that of either fruit bromelain or stem bromelain (14.0% and 21.9% reduction in FEC, respectively). In agreement with the effect on FEC, worm burdens were reduced by 93.5% in mice treated with PLS, but only 24.5% for stem bromelain and 22.4% for fruit bromelain. Statistically there was a significant main effect of TREATMENT on log10(x+10)-transformed worm counts (F 2,15= 137.2, P< 0.001) excluding the control group.

Fig. 7 In vivo efficacy of three plant-derived sources of CPs against H.bakeri. (A) Effect on faecal egg counts of fruit bromelain (△), stem bromelain (□), PLS (♦) and water (●); (B) effect on intestinal worm counts. Mice were treated for five consecutive days from day 20 with 240 nmol PLS, 60 nmol fruit bromelain, 240 nmol stem bromelain or water; error bars are standard error of the mean (S.E.M.).
Discussion
In this study we assessed the in vitro anthelmintic efficacies of fruit bromelain, stem bromelain and actinidain, since despite earlier publications (Berger & Asenjo, Reference Berger and Asenjo1939; Stepek et al., Reference Stepek, Buttle, Duce, Lowe and Behnke2005), still relatively little is known about their potential as drugs for the treatment of worm infections. The use of pineapple tissues and their extracts as sources of potential anthelmintics is not novel. Earlier studies have reported marked effects on larval motility and suppression of egg hatching in vitro; however, none of these studies have been conducted under conditions that would have enabled CPs to operate effectively (Hördegen et al., Reference Hördegen, Cabaret, Hertzberg, Langhans and Maurer2006; Domingues et al., Reference Domingues, Giglioti, Feitosa, Fantatto, Rabelo, de Sena Oliveira, de Oliveira, Barioni and Chagas2014). Moreover, in vivo trials with these extracts have generally failed to reduce worm burdens effectively or have given equivocal results (Hördegen et al., Reference Hördegen, Hertzberg, Heilmann, Langhans and Maurer2003; Githiori et al., Reference Githiori, Höglund, Waller and Baker2004; Domingues et al., Reference Domingues, Giglioti, Feitosa, Fantatto, Rabelo, de Sena Oliveira, de Oliveira, Barioni and Chagas2014). Our extracts from both pineapples and kiwi fruits did contain active CPs and our assays were conducted under conditions that would have enabled these enzymes to digest their target proteins. In addition to fruit bromelain, stem bromelain and actinidain, our preparations of these enzymes may have contained small quantities of other CPs. For instance, pineapple stem contains small amounts of ananain and comosain (Napper et al., Reference Napper, Bennett, Borowski, Holdridge, Leonard, Rogers, Duan, Laursen, Reinhold and Shames1994). Kiwi fruits may contain more than one form of actinidain (Larocca et al., Reference Larocca, Rossano and Riccio2010). Papaya latex contains four CPs: chymopapain, glycyl endopeptidase (also known as papaya proteinase IV), caricain (also known as papaya proteinase III) and papain, in order of abundance (Buttle et al., Reference Buttle, Dando, Coe, Sharp, Shepherd and Barrett1990). All these CPs are closely related in structure and enzyme mechanism as members of Family C1 of cysteine proteinases (see the Merops database, http://merops.sanger.ac.uk/). The mixture of CPs in PLS is known to be highly effective in removing intestinal worms (e.g. Luoga et al., Reference Luoga, Mansur, Buttle, Duce, Garnett and Behnke2012), and therefore we expected each of these new sources to show some efficacy. Indeed, we did find that fruit bromelain and stem bromelain both had detrimental effects on worm motility in vitro, although fruit bromelain was clearly more effective than stem bromelain at comparable molar concentrations and neither proved to be as potent as PLS. The mechanism of reduced motility was identical to that reported by Stepek et al. (Reference Stepek, Buttle, Duce, Lowe and Behnke2005), involving digestion of the cuticle, as illustrated clearly in the SEM images. The reduction in worm motility was noted from 30 min of incubation in the enzymes. These findings are consistent with those of Berger & Asenjo (Reference Berger and Asenjo1939), who reported complete digestion of Ascaris lumbricoides from a hog's intestine within 24 h when incubated in freshly squeezed pineapple juice, and who contrasted this observation with the loss of efficacy when worms were incubated in heat-inactivated pineapple juice, the worms remaining entirely active throughout the period of the experiment in this latter treatment.
Although stem bromelain was effective in reducing worm motility to some extent, relative to PLS its effect was comparatively poor, despite the use of enzyme preparations that had been standardized in terms of the concentration of active enzyme. Even at high concentrations, the inhibitory effect on motility was still comparatively low when compared with that of fruit bromelain. This is supported by the IC50 values, which were higher for stem bromelain than for PLS and fruit bromelain, and agrees with the results of Stepek et al. (Reference Stepek, Buttle, Duce, Lowe and Behnke2005) who reported IC50 values after 90 min of incubation and found that fruit bromelain had the lowest value, followed closely by papaya latex but with stem bromelain having a considerably higher value. Moreover, the intensity of the cuticular damage was seen to be higher in fruit bromelain than in stem bromelain, even though worms were incubated in higher molar concentrations of stem bromelain (200 μm) than fruit bromelain (150 μm). On the basis of these results we conclude that fruit bromelain is more efficacious than stem bromelain in vitro.
Actinidain caused some detrimental effect on worm motility in the current study, in contrast to previous reports indicating no effect at all on parasitic nematodes in vitro (Stepek et al., Reference Stepek, Buttle, Duce, Lowe and Behnke2005). The reduction of worm motility was not as high as in other CPs and this might have been partially due to the low concentration of the enzyme used, which was only 50 μm at most. However, at this molar concentration PLS caused about 40% reduction in mean motility at 30 min (extrapolation from their Figure 4A) and 50 μm crude papaya extract caused almost complete digestion of worms in 2 h, the time point used for calculation of the dose–response of actinidain in the present work (Stepek et al., Reference Stepek, Buttle, Duce, Lowe and Behnke2005). Like all Family C1 CPs, actinidain contains a free sulfhydryl group essential for its activity and, logically, a similar effect on target proteins might have been expected.
Our in vivo trials with enzymes from pineapple demonstrated much lower efficacy compared to PLS. In these trials stem bromelain and PLS were given at the same active molar concentration (240 nmol) and the results indicated that stem bromelain had a significantly poorer effect on FEC and worm burdens. In the case of the fruit bromelain, which matched PLS in terms of inhibition of worm motility in vitro, the lower in vivo efficacy may have been due to the lower concentration of the active enzyme administered to animals (60 nmol), as we found it technically difficult to obtain a sufficient concentration of active enzyme to match the level in our PLS preparation in a volume suitable for administration to mice. It is noteworthy that Stepek et al. (2007) carried out an in vivo dose–response study with papaya latex and H. bakeri and, by extrapolation from their Figure 2, it can be seen that with 60 nmol of active papaya enzyme we should expect a 50% reduction in worm burdens, so clearly, in comparison to papaya latex, fruit bromelain was relatively ineffective in removing worms.
So why do some CPs have anthelmintic effects while others have a poorer or no effect at all? It is possible that this relates to hydrolysis of particular peptide bond(s) in cuticle proteins that leads to loss of cuticular integrity. Although these CPs are very similar in structure, their substrate specificity can differ markedly. For example, it is known that fruit and stem bromelains have very different substrate specificities (Rowan et al., Reference Rowan, Buttle and Barrett1990) and are therefore unlikely to be targeting the same peptide sequence(s) in cuticle proteins. In contrast to fruit bromelain, and perhaps surprisingly, in the case of stem bromelain a clear dose-dependent effect of enzyme concentration on motility was not overtly evident (fig. 2, where a similar loss of motility can be seen for worms treated with 200 and 4000 μm), but nevertheless, with other factors taken into account, overall a statistically significant negative relationship between concentration and worm motility was found. Stem bromelain is known to have a different, perhaps more restricted, substrate specificity to fruit bromelain (Rowan et al., Reference Rowan, Buttle and Barrett1990) and so may hydrolyse a relatively small number of peptide bonds in the cuticle (such that even at higher enzyme concentrations no more hydrolysis takes place). In the case of PLS, which contains four proteinases, at least one of which (glycyl endopeptidase) has markedly different specificity to the others, the summative effect on cuticle disruption may be synergistic. Therefore, it may be that, in contrast to PLS, neither pineapple nor kiwi fruit CPs have the necessary combination of specificities for the key target proteins in nematode cuticles, a possibility that will only be resolved when the molecular targets are identified, purified and tested against each of the constituent enzymes in turn. This work is currently being undertaken. Similarly, even when in vitro results indicated similar, if not identical, levels of efficacy (e.g. PLS and fruit bromelain), in vivo the former was extremely effective and the latter hardly at all. In addition to the differences in dose used in the in vivo experiments, these results must reflect differences in the stability of these enzymes in the intestinal environment (with respect to intestinal serine proteinases, pH and competition with microflora; see for example Huet et al., Reference Huet, Looze, Bartik, Raussens, Wintjens and Boussard2006), and hence also bioavailability at the surface of the worms where they are required to make contact in order to damage the cuticle.
While, taken together, our results do not provide support for the idea that CPs derived from pineapples and kiwi fruits are likely to be successfully developed as novel anthelmintics for intestinal nematode infections, important questions still remain to be resolved. As indicated above, host environmental effects may also have contributed to the relatively weak efficacy of pineapple fruit enzymes in vivo. For example, PLS is known to work better in some strains of mice than others (Luoga, Reference Luoga2012). It may be necessary to assess the in vivo efficacy of pineapple CPs in other strains of mice, but whether this is justified on ethical grounds is debatable. Pineapple- and kiwi fruit-derived CPs may also show efficacy against other species of GI nematodes. For example Trichuris muris lives in the large intestine, where orally administered CPs are known to accumulate and to persist for longer (Stepek et al., Reference Stepek, Lowe, Buttle, Duce and Behnke2007a) than in the duodenum where H. bakeri is located. Other GI nematodes, perhaps lumen dwellers such as Ascaris spp., may prove to be more susceptible than H. bakeri. These are important questions to answer, given that bromelain is widely used as a natural plant-derived medicine for other ailments, is conveniently available commercially in large quantities and some groups have been evaluating the usefulness of pineapple by-products as fodder for domestic livestock (Hördegen et al., Reference Hördegen, Hertzberg, Heilmann, Langhans and Maurer2003).
Financial support
W.L. was supported by a postgraduate studentship from the government of Tanzania, and F.M. from the Government of Malaysia.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the national guides on the care and use of laboratory animals in the UK (The Animals (Scientific Procedures) Act 1986) and were locally approved by the Animal Welfare and Ethical Review Body of the University of Nottingham. Importantly all work was conducted within a recognized culture of care and compliance to meet the expectations of both the university and the UK Home Office Inspectorate. All animal procedures were carried out under UK Home Office licence numbers 40/2942 and 40/3138.
Dedication
We dedicate this paper to Dr Wenceslaus Luoga who sadly was killed in a road accident in July 2013 shortly after returning to Tanzania on the successful completion of his post-graduate studies at the University of Nottingham.