Introduction
The Olary Province of South Australia hosts a diversity of small uranium deposits (Dickinson et al., Reference Dickinson, Sprigg, King, Wade, Webb and Whittle1954; Campana and King, Reference Campana and King1958). These include Radium Hill, Crocker's Well, Mt Victoria and several smaller prospects. Most of the deposits are low grade and the only significant production has been from Radium Hill which was Australia's first uranium mine, discovered in 1906 and operated until 1961. The deposits are intrusive-type deposits, associated with Mesoproterozoic intrusives, mainly granite, alaskite, pegmatite and migmatite. The primary minerals are davidite-(La) and brannerite and only a small number of secondary uranium minerals have been recorded. Secondary uranium minerals have also been reported from numerous pegmatites and granites in the region and it is one of these that has produced the specimens containing baumoite. Baumoite is one of nine uranyl molybdate minerals and the first natural Ba, uranyl molybdate. Its structure is twinned and incommensurately modulated, one of several U6+ modulated structures that have been discovered recently along with swamboite-(Nd) and shinkolobweite (Olds et al., Reference Olds, Lussier, Oliver, Petříček, Plášil, Kampf, Burns, Dembowski, Carlson and Steele2017; Plášil et al., Reference Plášil, Petříček, Locock, Škoda and Burns2017; Plášil, Reference Plášil2018). Despite the weak intensities of the satellite reflections (only those of the 1st order were detected) on the CCD frames, the refinement in superspace converged to the reasonable values and it provided a reasonable structure model. Baumoite is named for its chemical composition. The mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2017-054, Elliott et al., Reference Elliott, Plášil, Petríček, Čejka and Bindi2017). The holotype specimen of baumoite is deposited in the mineral collection of the South Australian Museum, Adelaide, South Australia (registration number G34697).
Occurrence
The Olary Domain is contained within the Curnarnona Province that extends across northeastern South Australia and western New South Wales. The geology comprises a late Palaeoproterozoic metasedimentary and metavolcanic succession (Willyama Supergroup) with some meta-intrusives and early Mesoproterozoic volcanics and granitoid intrusives (Stevens, et al., Reference Stevens, Barnes, Forbes and Hughes1990; Flint and Parker, Reference Flint, Parker, Drexel, Preiss and Parker1993). The lithological and geophysical character of the Willyama Supergroup in the Olary Domain differs from the remainder of the Province with a higher proportion of shallow water sediments, lesser volcanics, widespread metasomatism, particularly albitisation, and an abundance of syntectonic to late-tectonic granitoids (Callen, Reference Callen1990; Forbes, Reference Forbes1991; Flint and Parker, Reference Flint, Parker, Drexel, Preiss and Parker1993). Rocks in the Radium Hill area are metasedimentary quartz–feldspathic gneiss, composite gneiss, quartz–feldspar–biotite schist and amphibolite of the Willyama Supergroup. Granitoid intrusives crop-out to the northwest of Radium Hill. Baumoite was found as thin crusts of minute crystals in thin seams on specimens collected in the late 1980s from a weathered granite outcrop, 4 km NW of the Radium Hill mine. Associated minerals are baryte, metatorbernite, phurcalite and kaolinite. The granite comprises pink orthoclase, quartz and muscovite. Baumoite has resulted from the alteration of baryte and primary U and Mo minerals by oxidising groundwaters. No primary U and Mo minerals have been observed in specimens containing baumoite, however, rocks in the surrounding area contain uraninite, davidite-(La) and molybdenite.
Appearance, physical and optical properties
Baumoite occurs as yellow to orange-yellow tabular to prismatic crystals up to 0.11 mm in size (Fig. 1). Crystals are translucent with a vitreous lustre, pale yellow streak, and show bright yellow green fluorescence under shortwave ultraviolet illumination. The Mohs hardness is ~2½. The density could not be measured as it exceeds that of available heavy liquids; the calculated density is 4.61 g/cm3 from the empirical formula and 4.68 g/cm3 from the ideal formula. Baumoite is brittle, the fracture is uneven and shows one excellent cleavage. Baumoite is optically biaxial (–), α = 1.716(4), β = 1.761(4) and γ = 1.767(4) measured in white light. Calculated 2V is 42.2°. The Gladstone–Dale compatibility index is 0.009, classed as excellent (Mandarino, Reference Mandarino1981).
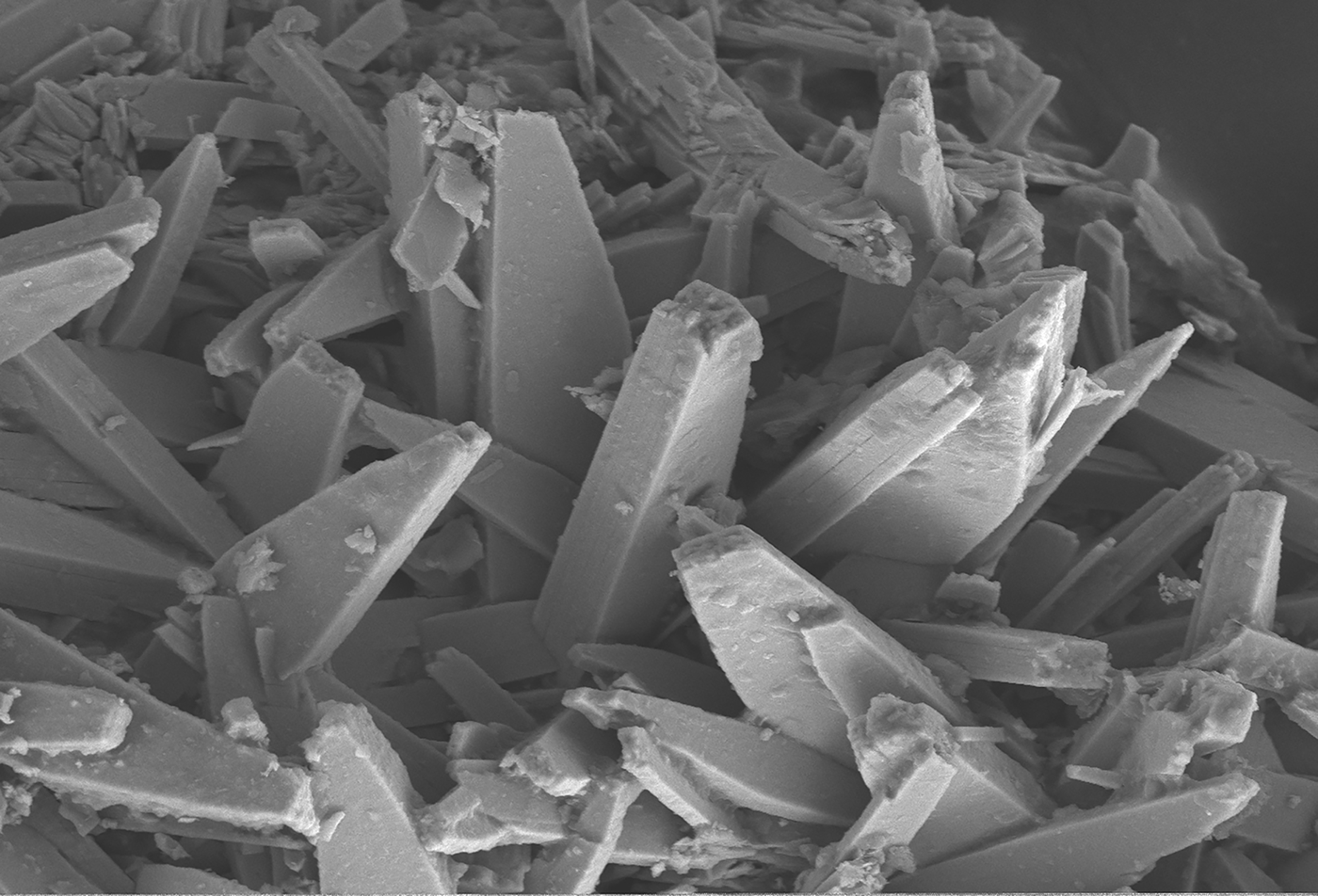
Fig. 1. Scanning electron microscopy photomicrograph showing crystals of baumoite. The field of view is 100 mm across.
Infrared spectroscopy
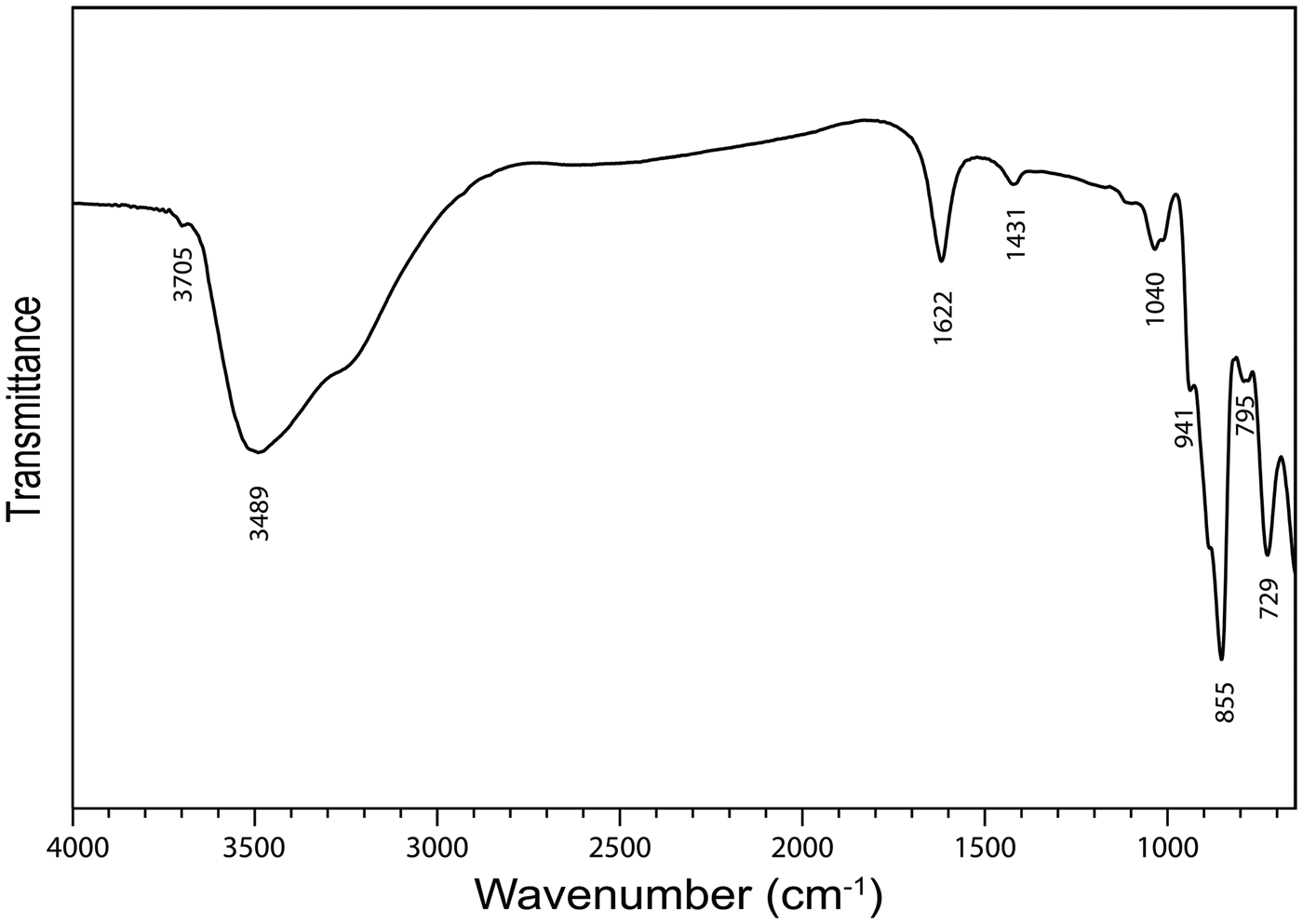
The infrared spectrum (Fig. 2) of powdered baumoite in the range 4000 to 650 cm−1 was obtained using a Nicolet 5700 FTIR spectrometer equipped with a Nicolet Continuum IR microscope and a diamond-anvil cell. The spectrum was interpreted with regard to the theoretical and experimental papers published by Hardcastle and Wachs (Reference Hardcastle and Wachs1990), Fomichev et al. (Reference Fomichev, Poloznikova and Kondratova1992), Čejka (Reference Čejka, Burns and Finch1999), Fedoseev et al. (Reference Fedoseev, Budantseva, Shirokova, Andreev, Yurik and Krupa2001), Sidorenko et al. (Reference Sidorenko, Chistyakova, Chukanov, Naumova and Rossulov2005), Frost et al. (Reference Frost, Čejka and Dickfos2008), Mączka et al. (Reference Mączka, Pietraszko, Paraguassu, Souza Filho, Freire, Mendes Filho and Hanuza2009), Nakamoto (Reference Nakamoto2009), Zhang and Guo (Reference Zhang and Guo2012) and Barik et al. (Reference Barik, Chatterjee and Choudhary2014). Moreover, we used data taken from Katscher et al. (Reference Katscher, Jehn and Kurtz1990), who reviewed infrared and/or Raman spectra of (MoOx)n– units in synthetic and mineral phases. Two bands at 3699 (vw) and 3489 (sb) and a shoulder at 3263 cm−1 are assigned to the ν OH stretching vibrations of hydrogen bonded to water molecules and hydrogen bonded hydroxyls. According to Libowitzky (Reference Libowitzky1999), approximate O–H···O hydrogen bond lengths vary from >3.2 to 2.73 Å. A medium strong band at 1616 cm−1 is attributed to the ν2 (δ) H2O bending vibration and weak bands at 1417 (shoulder), 1171 (vvw), 1100 (sh), 1030 (w-m), 1013 (w-m) may be connected with U–OH bending vibrations. A shoulder at 934 cm−1 and a very strong band at 851 cm−1 together with a shoulder at 880 cm−1 are attributed to the ν (Mo–O) stretching vibrations (Katscher et al., Reference Katscher, Jehn and Kurtz1990). However, an overlap (a coincidence) of this vibration with the ν3 (UO2)2+ antisymmetric stretching vibration is assumed. A shoulder at 787 cm−1 is connected with the ν1 (UO2)2+ antisymmetric stretching vibration. According to Bartlett and Cooney (Reference Bartlett and Cooney1989), approximate U–O bond lengths in uranyl, inferred from the wavenumbers of the (UO2)2+ stretching vibrations, are 1.82 Å (based on ν3) and 1.82 Å (based on ν1). Observed lengths are somewhat higher than the U–O lengths in uranyl ~1.8 Å, proposed by Lussier et al. (Reference Lussier, Lopez and Burns2016). A strong band at 723 cm−1 is attributable to the ν (Mo2O2) stretching vibration of the Mo = O2 = Mo unit bridging two Mo-octahedra and thus forming (Mo2O2) units (Katscher et al., Reference Katscher, Jehn and Kurtz1990).
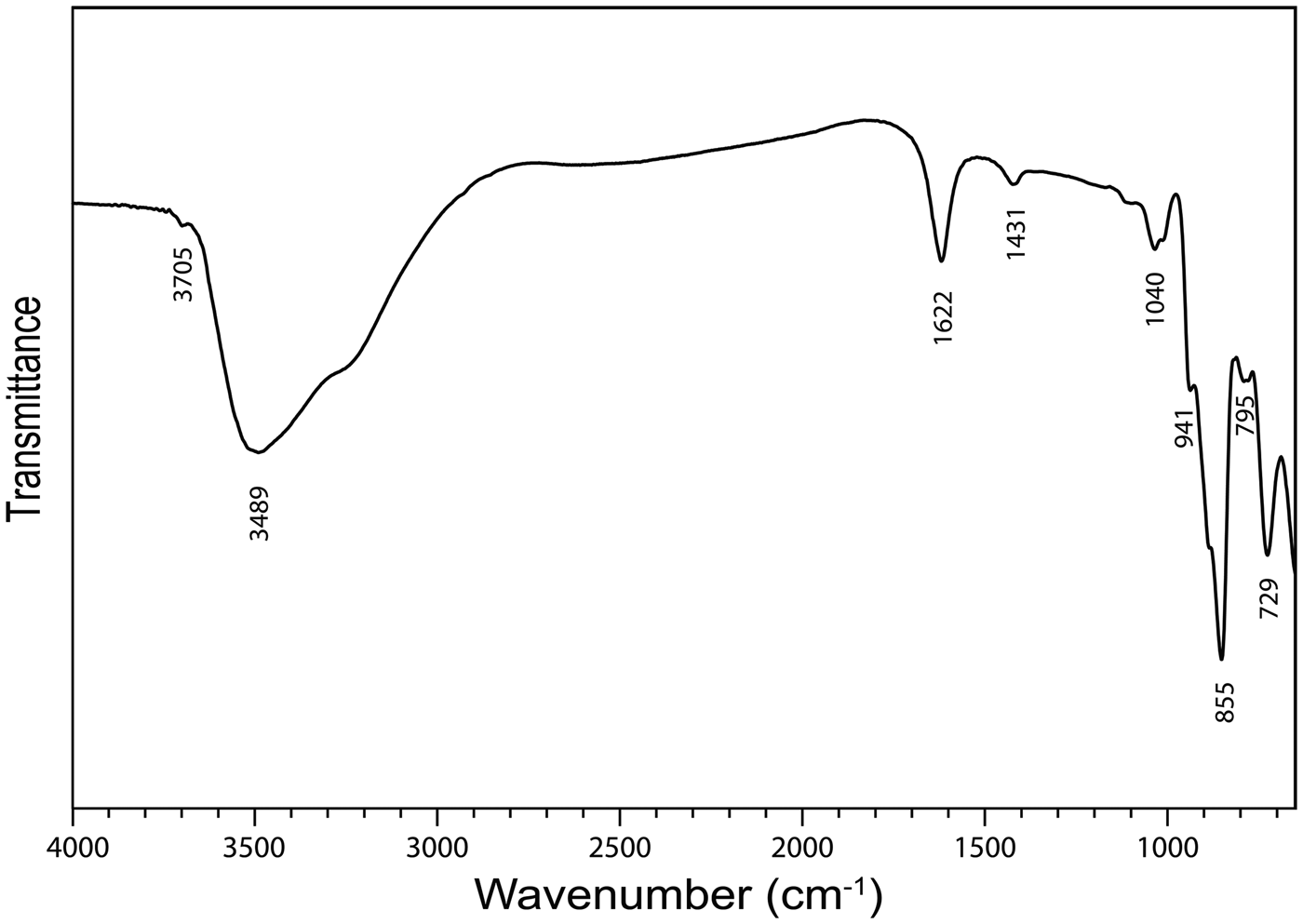
Fig. 2. Fourier-transform infrared spectrum of powdered baumoite.
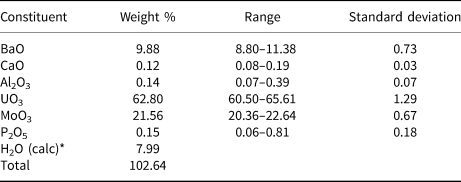
Chemical composition
The chemical composition of baumoite (20 points on one crystal aggregate) was determined using a Cameca SXFive electron microprobe operating in the wavelength-dispersive mode with an acceleration voltage of 20 kV, beam current of 20 nA, and 5 µm beam diameter. Data were processed using the φ(ϱz) correction procedure of Pouchou and Pichoir (Reference Pouchou, Pichoir and Armstrong1985). Analytical data (average of 20 points) are given in Table 1. The standards employed were: baryte (Ba), wollastonite (Ca), almandine (Al), U-metal (U), Mo-metal (Mo), and apatite (P). The empirical formula, calculated on the basis of 22 oxygen atoms and with H2O calculated to obtain charge balance, is Ba0.87Ca0.03Al0.04U2.97Mo2.02P0.03O22H11.99. The simplified formula is BaU3Mo2O16(H2O)6.
Table 1. Compositional data for baumoite.
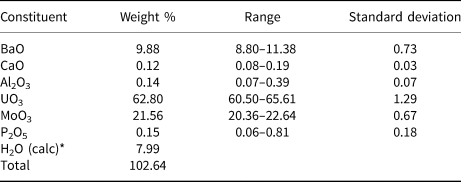
* calculated to obtain charge balance.
X-ray crystallography
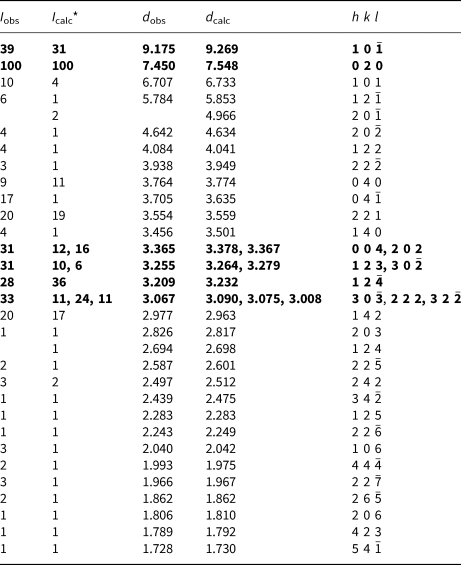
Powder X-ray diffraction
Powder X-ray studies (Table 2) were carried-out using a Rigaku Hiflux Homelab diffractometer, with monochromatised CuKα radiation (λ = 1.541870 Å). Unit-cell parameters refined using the Le Bail profile-fitting method (Le Bail et al., Reference Le Bail, Duroy and Fourquet1988; Hunter, Reference Hunter1998) and starting from the unit-cell parameters determined from the single-crystal study (see below), are a = 9.921(1), b = 15.080(1), c = 14.295(2) Å, β = 109.279(5)° and V = 2018.7(3) Å3.
Table 2. Powder X-ray diffraction data for baumoite.
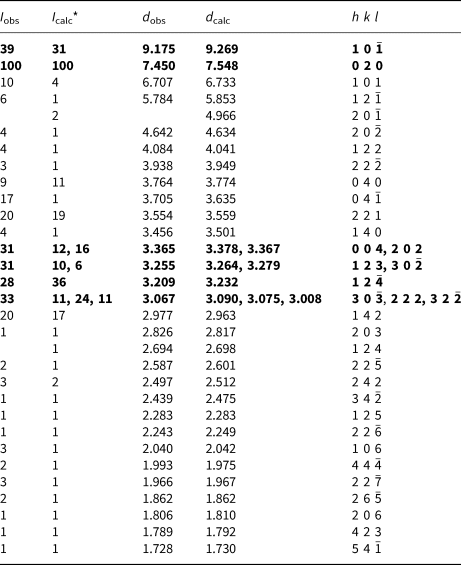
* Calculated intensities were obtained using the program Rietica (Hunter, Reference Hunter1998).
The strongest lines are given in bold
Single-crystal X-ray diffraction
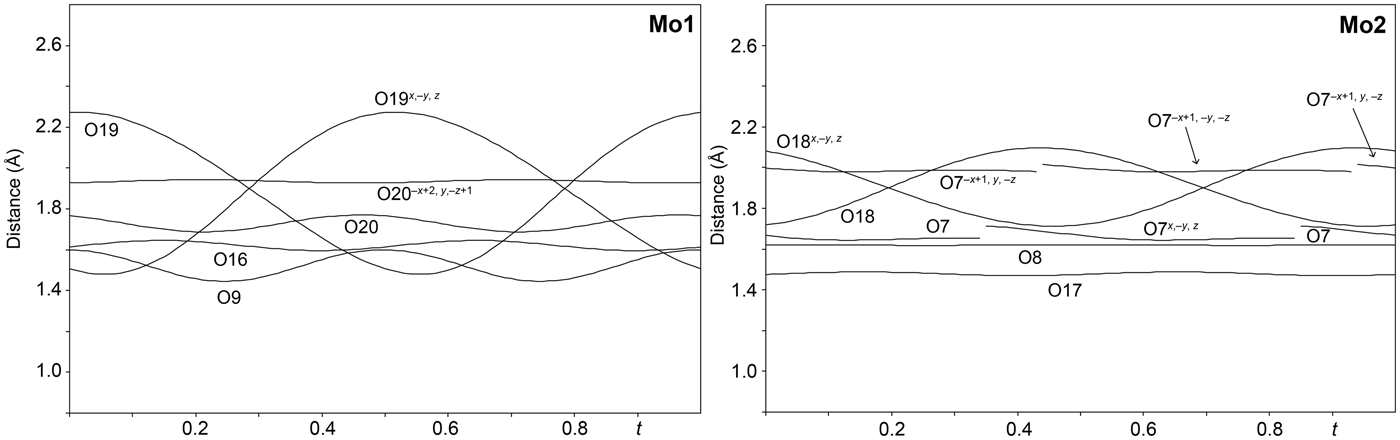
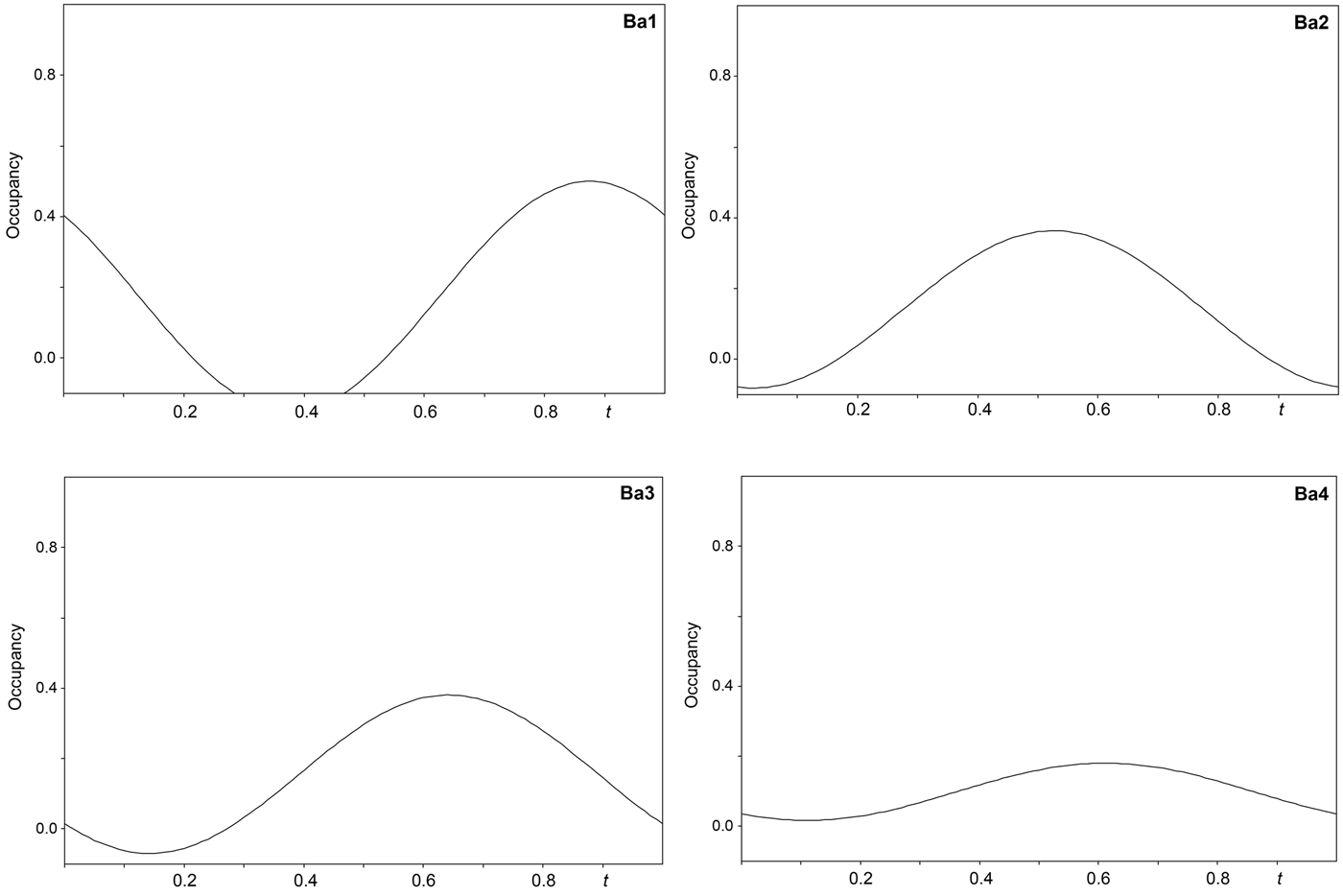
The single-crystal X-ray study was carried-out using intensity data collected at 293 K on a Rigaku SuperNova diffractometer (MoKα radiation from the microfocus X-ray tube, β = 0.71070 Å) equipped with an Atlas S2 CCD detector using a crystal 0.065 mm × 0.045 mm × 0.01 mm in size. Initial attempts to solve and refine the structure based on the preliminary data from an Oxford Diffraction Xcalibur E diffractometer (MoKα radiation from the conventional X-ray tube, λ = 0.71070 Å) equipped with an EoS CCD were done in the monoclinic space group C2/m, but this refinement returned only poor R values (R 1 ≈ 0.10 with a significantly large GoF ≈ 4), thus representing an average structure. By the careful inspection of the diffraction data it was found that the structure of baumoite is twinned and incommensurately modulated. The satellite reflections can be described by the modulation vector q = 0.718a* + 0.2803c*. Twin domains are related by the two-fold rotation along [001] axis represented by the twinning matrix [–1 0 –0.451/0 –1 0/0 0 1], which leads to a partial overlap of the reflections. Corrections for background, Lorentz, and polarisation effects were applied to the data during reduction in the CrysAlis package (Rigaku, 2018). The final data set was integrated as an hklm file (for the commensurate 3 + 1 case) using CrysAlis based on the list of reflections generated in Jana2006 (Petříček et al., Reference Petříček, Dušek and Palatinus2014). A correction for absorption was done using spherical harmonics by the Jana2006 program. Refinement of the modulated structure in the superspace group X2/m(a0g)0s with X = (0,½,0,½) was started using the atom coordinates obtained from the average structure solved by Superflip (Palatinus and Chapuis, Reference Palatinus and Chapuis2007). One modulation wave was set for the refinement. The modulations in the structure of baumoite were described using both continuous (harmonics) and discontinuous (crenel-like; Petříček et al., Reference Petříček, Eigner, Dušek and Čejchan2016) modulation functions. There are strong positional modulations of the U, Mo and O atoms that are linked to previously mentioned atoms and a strong occupational modulation of the Ba atoms and also less positional modulation of the Ba atoms and O atoms of the H2O sites. We have used the recently developed refinement technique in which the refinement is repeated several times starting from small randomly chosen modulation amplitudes. Crystallographic details, data collection and refinement parameters for the average and incommensurate structures, respectively, are given in Table 3. Fractional coordinates and atom displacement parameters, Fourier amplitudes of the occupational modulation are given in the accompanying crystallographic information files, which have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below). Selected interatomic distances as functions of t are displayed on Figs 3, 4 and 5; refined occupancies of Ba sites are displayed on Fig. 6.

Fig. 3. Bond distances around U1, U2 and U3 sites in the incommensurately modulated structure of baumoite as a function of the internal coordinate t.

Fig. 4. Bond distances around the Mo1 and Mo2 sites in the incommensurately modulated structure of baumoite as a function of the internal coordinate t.
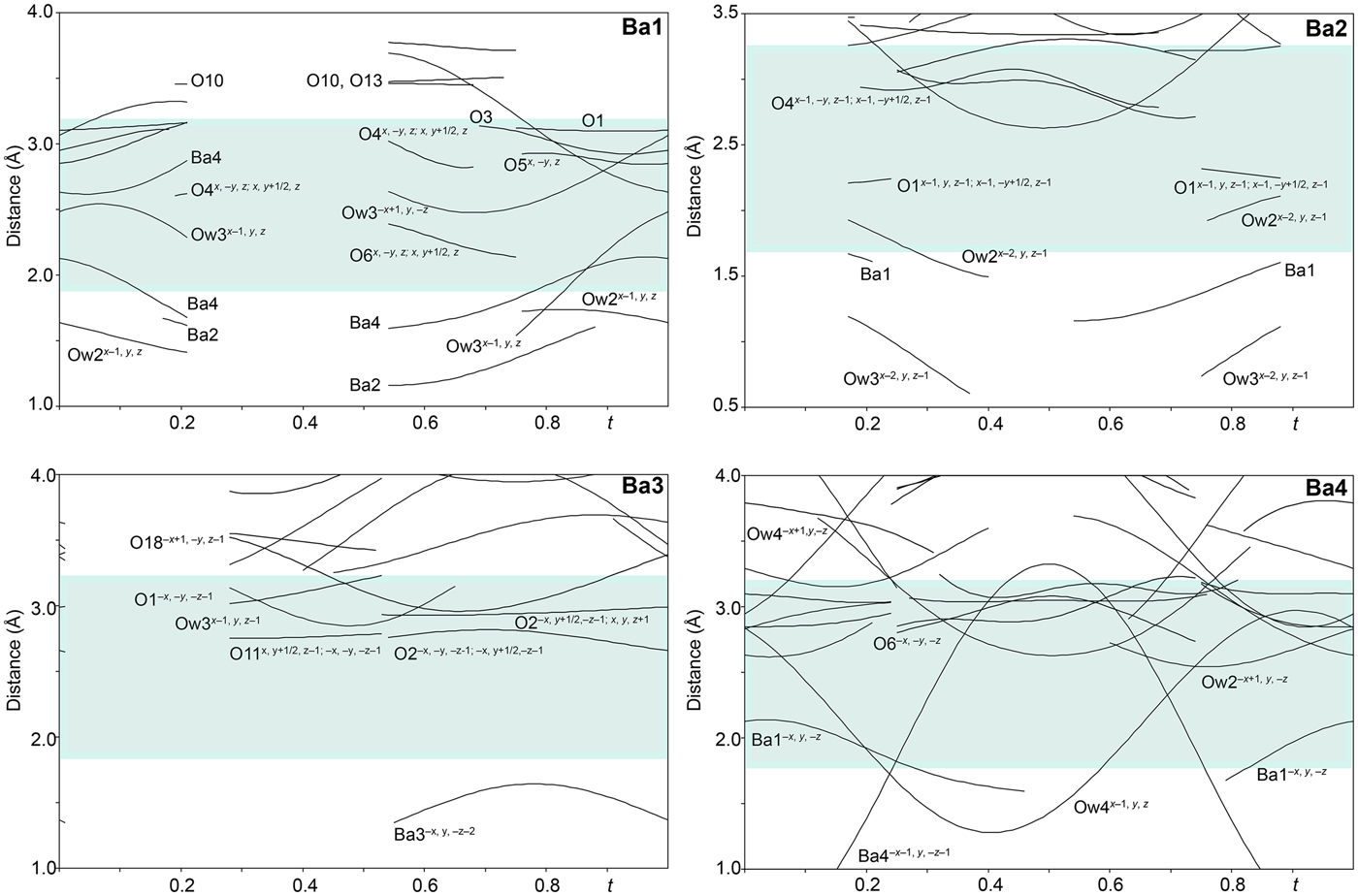
Fig. 5. Bond distances around the Ba sites in the incommensurately modulated structure of baumoite as a function of the internal coordinate t. The bluish region highlights the range of the most typical Ba–O distances in the crystalline solids.
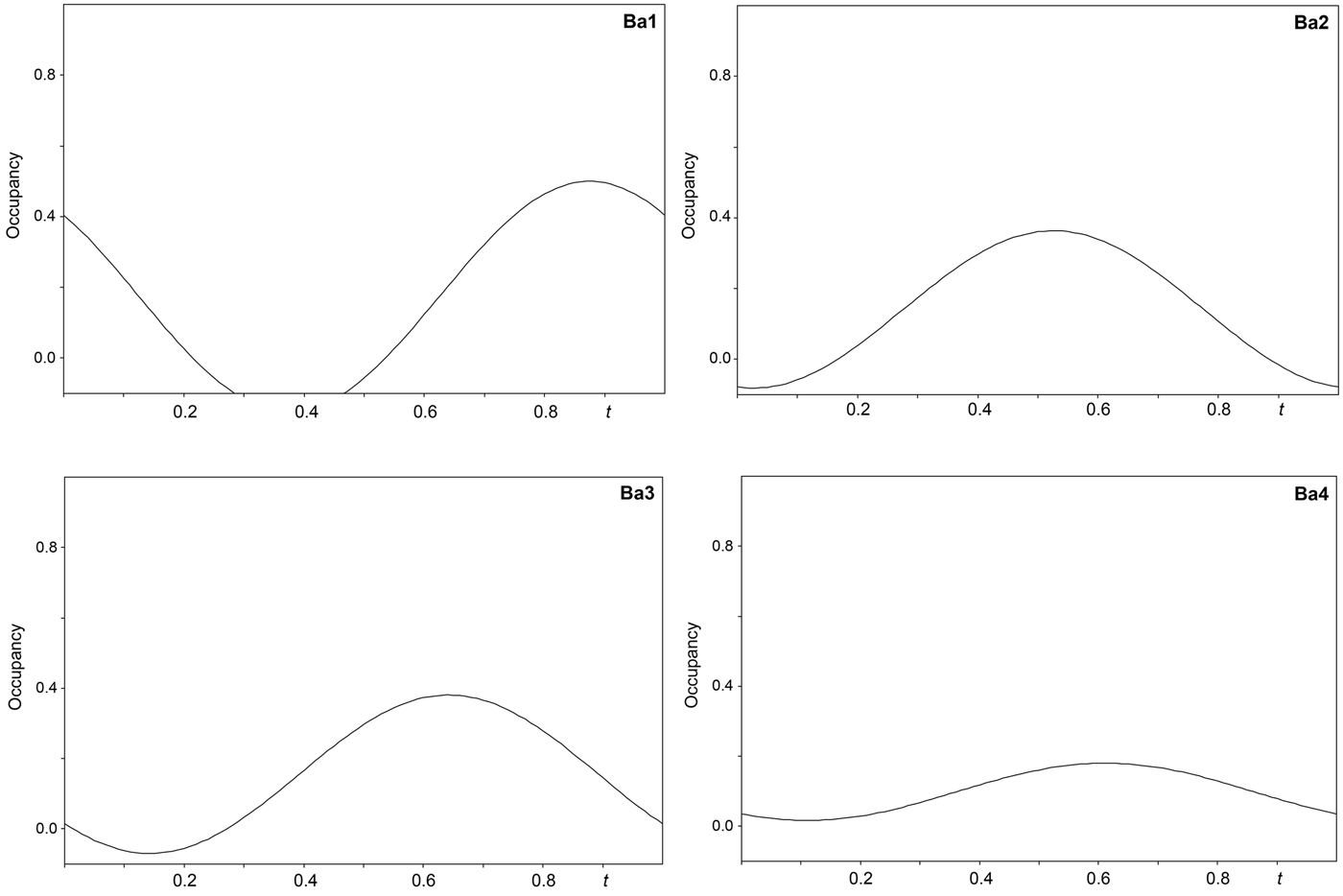
Fig. 6. Refined occupancies of the Ba sites in the incommensurately modulated structure of baumoite as a function of the internal coordinate t.
Table 3. Crystallographic and refinement parameters for baumoite.

Results and discussion
Description of the structure
The structure of baumoite contains three symmetrically distinct U sites, two Mo sites, four Ba sites and 22 O sites. All U sites in the structure are [7]-fold coordinated in the form of a pentagonal bipyramid, consisting of two strong U–O bonds, i.e. forming uranyl ion (UO2)2+, and five weaker bonds distributed in the equatorial plane of the pentagonal bipyramid (Fig. 3). All U in baumoite is present as hexavalent. The two Mo sites are [6]-coordinated in the form of irregular, distorted octahedra (Fig. 4). The Mo1 and Mo2 polyhedra share their common edge to form Mo2φ10 dimers. The baumoite structure is based upon uranyl-molybdate sheets. The fundamental building blocks of the sheets are trimers of edge-sharing U1, U2 and U3 polyhedra, which further share a common edge with other symmetrically related U3 polyhedra to form the six-membered clusters (Figs 7, 8a). These clusters are connected through the O11 equatorial atom of the U1 bipyramid to form irregular chains propagated along [![]() ${\bar 1}$01]. These chains of polyhedra are connected through Mo2φ10 dimers in a stair-case-like way (Fig. 8a).
${\bar 1}$01]. These chains of polyhedra are connected through Mo2φ10 dimers in a stair-case-like way (Fig. 8a).
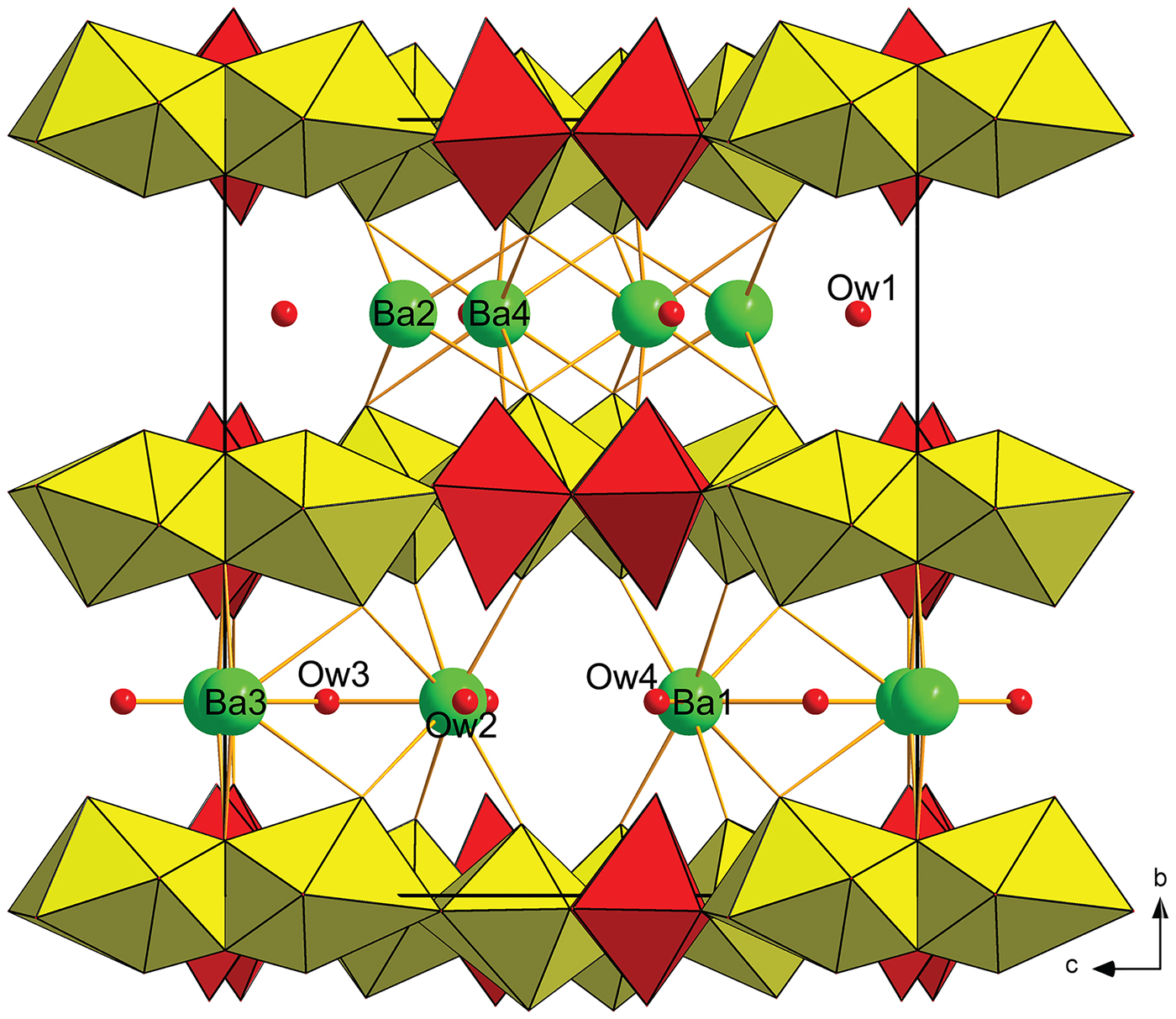
Fig. 7. Approximant structure of baumoite at t = 0. Uranyl-molybdate sheets, composed of uranyl pentagonal bipyramids (yellow) and Mo6+-octahedra (red), are alternating interlayer, where Ba atoms (green) and H2O molecules (Ow1–4) are localised.
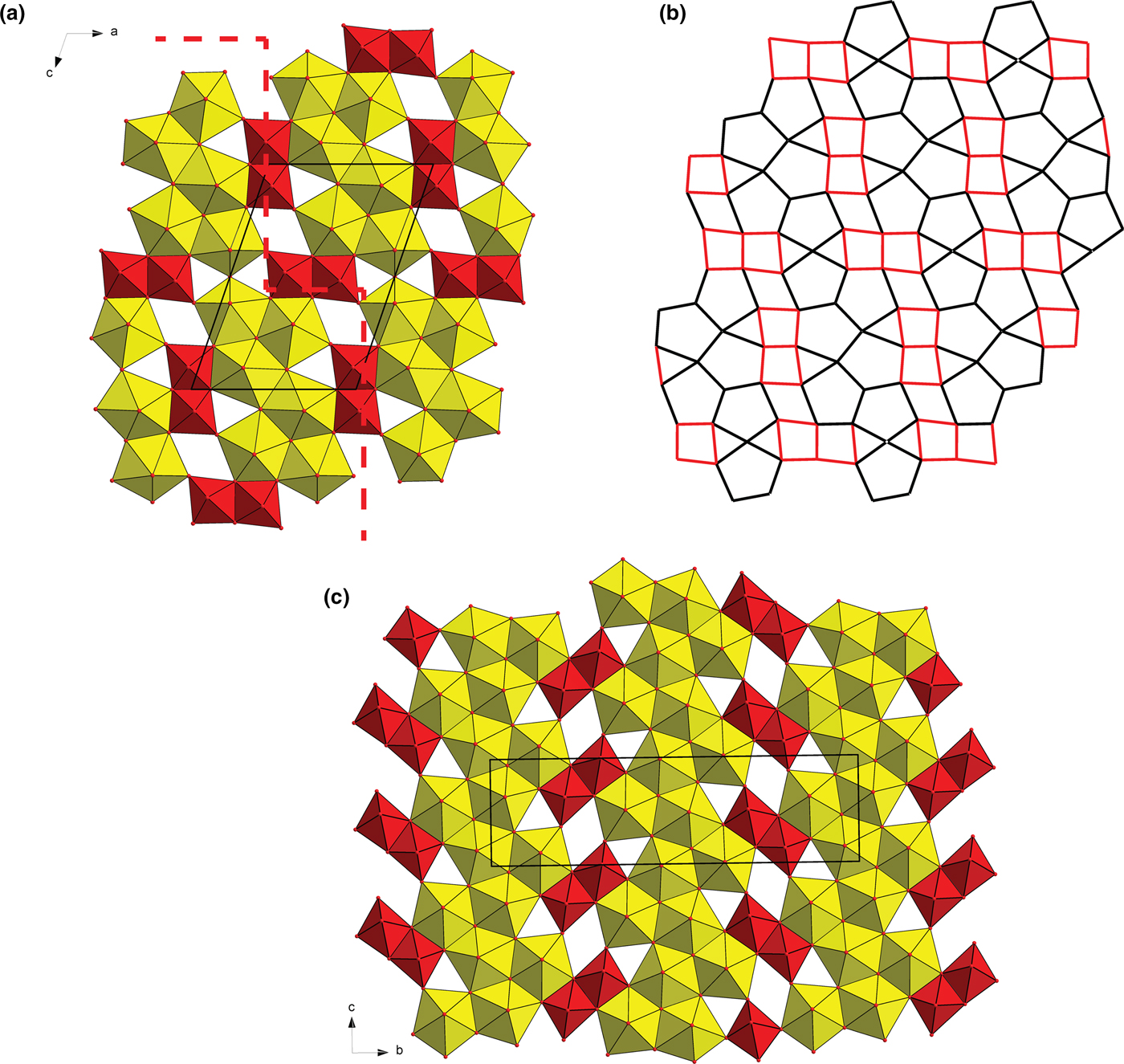
Fig. 8. Structural sheets in uranyl molybdates. (a) Uranyl molybdate sheet in baumoite at t = 0. UO7 bipyramids (yellow) share edges and vertices to form irregular infinite ribbons interconnected through dimers of Mo polyhedra (red), running through as stairs-like (represented by a dashed line). (b) Corresponding representation of the baumoite uranyl-anion topology. (c) Sheet in synthetic Ag10[(UO2)8O8(Mo5O20)] (Krivovichev and Burns, Reference Krivovichev and Burns2003).
Adjacent sheets are separated from each other at an interplanar distance of 7.5 Å (corresponding to half of the b-cell parameter). Between the sheets four symmetrically independent Ba atoms and four symmetrically independent H2O molecules are localised. All the Ba atoms are strongly occupationally and positionally modulated (Figs 5 and 6). The O sites of the corresponding H2O molecules are both occupationally and positionally modulated; the amplitude of their modulation depends on the presence of the corresponding Ba (to which they are coordinated) or presence of the under-and above-laying U-Mo sheet fragments.
Baumoite topology
Sheets in the structure of baumoite are topologically unique, when considering an approximant, idealised structure (Fig. 7). Another structure with similar high U:Mo ratio is a synthetic phase with composition Ag10[(UO2)8O8(Mo5O20)] (Krivovichev and Burns, Reference Krivovichev and Burns2003); however, in that phase there are infinite complex chains of edge-sharing pentagonal uranyl bipyramids which are linked through Mo2O7 dimers, as a part of Mo5O20 interconnecting adjacent sheets (with interplanar spacing ≈ 6.5 Å) (Fig. 8c). Another structure containing similar sheets with isolated dimers of Mo-octahedra (within the sheets) is that of synthetic K2Na8(UO2)8Mo4O24[(S,Mo)O4] (Krivovichev, Reference Krivovichev2014). All the mentioned molybdates are monoclinic.
Incommensurate modulation in baumoite most probably results from long-range positional ordering of Ba, U and Mo atoms. The nature of the baumoite structure resembles the behaviour of composite structures, known for sulfosalts (Petříček et al., Reference Petříček, Malý, Coppens, Bu, Císařová and Frost-Jensen1991; Makovicky et al., Reference Makovicky, Petříček, Dušek and Topa2011; Jaszczak et al., Reference Jaszczak, Rumsey, Bindi, Hackney, Wise, Stanley and Spratt2016). Such structures are based on two, interpenetrating lattice-periodic structures that are mutually incommensurate. Nevertheless, in the case of baumoite, it was not possible to check for such a possibility by the refinement due to limited quality of the data (only satellites of the first order were observed).
The idealised formula of baumoite inferred from the results of the structure refinement and bond-valence considerations is Ba0.5[(UO2)3O8Mo2(OH)3](H2O)~2.72 with Z = 4. However, from the refinement, it is clear that the picture is much more complex, as some of the O atoms within the equatorial plane of the U polyhedra are in fact protonated (as OH) and some of them can also be H2O in some regions of the superspace. This corresponds to the regions where Ba positions are not fully occupied, therefore the structure sheets tend to be electroneutral with an approximate composition [(UO2)3O6Mo2(OH)4(H2O)~1.0]0.
Acknowledgements
We are grateful to Vince Peisley and Glynn Francis for supplying us with specimens of baumoite. Ben Wade of Adelaide Microscopy, The University of Adelaide is thanked for assistance with the microprobe analysis. The infrared spectrum was acquired with the assistance of the Forensic Science Centre, Adelaide. JP and VP acknowledge the support through the project No. LO1603 under the Ministry of Education, Youth and Sports National sustainability program I of the Czech Republic. The authors thank Stuart Mills, Peter Leverett and two anonymous reviewers for helpful suggestions which improved the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.20.